Advances in Parkinson's Disease
Vol.2 No.1(2013), Article ID:27825,6 pages DOI:10.4236/apd.2013.21002
Pink1 and parkin demonstrate multifaceted roles when co-expressed with Foxo
![]()
Department of Biology, Memorial University of Newfoundland, St. John’s, Canada; *Corresponding Author: bestave@mun.ca
Received 26 November 2012; revised 28 December 2012; accepted 10 January 2013
Keywords: Pink1; Parkin; Foxo; Drosophila
ABSTRACT
Pink1 has been linked to both autosomal recessive and sporadic forms of Parkinson disease. The Pink1 protein is thought to be involved in mitochondrial protection by interacting with parkin to prevent oxidative damage, maintain mitochondrial integrity and regulate mitophagy. Pink1 and parkin have been linked to components of the insulin receptor (INR) pathway, including PTEN, Akt and Foxo, but their effects in the INR pathway have been largely overlooked. To further investigate the roles of Pink1/parkin, we have performed co-expression studies to determine the effects Pink1 and parkin on the Foxo-induced phenotype of developmental defects in the Drosophila eye. We examined directed expression of Pink1, parkin, Pink1 or parkin mutants, and Pink1 or parkin interfering RNAs (RNAi) with the overexpression of Foxo in the developing eye of Drosophila. Our findings show that reduction of Pink1 suppresses the effects of Foxo overexpression, where co-overexpression with Pink1 or parkin increases the severity of the phenotype. This suggests that Pink1 and parkin are able to increase the proapoptotic effects of Foxo. Contrary to the view that Pink1 and parkin act exclusively as protective proteins in the cell, it is likely that the Pink1/parkin pathway is involved in aspects of cell fate decisions other than degrading toxic proteins and maintaining mitochondrial integrity.
1. INTRODUCTION
Pink1 (PTEN induced putative kinase 1) encodes a serine-threonine kinase which has been linked to autosomal recessive and some sporadic forms of Parkinson disease [1-3]. Targeted to the mitochondria, Pink1 is thought to be involved in mitochondrial protection by preventing oxidative damage and maintaining mitochondrial integrity, where loss of function of Pink1, in humans and in Drosophila melanogaster, show substantial mitochondrial defects in sensitive tissues [4-8]. It is becoming increasingly apparent that protection during cell stress is due to the involvement of Pink1 in mitochondrial fission/fusion events [1,9]. This involvement implicates Pink1 as a key regulator of fission/fusion, acting upstream of the E3 ubiquitin ligase, parkin, to maintain proper mitochondrial integrity and function [4,6,10,11]. In this role, recruitment of parkin to the mitochondria by Pink1 results in the ubiquitination of various mitochondrial proteins, promoting fission and mitophagy [12-15]. In contrast, studies have found that loss of parkin or Pink1 function can also result in increased fission, promoting mitophagy [16,17]. Although the fission/fusion decision is not fully understood, results do highlight the importance of the Pink1/parkin pathway in maintaining mitochondrial homeostasis.
The Pink1/parkin pathway has been linked to components of the insulin receptor (INR) pathway, including: interaction of PTEN with Pink1 [18] and DJ-1 [19,20], an indirect interaction with Akt through parkin [21], an interaction with Akt through DJ-1 [22,23], and transacttivation of Pink1 by Foxo [24,25]. It has also been suggested that Pink1 may activate Foxo indirectly through Sir2 [26]. This is thought to be a protective mechanism, where Foxo activation results in the transcription of genes such as SOD2 and Thor. In addition to genes that promote stress resistance, under conditions of oxidative stress or starvation, Foxo transcription factors may also target genes that promote cell cycle arrest and apoptosis [27,28]. Overexprssion of Foxo has been linked to neurotoxicity [29,30] and overexpression in the developing Drosophila eye results in a characteristic phenotype with reductions in cell number and area [31]. Genetic expression studies using the fly eye have been enormously successful in the study of neurodegeneration. This is due to the conservation of key signaling pathways between humans and Drosophila, and the ease of quantifying degeneration of photoreceptor neurons associated with each Drosophila ommatidium. To further investigate the roles of Pink1/parkin, we have performed expression studies to determine the effects of Pink1 and parkin on the Foxo-induced phenotype of developmental defects in the Drosophila eye. We hypothesized that through an interaction with the INR pathway, or through mitochondrial protective effects, Pink1 and parkin would be capable of alleviating the detrimental effects of Foxo overexpression. In contrast, our findings show that reduction of Pink1 is able to suppress the effects of Foxo overexpression, where co-overexpression of Foxo with Pink1 or parkin results in an increased severity of the Foxo-induced phenotype. These findings suggest a complex role for the Pink1/parkin pathway in cell fate decisions.
