Food and Nutrition Sciences
Vol. 3 No. 1 (2012) , Article ID: 16920 , 7 pages DOI:10.4236/fns.2012.31002
DPPH Radical Scavenging Capacity of Phenolic Extracts from African Yam Bean (Sphenostylis stenocarpa)
![]()
1Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria; 2Department of Biological Sciences, Afe Babalola University, Ado-Ekiti, Nigeria.
Email: *venujiugha@yahoo.com
Received December 3rd, 2010; revised October 27th, 2011; accepted November 5th, 2011
Keywords: African Yam Bean; Phenolics; DPPH Scavenging; Antioxidant Properties; Solvent Extraction; Heat Treatment
ABSTRACT
The phenolic extracts of the seeds of African yam bean (Sphenostylis stenocarpa) were studied using different extraction solvents (70% ethanol, 80% acetone and acidic 70% acetone) and two heat treatment methods (dry heating on a hot plate with acid-washed sea sand at 135˚C for 25 min and wet heating in an autoclave at 120˚C for 20 min). The study examined the total phenolic content (TPC), total flavonoid content (TFC), and condensed tannin content (CTC) of the seed extracts, as well as their free radical scavenging and antioxidant properties. The raw African yam bean seed was dry heated in air oven at 100˚C for 5 min (control). Heat treatments application affected the phenolic contents of the seeds significantly (p < 0.05). The free radical scavenging activity of the phenolics were done using 2,2-diphenyl- 1-picrylhydrazyl (DPPH). The effectiveness of the extract was determined using DPPH at 50 mg/g, 10 mg/g and 5 mg/g of the extracts. At 5 mg/g, the extract was most effective indicating that higher concentration of extract gave higher antioxidant activity. The seed has high antioxidant capacity and an appreciable amount of phenolic extracts.
1. Introduction
Evaluation of the antioxidant activities of natural substances has been of interest in recent years. Antioxidants scavenge free radicals and reactive oxygen species and can be extremely important in inhibiting oxidative mechanisms that lead to degenerative diseases [1]. Free radicals have been implicated as playing a role in the etiology of cardiovascular disease, cancer, Alzheimer’s disease and Parkinson’s disease. The antioxidant capacity of most plant food sources is usually associated with their phenolic contents. Although plant polyphenols such as tannins and flavonoids have problems of astringency and protein binding which have grouped them under the category of anti-nutrients [2,3], they have been found useful as natural antioxidants in scavenging deleterious free radicals released in the body by fat metabolism [4]. Since the well-known synthetic antioxidants, butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are reported to confer some degree of carcinogenicity [5], current research efforts are channeled towards exploiting the antioxidant potentials of natural phenolics. Such compounds are found to be abundant in fruits, vegetables, cereal grains and legumes. In a previous research work, high concentrations of phenolics with strong free radical scavenging potentials were observed in a tropical underutilized legume seed [4].
The African yam bean (Sphenostylis stenocarpa Hochst. Ex A. Rich), a tropical under-exploited grain legume, is grown in West Africa, as a security crop by peasant farmers, for both its edible seeds and tubers. The seed is usually cooked as a local porridge mixed with other ingredients. The mature seed contains on dry weight basis, 20.67% protein, 5.81% fat, 3.87% ash and 60.09% carbohydrates [6]. It has a characteristic problem of being hard-to-cook and is largely underutilized as a minor grain legume, even when it is an important food crop in tropical Africa. The African yam bean will continue to be a valuable constituent of African peasant agriculture. Rural producers of bean pudding (moin-moin) and bean cake (akara) sometimes replace cowpea with African yam bean. Its inherent ability to adapt to diverse environments might have contributed largely to its survival and widespread use. There is no known work on its innate anti-oxidative properties. The present research examines the effect of heat treatment and extraction solvent on the DPPH radical scavenging capacity of the yam bean phenolics.
