American Journal of Plant Sciences
Vol.06 No.10(2015), Article ID:57586,8 pages
10.4236/ajps.2015.610164
Co-Inoculation of Soybean with Bradyrhizobium and Azospirillum Promotes Early Nodulation
Amaral Machaculeha Chibeba1,2, Maria de Fátima Guimarães1, Osmar Rodrigues Brito1, Marco Antonio Nogueira1,2, Ricardo Silva Araujo3, Mariangela Hungria2*
1Department of Agronomy, Universidade Estadual de Londrina (UEL), Londrina, Brazil
2Embrapa Soja, Londrina, Brazil
3Total Biotecnologia Indústria e Comércio Ltda, Curitiba, Brazil
Email: amaral_chibeba@yahoo.com, mfatima@uel.br, osmar@uel.br, marco.nogueira@embrapa.br, rsaraujo@totalbiotecnologia.com.br, *mariangela.hungria@embrapa.br
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 April 2015; accepted 27 June 2015; published 30 June 2015
ABSTRACT
Soybean inoculation with elite strains of Bradyrhizobium to improve nodulation, N2 fixation, and grain yield is well established worldwide. However, when grown in soils where N is deficient, soybean undergoes an initial phase of N starvation that may last up to 20 days after seedling germination due to the lack of synchronism between the phase when seed N reserves are exhausted and the moment when plants begin to benefit from the nitrogen fixed by the bacteria. Practices that promote early nodulation may play a key role in reducing the N starvation period. Azospirillum is a plant growth promoting rhizobacteria (PGPR) that can stimulate root hair formation and root growth, creating more sites for early root infection and nodule formation by N2-fixing Bradyrhizobium spp. In this study, the effects of co-inoculating soybeans with Bradyrhizobium spp. and Azospirillum brasilense on nodulation precocity and N2 fixation were evaluated under greenhouse and field conditions. Nodule number and dry weight, as well as plant and root dry weight and N accumulated in shoots at 15, 18, 21, 24 and 30 days after emergence (DAE) were evaluated in response to inoculation with Bradyrhizobium spp. alone or when co-inoculated with Azospirillum sp. In the greenhouse, co-inoculated plants nodulated precociously as indicated by a significant increase (p < 0.05) in nodule biomass observed at (include) 21 DAE. More pronounced effects of co-inocula- tion were observed in the field as early as 18 DAE, suggesting that the presence of Azospirillum helps plants to overcome environmental stresses.
Keywords:
Biological Nitrogen Fixation, Inoculation, Soybean, Symbiosis

1. Introduction
The practice of seed inoculation with nitrogen-fixing bacteria with the objective of increasing soybean [Glycine max (L.) Merr.] yield has long been successfully established worldwide. In Brazil, 100% of the areas grown with soybeans today have been inoculated at least once, and about 60% of these areas are re-inoculated every year [1] [2] . However, frequent climatic limitations and increased incidence of pests and diseases at sowing, among other factors, may reduce soybean nodulation, thus compromising nitrogen (N) supply to the crop.
When grown in soils where N is deficient, even when effective rhizobia are present, legumes may undergo an initial phase of N starvation that may last up to 15 to 20 days after seedling emergence [3] -[5] . This is due to the absence of synchronism between the phase when seed N reserves are exhausted and the moment when plants begin to benefit from the nitrogen fixed by the bacteria in the recently formed root nodules [3] [4] [6] . Therefore, any practice that promotes earlier nodulation may help to reduce the N starvation period.
Besides rhizobia, another group of beneficial soil microorganisms contains associative plant growth promoting rhizobacteria (PGPR) [7] which have also been exploited in agriculture with remarkable success. PGPR perform an array of biological processes that are beneficial to plants, including the production of plant growth hormones [8] [9] , such as auxins [10] [11] , gibberellins [12] , and cytokinins [11] [13] . Other PGPR attributes in- clude inducing plant resistance to stresses and diseases [14] , solubilization of phosphates [15] -[18] , and atmospheric N2 fixation [19] .
Among the PGPR, the genus Azospirillum has long been employed in plant inoculants worldwide, and, more recently, in Brazil too [20] . A clear result of the action of Azospirillum spp. is an increase in the production of root hairs [21] [22] and in root growth, benefiting plants with better absorption of water and nutrients [23] . The co-inoculation of soybeans with Azospirillum and Bradyrhizobium is known to result in increased N2 fixation and grain yield [24] -[27] .