2. MATERIALS AND METHODS
2.1. Fly Stocks and Culture
The UAS-Pink1 transgenic line was created from the GH20931 Drosophila melanogaster Pink1 clone [32]. The UAS-murine Foxo1 (UAS-Foxo) and UAS-murine Foxo1AA (UAS-FoxoAA) transgenes are described in Kramer et al. [31] and the GMR-Gal4 UAS-Foxo and GMRGal4;UAS-FoxoAA lines were established through standard means. UAS-parkin was created previously in our laboratory [33]. The Pink1B9 mutant line was provided by Dr. J. Chung [6]. The UAS-Pink1RNAi and UASparkinRNAi lines were provided by Dr. B. Lu [7,34]. The UASGFP control was obtained from the Bloomington stock centre. The parkin45 mutant line was provided by Dr. L. Pallanck [35]. All crosses were performed using standard techniques. All flies were cultured on standard cornmeal/yeast/molasses/agar media at 25˚C.
2.2. Scanning Electron Microscopy of the Drosophila Eye
Flies were aged three days past eclosion on standard cornmeal/yeast/molasses/agar media at 25˚C. Flies were then frozen at −80˚C and examined under dissecting microscope. Flies were mounted, desiccated overnight and coated in gold before photography at 170 times magnification with a Hitachi S-570 SEM. Area of the eye was measured as per the ocular area, regardless of the presence of ommatidia. This was determined by outlining the ocular margin and/or ridge bristles indicating the postocular area. Eye areas and ommatidial counts were compared using GraphPad Prism 5, using unpaired t-test with a significance level of 0.05.
3. RESULTS
3.1. Parkin Increases the Severity of the Foxo-Induced Phenotype
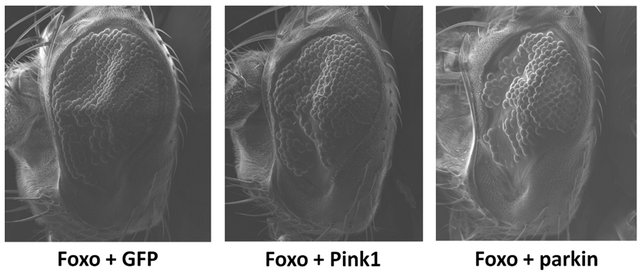
Overexpression of Foxo in the developing Drosophila eye results in a characteristic phenotype with reductions in cell number and area [31]. When co-overexpressed with parkin, there is a significant increase in the severity of the Foxo-induced phenotype (Figure 1), including a significant reduction in number of ommatidia and overall area of the eye (p < 0.0001, df = 31). This suggests that the addition of parkin further reduces the number of viable cells available during eye development. Co-overexpression with Pink1 shows no significant increase in the Foxo-induced reduction of ommatidia (p = 0.1150, df = 29) and area (p = 0.2335, df = 29) (Figure 1).