2. Materials and Methods
2.1. Chemicals and Reagents
Folin-ciocalteu reagent, gallic acid, catechin, acetone, ethanol, acetic acid, tannic acid, NaNO3, AlCl3, methanol, Vanillin, H2SO4 hydrochloric acid (HCl), 2,2-diphenly-1- picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), linoleic acid and β- carotene. These chemicals were purchased from Lixokein chemical Co. (Akure, Nigeria). All other reagents were of analytical grade.
2.2. Preparation of Raw and Dry Heated African Yam Bean
The matured seeds of Sphenostylis stenoscarpa were purchased from the popularly known Oja Oba located at Akure, Nigeria. The seeds (150 g) were dry heated with acid-washed sea sand on an open hot plate (Tecator 1022, Sweden) at 135˚C ± 2˚C for 25 min. During the heat processing, frequent agitation of the seeds together with the sand was done for 3 min using a glass rod for uniform heating of the seeds. The seeds were separated by sieving and cleaned thoroughly. The raw and dry heated seed samples were ground to a fine powder and stored in a separate screw cap bottle at –20˚C before analysis.
2.3. Preparation of Wet Heated African Yam Bean
As done to the raw and dry heat seed, the same processes are followed in the wet heat seed. For the pressure – cooking treatment, the seeds (100 g) were soaked in distilled water in the ratio of 1:10 (seed: water, W/V) for 24 h at room temperature (25˚C). After decanting the water, the soaked seeds (seed: water 1:5 w/v) were subjected to autoclaving for 20 min at 120˚C. Soon after decanting the liquid, the autoclaved seeds were freeze dried. The seeds were ground to a fine powder (particle size of about 0.25 mm) and stored in separate screw cap bottles at –20˚C before analysis as found in raw and dry heated seed.
2.4. Solvent Extraction
Raw and processed ground seed samples (10 g) were extracted by stirring with 100ml of acidic 70% acetone, 80% acetone and 70% ethanol at 25˚C for 24 hr and filtered through Whatman No. 4 filter paper. The residues were re-extracted with an additional 50 ml of 70% acetone for 3 hr. The extract was evaporated under reduced pressure, using a rotary vacuum-evaporator at 40˚C and the remaining water was removed by lyophilization. The freeze-dried extract thus obtained was used directly for total phenolics, total flavonoids and condensed tannins estimation and also for the assessment of antioxidant capacity through DPPH chemical assay.
2.5. Determination of Total Phenolic Content
The total phenolic content (TPC) was determined by a Folin-ciocalteu assay [7,8] using gallic acid (GA) as the standard. The mixture of the sample solution (50 μL), distilled water (3 ml), 250 μL of Folin-ciocalteu’s reagents solution and 70% Na2CO3 (750 μL) was vortexed and incubated for 8min at room temperature. Then, a dose of 950 μL of distilled water was added. The mixture was allowed to stand for 2 hr at room temperature. The absorbance was measured at 765 nm against distilled water as a blank. The total phenol content was expressed aus gallic acid equivalents (mg of GAE/g sample) through the calibration curve of gallic acid. Linearity calibration curve was 50 to 1000 µg/ml (r = 0.99).
2.6. Determination of Total Flavonoid Content
Total flavonoid content was determined using a colorimetric method described previously [4]. Briefly, a dose of 0.25 ml of the seed extract or (+) – catechin standard solution was mixed with 1.25 ml of distilled water in a test tube, followed by adding 75 μL of a 50% NaNO2 solution. After 6 min, 150 μL of a 10% AlCl3∙6H2O solution was added and was allowed to stand for another 5 min before adding 0.5 ml of 1 M NaOH. The mixture was brought to 2.5 ml with distilled water and mixed well. The absorbance was measured immediately against the blank (the same mixture without the sample) at 510 nm using a UV-Visible spectrophotometer (UV 160, shimadzu, Japan). The results were calculated and expressed as micrograms of (+) – catechin equivalents (mg of CAE/g sample) using the calibration curve of (+) – catechin. Linearity range of the calibration curve was 10 to 1000 μg/ml (r = 0.99). The extraction was conducted in triplicate.