3.2. Reduction in Pink1 Decreases the Severity of the Foxo-Induced Phenotype
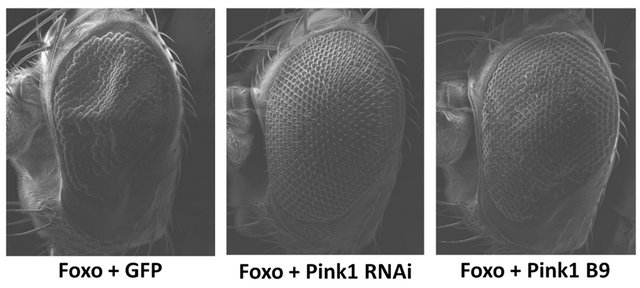
Overexpression of Foxo in a Pink1 mutant background (Pink1B9) results in a significant increase in ommatidia number (p = 0.0008, df = 21) and eye area (p = 0.0015, df = 21) (Figure 2). In addition, co-overexpression of Foxo with Pink1RNAi shows an even greater effect, with significant increases in ommatidia number and area (p < 0.0001, df = 30) (Figure 2). These results suggest that the absence or depletion of Pink1 during eye development is able to alleviate the detrimental effects of Foxo. Overexpression of Foxo in a parkin mutant background (parkin45) or co-overexpression with parkinRNAi resulted
 (a)
(a)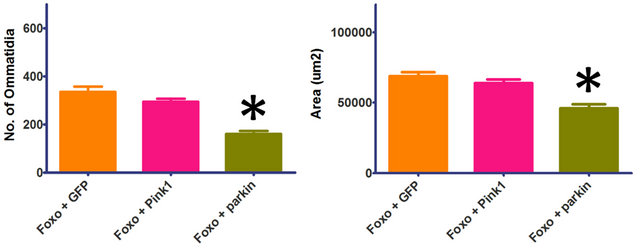 (b)
(b)
Figure 1. Parkin increases the severity of the Foxo-induced phenotype. Co-overexpression of Foxo with parkin shows a significant reduction in number of ommatidia and overall area of the eye. Co-overexpression of Foxo with Pink1 shows no significant increase in the Foxo-induced reduction of ommatidia and area. Genotypes shown include GMR-Gal4 UASFoxo/UAS-GFP (Foxo + GFP), GMR-Gal4 UAS-Foxo/+; UASPink1/+ (Foxo + Pink1), GMR-Gal4 UAS-Foxo/+; UAS-parkin/+ (Foxo + parkin). Error bars indicate standard error of the mean.
 (a)
(a)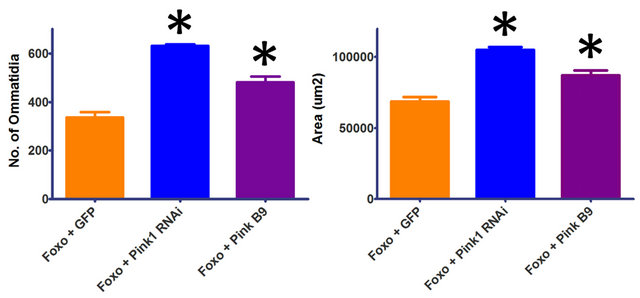 (b)
(b)
Figure 2. Reduction in Pink1 decreases the severity of the Foxo-induced phenotype. Overexpression of Foxo in the Pink1 mutant background (Pink1 B9) results in a significant increase in ommatidia number and eye area. Co-overexpression of Foxo with Pink1RNAi (Pink1 RNAi) shows significant increases in ommatidia number and area. Overexpression of Foxo in a parkin mutant background (GMR-Gal4 UAS-Foxo/+; parkin45/ parkin45) or co-overexpression with parkinRNAi (GMR-Gal4 UAS-Foxo/+; parkinRNAi/+) resulted in apparent synthetic lethality. Genotypes shown include GMR-Gal4 UAS-Foxo/UASGFP (Foxo + GFP), GMR-Gal4 UAS-Foxo/+; UAS-Pink1RNAi/ + (Foxo + Pink1 RNAi), Pink1B9/y; GMR-Gal4 UAS-Foxo/+ (Foxo + Pink1 B9). Error bars indicate standard error of the mean.
in apparent synthetic lethality with no surviving progeny. This implies that the broad protective functions of parkin are necessary to maintain a viable organism during this development.
3.3. Effects of Pink1 and Parkin on the Foxo-Induced Phenotype are Independent of Akt Signalling
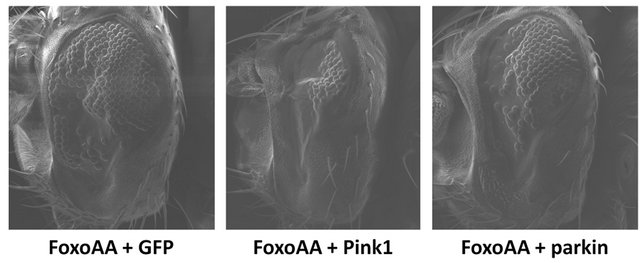
The constitutively active version of Foxo (FoxoAA) contains an alanine substitution at the T1 (T24A) and S1 (S253A) Akt phosphorylation sites [36]. Using FoxoAA, the severity of the Foxo-induced phenotype was seen to increase with Pink1 or parkin co-overexpression (Figure 3). Co-overexpression of Pink1 with FoxoAA results in significant decreases in number of ommatidia and eye area (p < 0.0001, df = 30). Co-overexpression of parkin with FoxoAA also results in significant decreases in number of ommatidia (p = 0.0090, df = 29) and eye area (p = 0.0190, df = 29). The apparent rescue of the Foxo-induced phenotype, seen when co-overexpressing Foxo
 (a)
(a)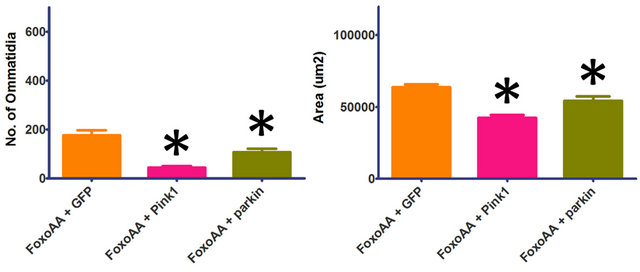 (b)
(b)
Figure 3. Effects of Pink1 and parkin on the Foxo-induced phenotype, independent of Akt signalling. Co-overexpression of FoxoAA (FoxoAA) with Pink1 results in significant decreases in number of ommatidia and eye area. Co-overexpression of FoxoAA with parkin results in significant decreases in number of ommatidia and eye area. Genotypes shown include GMRGal4/UAS-GFP;UAS-FoxoAA/+ (FoxoAA + GFP), GMR-Gal4/ +; UAS-FoxoAA/UAS-Pink1 (FoxoAA + Pink1), GMR-Gal4/+; UAS-FoxoAA/UAS-parkin (FoxoAA + parkin). Error bars indicate standard error of the mean.
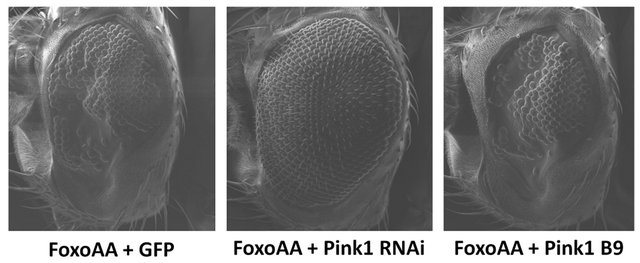
with Pink1RNAi (Figure 2), is maintained when using the constitutively active version, FoxoAA (Figure 4). Cooverexpression of FoxoAA with Pink1RNAi results in a dramatic increase in ommatidia number and eye area (p < 0.0001, df = 30). These results indicate that the Pink1/ parkin interaction with Foxo is independent of Akt signalling. In contrast, there is no significant difference in ommatidia number (p = 0.2131, df = 29) or eye area (p = 0.8027, df = 29) when FoxoAA is overexpressed in the Pink1B9 mutant background (Figure 4). As seen with Foxo overexpression, co-overexpression of FoxoAA with parkinRNAi resulted in apparent synthetic lethality with no surviving progeny.