2.7. Determination of Condensed Tannin Content
Analysis of condensed tannin content (CTC) was carried out according to the method of Broadhurst and Jones [9] and as modified by Xu and Chang [10]. To 50 μL of the suitably diluted sample, 3 ml of a 4% methanol vanillin solution and 1.5 ml of concentrated hydrochloric acid were added. The mixture was allowed to stand for 15 min and the absorption was measured at 500 nm against methanol as a blank. The amount of condensed tannin was calculated and expressed as mg catechin equivalents (mg of CAE/g sample) using the calibration curve of (+) – catechin. Linearity range of the calibration curve was 50 to 1000 μg/ml (r = 0.99). For each specific sample, triplicate extractions were performed and used for analyses.
2.8. Radical DPPH Scavenging Activity
DPPH-free radical scavenging capacity of legume extracts was evaluated according to the method of Chen and Ho [11] as modified by Xu and Chang [10]. Briefly, a dose of 0.2 ml of the tested seed extracts was added to 3.8 ml ethanol solution of DPPH radical (final concentration was 0.1 mM). The mixture was shaken vigorously for 1min by vortexing and left to stand at room temperature in the dark for 30 min. Thereafter, the absorbance for the sample (Asample) was measured using the UV 160 spectrophotometer at 517 nm against ethanol blank. A negative control (Acontrol) was taken after adding DPPH solution to 0.2 ml of the respective extraction solvent. The percent of DPPH discolouration of the sample was calculated according to the equation:
% discolouration = [1 – (Asample/Acontrol)] × 100.
The free radical scavenging capacity of the seed extracts was expressed as an equivalent of that of Trolox. Every sample was extracted in triplicate and the results were calculated and expressed as micromoles curve of Trolox equivalents (TE) per gram of seed using the calibration curve of Trolox. Linearity range of the calibration curve was 20 to 1000 μM (r = 0.99).
2.9. Statistical Analysis
Analyses were performed in triplicates. The data were statistically evaluated using analysis of variance (ANOVA) with SPSS 15.0. Duncan’s multiple range test was carried out in order to test any significant differences between the solvents used and the treatment methods. Also, the DPPH was calculated in percentage. Significance levels were defined using p = 0.05.
3. Results and Discussion
3.1. Total Phenolic Content
Natural phenolics exert their beneficial health effects mainly through their antioxidant activity [12]. These compounds are capable of decreasing oxygen concentration, intercepting singlet oxygen, preventing 1st—chain initiation by scavenging initial radicals such as hydroxyl radicals, binding metal ion catalysts, decomposing primary products of oxidation to non radical species and breaking chains to prevent continued hydrogen abstracttion from substances [13]. Phenolic compounds contribute to the overall antioxidant activities of the plant foods.
Total phenolic contents (TPC) of the extracts from raw, dried and autoclaved samples of African yam bean using different solvents are presented in Table 1. Seed extracts from different extraction solvents differed significantly
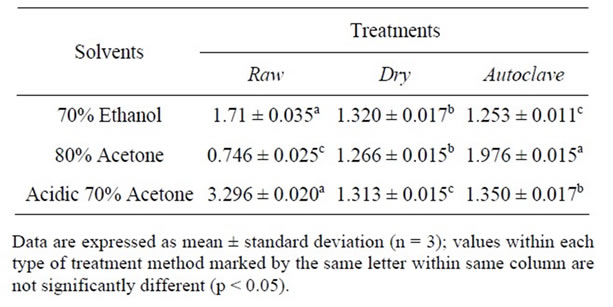
Table 1. Effects of different extraction solvents and treatments methods on phenolic profiles of African yam bean.
(p < 0.05) in their TPC. Meanwhile different extraction method exhibited different TPC. The total phenolic contents of raw sample extracted by different solvents ranged from 0.746 to 3.29 mg GAE/g, dry heated sample ranged from 1.266 to 1.320 mg GAE/g and autoclaved sample from 1.253 to 1.976 mg GAE/g.