4. DISCUSSION
Under cell stress conditions, Foxo transcription factors are activated and target genes that promote cell survival and/or apoptosis [27,28]. The transactivation of Pink1 by Foxo [24,25] suggests that there may be recruitment of the Pink1/parkin pathway to help maintain mitochondrial homeostasis during cell stress. Acting in this protective role, we hypothesized that Pink1 and parkin may alleviate the Foxo-induced phenotype of developmental defects in the Drosophila eye. This would presumably be
 (a)
(a)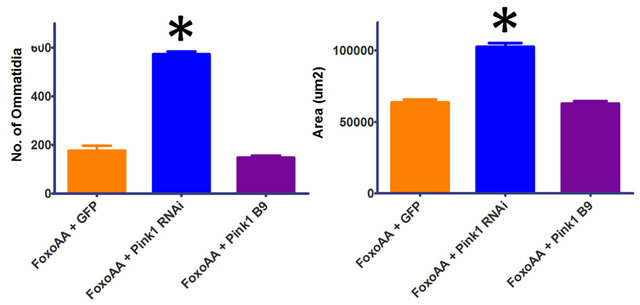 (b)
(b)
Figure 4. Effects of reductions in Pink1 on the Foxo-induced phenotype, independent of Akt signalling. Co-overexpression of FoxoAA (FoxoAA) with Pink1RNAi (Pink1 RNAi) results in a dramatic increase in ommatidia number and eye area. Overexpression of FoxoAA in the Pink1 mutant background (Pink1 B9) is not significantly different from the control. Co-overexpression of FoxoAA with with parkinRNAi (GMR-Gal4/+; UAS-FoxoAA/ parkinRNAi/+) resulted in apparent synthetic lethality. Genotypes shown include GMR-Gal4/UAS-GFP; UAS-FoxoAA/+ (FoxoAA + GFP), GMR-Gal4/+; UAS-FoxoAA/UAS-Pink1RNAi (FoxoAA + Pink1 RNAi), Pink1B9/y; GMR-Gal4/+; UAS-FoxoAA/ + (FoxoAA + Pink1 B9). Error bars indicate standard error of the mean.
due to the regulation of mitochondrial fission/fusion events and through mitophagy to degrade dysfunctional mitochondrial fragments, maintaining the overall mitochondria health of the cell [1,9]. In contrast, our results indicate that the Pink1/parkin pathway may be involved in aspects of cell fate other than protection. Our findings show that co-overexpression of Pink1 or parkin results in an increased severity of the Foxo-induced phenotype, and that a reduction in Pink1 is able to improve on the phenotype. This suggests that there may be a more complex role for the Pink1/parkin pathway under cell stress conditions.
Many transcriptional targets of Foxo have been identified, including molecules involved in metabolism, oxidative stress resistance, cell cycle arrest and apoptosis [27,28]. The Foxo-induced phenotype of developmental defects in the Drosophila eye is likely due to the transcription of pro-apoptotic gene targets. Drosophila studies link phosphorylation of Foxo to neurodegeneration, and have identified the pro-apoptotic Hid gene as one responsible target, where overexpression of Hid causes dramatic eye degeneration [29,30,37]. In contrast, Foxo has also been shown to prevent mitochondrial dysfunction and neurodegeneration, and is proposed to function downstream of Pink1 [26]. In this protective role, Foxo is thought to act through targets including the mitochondrial superoxide dismutase SOD2, a gene involved in stress resistance. With the ability to promote cell survival or apoptosis, changes in Foxo activity may be the mechanism behind the effects of Pink1 and parkin on the Foxo-induced phenotype. Co-overexpression of Pink1 or parkin may affect Foxo activity to increase the transcription of pro-apoptotic targets, thus increasing the severity of the phenotype. Improvement of the Foxoinduced phenotype seen with reductions in Pink1 may also indicate a change in Foxo activity, suggesting that the relationship between Pink1 and Foxo is more complex than Pink1 indirectly activating Foxo downstream. It is also possible that Pink1 and parkin are acting outside of the INR pathway to affect cell survival. The influence of Pink1 and parkin on mitofission/mitofusion events is not fully understood, and some mechanisms involved are closely tied to apoptosis. It may be that under certain conditions these mechanisms are utilized to promote apoptosis instead of cell protection. One example would be the ubiquitination of VDAC by parkin [14]. VDAC is a major component of the permeability transition pore (PTP), and is involved with mitochondrial outer membrane permeabilization (MOMP) through interacttions with pro-apoptotic Bcl-2 proteins [38]. With both the PTP and MOMP implicated as initiators of apoptosis, mitofission events triggered by ubiquitination of VDAC must be controlled so to prevent release of apoptotic factors from the mitochondria. Compounding factors, such as the effects of Hid in the Foxo-induced phenotype, may result in overwhelming instability during increases in Pink1 or parkin expression, making this degree of control impossible. In this instance, Pink1 and parkin may actively participate in the initiation of apoptosis, a novel role for the Pink1/parkin pathway that warrants further investigation.