Overall, the TPC of the treatment methods (raw, dry and autoclave) was less affected by the different solvents used as evidenced by their smaller variations in the range of the TPC yields. The TPC extracted by the selected solvents were in the following order from high to low; acidic 70% acetone > 70% ethanol > 80% acetone for raw sample, 70% ethanol > acidic 70% acetone > 80% acetone for dry heated sample and 80% acetone > acidic 70% acetone > 70% ethanol for autoclaved treatment sample.
These results suggested that acidic 70% acetone gave the highest yields among the 3 solvents for extracting total phenolics from raw sample and 70% ethanol was the best for dry heated sample, where as 80% acetone was the best for autoclaved seeds of African yam bean. However, the extractable total phenolics (1.716 mg GAE/g and 3.296 mg GAE/g) in raw samples using 70% ethanol and acidic 70% acetone respectively were higher than those of processed samples. Similarly, when drying red grape pomace peels at a temperature of 100 and 140˚C, a significant reduction in both the total extractable polyphenols (18.6% and 32.6% respectively was found [14]. Nonetheless, the dry heated seed sample had the lowest concentration of the respective phenolic fractions possibly due to the poor extractability of phenolics by the formation of cell wall polysaccharide complexes.
3.2. Total Flavonoid Content
Flavonoids are widespread plant secondary metabolites, including flavones, flavanols and condensed tannins. Epidermiological studies suggest that the consumption of flavonoid-rich foods protects against human diseases associated with oxidative stress. In vitro, flavonoids from several plants sources have shown free-radical scavenging activity and protection against oxidative stress. As components of vegetables and fruits, they are regularly contained in human food. However, there were only a few reports on identification and quantification of flavonoids in food legumes. For example, only several reports on common beans [15,16] and peas [17] were documented.
In order to estimate the potential role of flavonoids on antioxidant activity of raw, dry heated and autoclaved samples, TFCs of the extracts were analysed and the results are presented in Table 2. Seed extracts from different extraction solvents differed significantly (p < 0.05) in their TFC. The TFC of raw sample ranged from 0.076 to 0.223 mg CAE/g; dry heated from 0.190 to 1.016 mg CAE/g and autoclaved sample ranged from 0.143 to 0.166mg CAE/g. The TFC yields by the extraction solvents were in the following order from high to low; 70% ethanol, 80% acetone and acidic 70% acetone for raw samples, 80% acetone, acidic 70% acetone, 70% ethanol for dry heated samples and 80% acetone, 70% ethanol, acidic 70% acetone for autoclaved sample. These results suggested that 70% ethanol was the best among the 3 selected solvents for extracting flavonoids from raw sample and 80% acetone was the best for extracting total flavonoids from dry heated and autoclaved samples.
However, the extractable total flavonoids (0.223 mg CAE/g) in raw seed sample using 70% ethanol was higher than those of processed samples. Makkar and Singh [18] reported that there was a decrease in the content of total proanthocyanidins in cassava and Leucaena leaves (10.1% and 21.4%, respectively) when heated at 90˚C for 24 hours. Heat treatment could therefore be said to negatively affect the flavonoid content of similar underutilized leguminous crop seeds. This trend was observed in a previous study using African locust bean seeds (Parkia biglobosa) [4]. However, high temeperature short time (HTST) process would most likely minimize the effect of heat treatment on flavonoids.
3.3. Condensed Tannin Content
The tannins of extracts obtained from raw, dry heated and autoclaved seed samples of African yam bean using
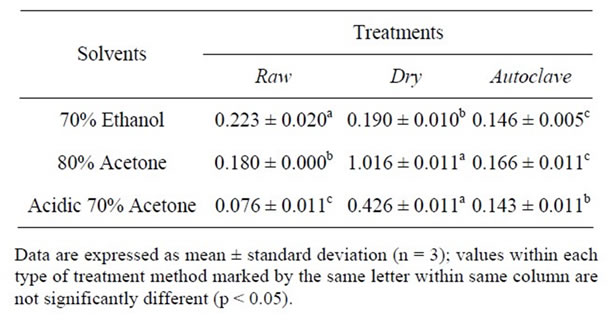
Table 2. Total Flavonoid content (mg catechin equivalents/ g).
70% ethanol, 80% acetone and acidic 80% acetone are shown in the Table 3. Tannins are produced through the process known as condensation of simple phenolics and have a variety of molecular structures. Generally, they are divided into hydrolysable and condensed proanthocyanidins (polymers of flavan-3-ols) [19]. Tannins are biologically active compounds and may have beneficial or adverse nutritional effects. Salunkhe et al. [20] suggested that phenolic substances occur primarily in the seeds of certain pigmented cultivars of sorghum, millets and legumes. Condensed tannins, the predominant phenolic compounds in legumes seeds were widely found in lentil, pea, coloured soybean and common bean [21-24]. Tannins are found mainly in the testa and play an important role in the defense system of seeds that are exposed to oxidative damage by many environmental forces [25].
The effects of various solvent extractions systems on the recovery of condensed tannins from raw, dry heated and autoclaved seed sample of African yam bean are presented in Table 3. Legume extracts from different extraction solvents differed significantly (p < 0.05) in their condensed tannin content (CTC). The (CTC) of raw sample ranged from 0.103 to 0.110 mg CAE/g; dry heated sample ranged from 0.073 to 0.133 mg CAE/g and autoclaved sample ranged from 0.060 to 0.090 mg CAE/g. The CTC yields by the extracting solvents were in the following order from high to low; acidic 70% acetone > dried sample and 80% acetone > acidic 70% acetone > 70% ethanol for autoclaved sample.
These results suggested that acidic 70% acetone was the best for the extraction condensed tannins from raw samples and 70% ethanol for dry heated seed samples whereas 80% acetone was best for autoclaved sample. The tannins extracted (0.110 mg CAE/g) from the raw seed sample using acidic 70% acetone was higher than the processed seed samples. The tannins which were observed in raw sample were also reported previously by Enujiugha [2]. Nonetheless, the low concentration of dry heated treatment of respective phenolic fraction possibly due to the poor extractability of phenolics by the formation of insoluble tannin-protein and tannin-carbohydrate.
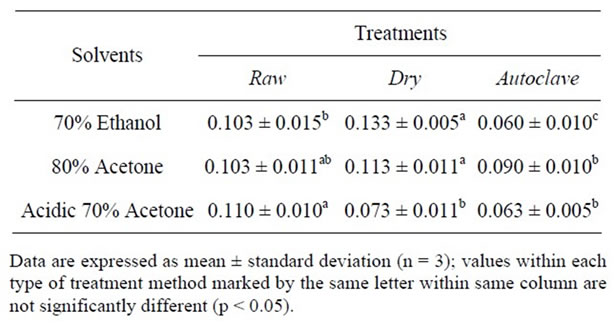
Table 3. Condensed Tannin content (mg catechin equivalents/g).
3.4. Radical DPPH Scavenging Activity
The DPPH (50 mg/g of the extract) value of raw seed sample as presented in Table 4 ranged from 0.083 to 0.090 μmol TE/g, dried sample from 0.076 to 0.083 μmol TE/g and autoclaved sample from 0.076 to 0.083 μmol TE/g. The DPPH value was affected by the extracting solvents in the following order from high to low; acidic 70% acetone > 70% ethanol > 80% acetone of raw sample and acidic 70% acetone > 80% acetone > 70% ethanol of both dried and autoclaved samples. The result further suggested that acidic 70% acetone was the best for extracting and DPPH antioxidant assay from raw, dry heated and autoclaved seed samples.