Expression of the constitutively active version of Foxo (FoxoAA) with co-overexpression of Pink1, parkin or Pink1RNAi seems to indicate that the Pink1/parkin effect on the Foxo-induced phenotype is independent of Akt signalling, supporting the idea that Pink1 and parkin may be acting outside of the INR pathway. In contrast, the change in significance when expressing FoxoAA in the Pink1B9 mutant background suggests that there is Akt involvement. Interestingly, this may indicate that there is a role for Pink1 in the cell that is independent of its kinase function, and that this additional role is somehow involved in the Akt signalling pathway. In this respect, the apparent rescuing effect of the Foxo-induced phenoltype during decreases in Pink1 expression would be partially due to the decrease in kinase activity, and partially due to the presence of the Pink1 protein. Future studies looking into an additional role for Pink1, apart from its kinase function, may yield new interactions and targets in the Pink1/parkin pathway.
5. CONCLUSION
In conclusion, our results show that Pink1 and parkin are able to increase the effects of Foxo in Drosophila, highlighting a possible role for the Pink1/parkin pathway in cell death. In addition, the constitutively active version of Foxo allows us to exclude a general requirement for Akt during increased expression of Pink1 or parkin, however, suggests that there may be an additional role for Pink1 apart from its kinase function. Further studies looking at the effect of Pink1 and/or parkin on Foxo activity, and the role of the Pink1/parkin pathway in mitochondrial fission/fusion events, may uncover underlying mechanisms that mediate a shift towards apoptosis. Moreover, it is likely that the Pink1/parkin pathway is involved in various aspects of cell fate decisions, contrary to the view that Pink1 and parkin act exclusively as protective proteins.
6. ACKNOWLEDGEMENTS
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant and Parkinson Society Canada Friedman Pilot Project Grant to BES. We thank the family of Jerry Friedman for their generosity. Student support was received from the Department of Graduate Studies at Memorial University to AMT. We thank Dr. Liqiu Men (Bio-molecular Imaging Cluster, Memorial University of Newfoundland) for help with SEM.
![]()
![]()
REFERENCES
- Jendrach, M., Gispert, S., Ricciardi, F., Klinkenberg, M., Schemm, R. and Auburger, G. (2009) The mitochondrial kinase Pink1, stress response and Parkinson’s disease. Journal of Bioenergetics Biomembranes, 41, 481-486. doi: 10.1007/s10863-009-9256-0
- Valente, E.M., Abou-Sleiman, P.M., Caputo, V., Muqit, M.M., Harvey, K., Gispert, S., et al. (2004) Hereditary early-onset Parkinson’s disease caused by mutations in Pink1. Science, 304, 1158-1160. doi: 10.1126/science.1096284
- Valente, E.M., Salvi, S., Ialongo, T., Marongiu, R., Elia, A.E., Caputo, V., et al. (2004) Pink1 mutations are associated with sporadic early-onset parkinsonism. Annals of Neurology, 56, 336-341. doi: 10.1002/ana.20256
- Clark, I.E., Dodson, M.W., Jiang, C., Cao, J.H., Huh, J.R., Seol, J.H., et al. (2006) Drosophila Pink1 is required for mitochondrial function and interacts genetically with parkin. Nature, 441, 1162-1166. doi: 10.1038/nature04779
- Exner, N., Treske, B., Paquet, D., Holmstrom, K., Schiesling, C., Gispert, S., et al. (2007) Loss-of-function of human Pink1 results in mitochondrial pathology and can be rescued by parkin. The Journal of Neuroscience, 27, 12413-12418.
- Park, J., Lee, S.B., Lee, S., Kim, Y., Song, S., Kim, S., et al. (2006) Mitochondrial dysfunction in Drosophila Pink1 mutants is complemented by parkin. Nature, 441, 1157- 1161.
- Yang, Y., Gehrke, S., Imai, Y., Huang, Z., Ouyang, Y., Wang, J.W., et al. (2006) Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proceedings of the National Academy of Sciences of United States of America, 103, 10793-10798. doi: 10.1073/pnas.0602493103
- Hoepken, H.H., Gispert, S., Morales, B., Wingerter, O., Del Turco, D., Mulsch, A., et al. (2007) Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiology of Disease, 25, 401-411. doi: 10.1016/j.nbd.2006.10.007
- Chu, C.T. (2010) A pivotal role for Pink1 and autophagy in mitochondrial quality control: Implications for Parkinson disease. Human Molecular Genetics, 19, R28-37. doi: 10.1093/hmg/ddq143
- Yang, Y., Ouyang, Y., Yang, L., Beal, M.F., McQuibban, A., Vogel, H., et al. (2008) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proceedings of the National Academy of Sciences of United States of America, 105, 7070-7075. doi: 10.1073/pnas.0711845105
- Poole, A.C., Thomas, R.E., Andrews, L.A., McBride, H.M., Whitworth, A.J. and Pallanck, L.J. (2008) The Pink1/Parkin pathway regulates mitochondrial morphology. Proceedings of the National Academy of Sciences of United States of America, 105, 1638-1643. doi: 10.1073/pnas.0709336105
- Gegg, M.E., Cooper, J.M., Chau, K.Y., Rojo, M., Schapira, A.H. and Taanman, J.W. (2010) Mitofusin 1 and mitofusin 2 are ubiquitinated in a Pink1/parkin-dependent manner upon induction of mitophagy. Human Molecular Genetics, 19, 4861-4870. doi: 10.1093/hmg/ddq419
- Vives-Bauza, C., Zhou, C., Huang, Y., Cui, M., de Vries, R.L., Kim, J., et al. (2010) Pink1-dependent recruitment of Parkin to mitochondria in mitophagy. Proceedings of the National Academy of Sciences of United States of America, 107, 378-383. doi: 10.1073/pnas.0911187107
- Geisler, S., Holmstrom, K.M., Skujat, D., Fiesel, F.C., Rothfuss, O.C., Kahle, P.J., et al. (2010) Pink1/Parkinmediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature Cell Biology, 12, 119-131. doi: 10.1038/ncb2012
- Ziviani, E., Tao, R.N. and Whitworth, A.J. (2010) Drosophila parkin requires Pink1 for mitochondrial translocation and ubiquitinates mitofusin. Proceedings of the National Academy of Sciences of United States of America, 107, 5018-5023. doi: 10.1073/pnas.0913485107
- Dagda, R.K., Cherra, S.J., 3rd, Kulich, S.M., Tandon, A., Park, D. and Chu, C.T. (2009) Loss of Pink1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. The Journal of Biological Chemistry, 284, 13843-13855. doi: 10.1074/jbc.M808515200
- Lutz, A.K., Exner, N., Fett, M.E., Schlehe, J.S., Kloos, K., Lammermann, K., et al. (2009) Loss of parkin or Pink1 function increases Drp1-dependent mitochondrial fragmentation. The Journal of Biological Chemistry, 284, 22938-22951. doi: 10.1074/jbc.M109.035774
- Unoki, M. and Nakamura, Y. (2001) Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene, 20, 4457-4465. doi: 10.1038/sj.onc.1204608
- Kim, R.H., Peters, M., Jang, Y., Shi, W., Pintilie, M., Fletcher, G.C., et al. (2005) DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell, 7, 263-273. doi: 10.1016/j.ccr.2005.02.010
- Kim, Y.C., Kitaura, H., Taira, T., Iguchi-Ariga, S.M. and Ariga, H. (2009) Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. International Journal of Oncology, 35, 1331-1341.