The DPPH value using 10 mg/g of the extract of the raw sample as shown in Table 5 ranged from 0.086 to 0.090 μmol TE/g; dry from 0.080 to 0.090 μmol TE/g; and 0.080 to 0.090 μmol TE/g for autoclaved sample. The DPPH value was affected by the extracting solvents in the following order from high to low; acidic 70% acetone = 80% acetone > 70% ethanol of raw sample and 70% ethanol = 80% acetone > acidic 70% acetone for dry heated sample, whereas acidic 70% acetone > 80% acetone > 70% ethanol for autoclaved sample. This suggested that acidic 70% acetone was the best for extracting and DPPH antioxidant assay of raw sample.
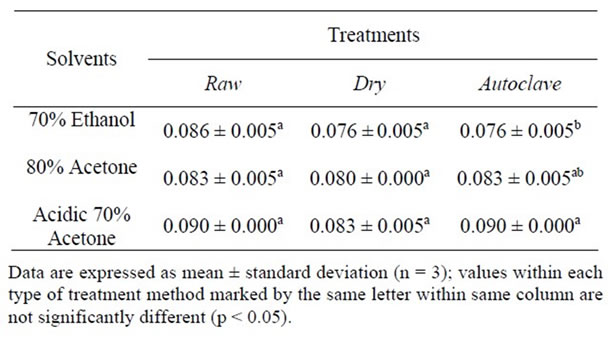
Table 4. DPPH scavenging capacity at 50 mg/g of extract (µmol trolox equivalents/g).

Table 5. DPPH scavenging capacity at 10 mg/g of extract (µ mol trolox equivalents/g).
For 5 mg/g of the extract, the DPPH value was affected by the extracting solvents in the following order from high to low; acidic 70% acetone > 80% acetone > 70% ethanol for raw sample; 80% acetone > 70% ethanol > acidic 70% acetone for dry heated sample whereas, 70% ethanol > 80% acetone > acidic 70% acetone in autoclaved sample. Table 6 results suggest that acidic 70% acetone was the best for extracting and for DPPH antioxidant assay from raw, dry heated and autoclaved seed sample. Such a radical scavenging activity of untreated and treated seed extracts would be related to substitution of hydroxyl groups in the aromatic rings of phenolics, thus contributing to their hydrogen donating ability [26]. However, it could be inferred from the results that increasing the concentration of the phenolic extract or reducing the level of the free radical (in this particular instance, 2,2-diphenyl-1-picrylhydrazyl) in the reaction mixture would ultimately raise the potency to scavenge or chelate free radicals by the seed extract; hence an increase in the antioxidant properties.
4. Conclusions
The results obtained indicated that the use of appropriate solvent gives an effective determination of phenolic yield and antioxidant activity. This study has shown that phenolic extracts from african yam bean seed have antioxidant power and the ability to scavenge free radicals. Acidic 70% acetone was the most effective in the extraction of phenolic content. Extracts obtained from raw seeds registered higher DPPH radical-scavenging activity than both the dryand wet-heat treated seed samples. The study also showed that the higher the concentration of extracts, the higher the capacity to scavenge free radicals. Siddhuraju et al. [27] reported the elevated DPPH radical quenching capacity of tannins extracted from the stem bark of Cassia fistula. The DPPH radical-scavenging efficiency of extracts from heated seed samples might have also been partly contributed by the Maillard reaction products other than the phenolic constituents.
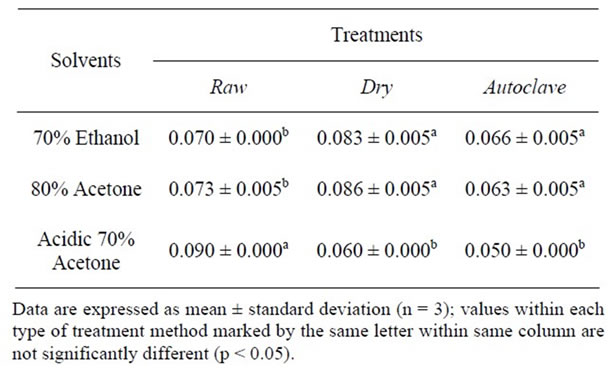
Table 6. DPPH scavenging capacity at 5 mg/g of extract (µmol trolox equivalents/g).