- Fallon, L., Belanger, C.M., Corera, A.T., Kontogiannea, M., Regan-Klapisz, E., Moreau, F., et al. (2006) A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nature Cell Biology, 8, 834-842. doi: 10.1038/ncb1441
- Aleyasin, H., Rousseaux, M.W., Marcogliese, P.C., Hewitt, S.J., Irrcher, I., Joselin, A.P., et al. (2010) DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proceedings of the National Academy of Sciences of United States of America, 107, 3186-3191. doi: 10.1073/pnas.0914876107
- Yang, Y., Gehrke, S., Haque, M.E., Imai, Y., Kosek, J., Yang, L., et al. (2005) Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proceedings of the National Academy of Sciences of United States of America, 102, 13670-13675. doi: 10.1073/pnas.0504610102
- Mei, Y., Zhang, Y., Yamamoto, K., Xie, W., Mak, T.W. and You, H. (2009) Foxo3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proceedings of the National Academy of Sciences of United States of America, 106, 5153-5158. doi: 10.1073/pnas.0901104106
- Sengupta, A., Molkentin, J.D., Paik, J.H., Depinho, R.A. and Yutzey, K.E. (2011) Foxo transcription factors promote cardiomyocyte survival upon Induction of Oxidative Stress. The Journal of Biological Chemistry 286, 7468- 7478. doi: 10.1074/jbc.M110.179242
- Koh, H., Kim, H., Kim, M.J., Park, J., Lee, H.J. and Chung, J. (2012) Silent information regulator 2 (Sir2) and forkhead box O (Foxo) complement mitochondrial dysfunction and dopaminergic neuron loss in Drosophila PTEN-induced kinase 1 (Pink1) null mutant. The Journal of Biological Chemistry, 287, 12750-12758. doi: 10.1074/jbc.M111.337907
- Greer, E.L. and Brunet, A. (2005) Foxo transcription factors at the interface between longevity and tumor suppression. Oncogene, 24, 7410-7425. doi: 10.1038/sj.onc.1209086
- van der Horst, A. and Burgering, B.M. (2007) Stressing the role of Foxo proteins in lifespan and disease. Nature Reviews: Molecular Cell Biology, 8, 440-450. doi: 10.1038/nrm2190
- Kanao, T., Venderova, K., Park, D.S., Unterman, T., Lu, B. and Imai, Y. (2010) Activation of Foxo by LRRK2 induces expression of proapoptotic proteins and alters survival of postmitotic dopaminergic neuron in Drosophila. Human Molecular Genetics, 19, 3747-3758. doi: 10.1093/hmg/ddq289
- Kanao, T., Sawada, T., Davies, S.A., Ichinose, H., Hasegawa, K., Takahashi, R., et al. (2012) The nitric oxidecyclic GMP pathway regulates Foxo and alters dopaminergic neuron survival in Drosophila. PLoS One, 7, e30958. doi: 10.1371/journal.pone.0030958
- Kramer, J.M., Davidge, J.T., Lockyer, J.M. and Staveley, B.E. (2003) Expression of Drosophila Foxo regulates growth and can phenocopy starvation. BMC Developmental Biology, 3, 5. doi: 10.1186/1471-213X-3-5
- Todd, A.M. and Staveley, B.E. (2008) Pink1 suppresses alpha-synuclein-induced phenotypes in a Drosophila mo- del of Parkinson’s disease. Genome, 51, 1040-1046. doi: 10.1139/G08-085
- Haywood, A.F. and Staveley, B.E. (2004) Parkin counteracts symptoms in a Drosophila model of Parkinson’s disease. BMC Neuroscience, 5, 14. doi: 10.1186/1471-2202-5-14
- Yang, Y., Nishimura, I., Imai, Y., Takahashi, R. and Lu, B. (2003) Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron, 37, 911-924. doi: 10.1016/S0896-6273(03)00143-0
- Greene, J.C., Whitworth, A.J., Kuo, I., Andrews, L.A., Feany, M.B. and Pallanck, L.J. (2003) Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proceedings of the National Academy of Sciences of United States of America, 100, 4078-4083. doi: 10.1073/pnas.0737556100
- Biggs, W.H., Meisenhelder, J., Hunter, T., Cavenee, W.K. and Arden, K.C. (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proceedings of the National Academy of Sciences of United States of America, 96, 7421-7426. doi: 10.1073/pnas.96.13.7421
- Wilson, R., Goyal, L., Ditzel, M., Zachariou, A., Baker, D.A., Agapite, J., et al. (2002) The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nature Cell Biology, 4, 445-450. doi: 10.1038/ncb799
- Green, D.R. and Kroemer, G. (2004) The pathophysiology of mitochondrial cell death. Science, 305, 626-629.