REFERENCES
- A. Cardador-Martinez, G. Loacra-Pina and B. D. Oomah, “Antioxidant Activity in Common Beans (Phaseolus vulgaris L.),” Journal of Agricultural and Food Chemistry, Vol. 50, No. 24, 2002, pp. 6975-6980. doi:10.1021/jf020296n
- V. N. Enujiugha, “Quality Dynamics in the Processing of Underutilized Legumes and Oilseeds,” In: R. Dris, ed., Crops: Growth, Quality and Biotechnology, WFL Publisher, Helsinki, 2005, pp. 732-746.
- V. N. Enujiugha and O. Ayodele-Oni, “Evaluation of Nutrients and Some Anti-nutrients in Lesser-known Underutilized Oilseeds,” International Journal of Food Science and Technology, Vol. 38, No. 5, 2003, pp. 525-528. doi:10.1046/j.1365-2621.2003.00698.x
- V. N. Enujiugha, “The Antioxidant and Free RadicalScavenging Capacity of Phenolics from African Locust Bean Seeds (Parkia biglobosa),” Advances in Food Sciences, Vol. 32, No. 2, 2010, pp. 88-93.
- N. Ito, A. Hagiwara, O. Shibata, T. Ogiso and A. Fukushima, “Induction of squamous Cell Carcinoma in the Fore Stomach of F344 Rats Treated with Butylated Hydroxyanisole,” Gann, Vol. 73, 1982, pp. 332-334.
- A. A. Oshodi, K. O. Ipinmoroti and E. I. Adeyeye, “Functional Properties of Some Varieties of African Yam Bean (Sphenostylis stenocarpa) Flour III,” International Journal of Food Sciences and Nutrition, Vol. 48, No. 4, 1997, pp. 243-250. doi:10.3109/09637489709028568
- P. Siddhuraju, P. S. Mohan and K. Becker, “Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.), a preliminary assessment of crude extracts from Stem bark, leaves, flowers and fruit pulp,” Journal of Food Chemistry, Vol. 79, No. 1, 2002, pp. 61-67. doi:10.1016/S0308-8146(02)00179-6
- V. I. Singleton and R. M. Lamuela-Raventos, “Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent,” Method in Enzymology, Vol. 299, 1999, pp.152-178. doi:10.1016/S0076-6879(99)99017-1
- R. B. Broadhurst and W. T. Jones, “Analysis of Condensed Tannins Using Acidified Vanillin I,” Science Food Agriculture, Vol. 29, No. 9, 1978, pp. 788-794. doi:10.1002/jsfa.2740290908
- B. J. Xu and S. K. C. Chang, “A Comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents,” Journal of Food Science, Vol. 72, No.2, 2007, pp. 160-161.
- C. W. Chen and C. T. Ho, “Antioxidant properties of Polyphenols extracted from green and black teas,” Journal of Food Lipids, Vol. 2, No. 1, 1995, pp. 35-46. doi:10.1111/j.1745-4522.1995.tb00028.x
- Y. Z. Fang, S. Yang and G. Wu, “Free Radicals Antioxidant and Nutrition,” Nutrition Journal, Vol. 18, No. 10, 2002, pp. 872-879. doi:10.1016/S0899-9007(02)00916-4
- F. Shahidi and M. Naczk, “Antioxidant properties of food phenolics,” In: F. Shahidi and M. Naczk, Eds., Phenolics in food and nutraceuticals, CRC Press, Boca Raton, 2003, pp. 1-403.
- J. A. Larrauri, P. Ruperez and F. Saura-Calixto, “Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape promace peels,” Journal of Agricultural and Food Chemistry, Vol. 45, No. 4, 1997, pp. 1390-1393. doi:10.1021/jf960282f
- J. Hempel and H. Bolin, “Quality and quantity of prevailing flavonoid glycosides of yellow and green French beans (Phaseolus vulgaris L.),” Journal of Agricultural and Food Chemistry, Vol. 44, No. 8, 1996, pp. 2114-2116. doi:10.1021/jf9507001
- A. Romani, C. Vignolini Galardi, N. Mulinacci, S. Benedettelli and D. Heimler, “Germplasm characterization of Zolfino landraces (Phaseolus vulgaris L.) by flavonoid content,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 12, 2004, pp. 3838-3842. doi:10.1021/jf0307402
- A. Troszynska, I. Esterella, M. L. Lopez-Amores and T. Hernandez, “Antioxidant activities of pea (Pisum savitum L.), seed coat acetone extract,” LWT—Food Science and Technology, Vol. 35, No. 2, 2002, pp.158-164.
- H. P. S. Makkar and B. Singh, “Effect of Drying Conditions on Tannin, Fibre and Lignin Levels of Mature Oak (Quercus incana) Leaves,” Journal of Food Science and Agriculture, Vol. 54, No. 3, 1991, pp. 323-328. doi:10.1002/jsfa.2740540302
- E. Haslam, “Plant Polyphenols, Vegetable Tannins Revisited,” Cambridge University Press, Cambridge, 1989.
- D. K. Salunkhe, S. I. Jadhav, S. S. Kadam and J. K. Chavan, “Chemical, Biochemical and Biological Significance of Polyphenols in cereals and legumes,” Critical Reviews in food science and Nutrition, Vol. 17, No. 3, 1982, pp. 277-305.
- L. G. Elias, D. G. Fernandez and R. Bressani, “Possible Effects of Seed Coat Polyphenolics on the Nutritional Quality of Bean Protein,” Journal of Food Science, 44, No. 2, 1979, pp. 524-531. doi:10.1111/j.1365-2621.1979.tb03827.x
- C. Vidal-Valverde, J. Frias, I. Estrella, M. J. Gorospe, R. Ruiz and J. Bacon, “Effect of processing on some antinutritional factors of lentils,” Journal Agricultural and Food Chemistry, Vol. 42, No. 10, 1994, pp. 2991-2995. doi:10.1021/jf00046a039
- Y. Takahata, M. Ohnishi-Kameyama, S. Furuta, M. Takahashi and I. Suda, “Highly polymerized procyanidins in brown soybean seed coat wit a high radical-scavenging activity,” Journal Agricultural and Food Chemistry, Vol. 49: No. 12, 2001, pp. 5843-5847. doi:10.1021/jf010307x
- R. Amarowicz, A. Troszynska, N. Braylko-Pikielna and F. Shahidi, “Polyphenolics extracts from legume seeds: correlations between total antioxidant activity, total phenolic content, tannins content and astringency,” Journal of Food Lipids, Vol. 11, No. 4, 2004, pp. 278-286. doi:10.1111/j.1745-4522.2004.01143.x
- A. Troszynska and E. Ciska, “Phenolic compounds of seed coats of white and coloured varieties of pea (Pisum sativum L.) and their total antioxidant activity,” Czech Journal of Food Science, Vol. 20, No. 1, 2002, pp. 15-22.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, “Use of a Free Radical Method to Evaluate Antioxidant Activity,” LWT—Food Science and Technology, Vol. 28, No. 1, 1995, pp. 25-30.
- P. Siddhuraju, P. S. Mohan and K. Becker, “Studies on the Antioxidant Activity of Indian Laburnum (Cassia fistula L.), a Preliminary Assessment of Crude Extracts from Stem bark, Leaves, Flowers and Fruit Pulp,” Journal of Food Chemistry, Vol. 70, No. 1, 2002, pp. 61-67. doi:10.1016/S0308-8146(02)00179-6
NOTES
*Corresponding author.