Given that the formation of legume root nodules is initiated in root hairs [28] , it is possible that co-inoculation with Azospirillum and Bradyrhizobium may result in earlier nodule formation in soybean due to the increased number of root hairs available to be infected by rhizobia. However, early nodulation is difficult to evaluate in the field because of the presence of previously established populations of both bacteria genera. Therefore, the objective of this study was to evaluate, under controlled conditions in the greenhouse, the effect of co-inocula- tion of soybean grown in sterile substrate with both Azospirillum brasilense and Bradyrhizobium spp., on the precocity of nodulation. The results obtained under the greenhouse were then validated in field conditions.
2. Materials and Methods
2.1. Experimental Site
Both the greenhouse and field experiments were conducted during the summer season of 2012/2013, at the experimental station of Empresa Brasileira de Pesquisa Agropecuária, Centro Nacional de Pesquisa de Soja (Embrapa Soja). The station is located in Londrina (23˚11'S, 51˚11'W, 620 m altitude, Köpen-Geiger climate type Cfa), in the State of Paraná, Brazil.
The field trial was performed in an oxisol (Latossolo Vermelho Eutroférrico, Brazilian classification; Rhodic Eutrudox, American classification). At the beginning of the experiment, twenty soil subsamples (0 - 20 cm) were taken to evaluate soil chemical properties and soil granulometry, as described before [29] . Soil chemical properties, granulometry and bacterial population are shown in Table 1.
2.2. Bacterial Inoculants and Plant Cultivars Employed
Inoculants employed in this study were made of strains SEMIA 5079 (=CPAC 15) of Bradyrhizobium japonicum, SEMIA 5080 (=CPAC 7) of B. diazoeffficiens, both at 2 × 109 cells∙mL−1, and Ab-V5 and Ab-V6 of Azospirillum brasilense, both at 1 × 108 cells∙mL−1. All strains are used for commercial production of inoculants in Brazil. Inoculation treatments consisted of 1) a combination of both strains of Bradyrhizobium, without Azospirillum; and 2) a combination of inoculants containing the two strains of Bradyrhizobium and with the two strains of Azospirillum. For all experiments, the commercial soybean cultivar BRS 295RR, well adapted to the conditions of Londrina, was the host of choice.
2.3. Greenhouse Experiment
Seeds were surface disinfested by immersion in 80% ethanol for two to three minutes, followed by immersion in a 10% sodium hypochlorite (NaClO) solution for three to four minutes and then washed with sterile distilled water several times to remove all traces of hypochlorite [30] .
Treatments consisted of the inoculation with 1) 1.2 × 106 cells seed−1 of Bradyrhizobium spp. and 2) 1.2 × 106 cell seed−1 of Bradyrhizobium spp. + 1.2 × 105 cells seed−1 of Azospirillum brasilense. Seed inoculation was accomplished by mixing seeds and inoculants and leaving the mixtures in contact for half an hour before sowing.
After inoculation, four seeds were sown in each of the pre-sterilized modified Leonard jars [30] containing a 1:1 (v:v) mixture of sand and vermiculite and N-free nutrient solution with pH adjusted to 6.6 - 6.8 [31] . Seven days after sowing seedlings were thinned to two per jar. All along the experiment, jars received sterile N-free nutrient solution as needed.
Temperature and relative humidity of the air inside the greenhouse, and temperature at 2 - 3 cm inside the substrate in the Leonard jars were measured in the morning and afternoon throughout the duration of the experiment. Daily average air temperatures at 9 a.m. and 3 p.m. were 26.0˚C ± 1.7˚C and 33.5˚C ± 1.6˚C, respectively. Daily average relative humidity of the air inside the greenhouse at 9 a.m. and 3 p.m. were 66.4% ± 13.7% and 52.5% ± 7.9%, respectively. Daily average temperatures of the substrate in the jars at 9 a.m. and 3 p.m. were 26.4˚C ± 1.7˚C and 30.4˚C ± 3.0˚C, respectively.
The experiment was conducted under a completely randomized design with a 2 × 5 factorial, being two inoculants, Bradyrhizobium spp. and Bradyrhizobium spp. + A. brasilense, and five sampling times, 15, 18, 21, 24, and 30 days after emergence (DAE), with four replicates.
2.4. Field Experiment
Before sowing the experiment, soil rhizobial population was estimated (Table 1) by the most-probable-number (MPN) technique using cultivar BRS 295RR as the trap host as described before [30] . For the estimation of the populations of free-living diazotrophic bacteria, counts were made in semi-solid NFb medium [32] . Soil pH (Table 1) was previously corrected to reach 5.5 - 5.8 at sowing. One week before sowing fertilizer was applied at a rate of 300 kg∙ha−1 of a 0-28-20 NPK; no N fertilizer was applied. The same treatments and concentrations of cells per seed of the greenhouse experiment were tested in the field experiment. Seed inoculation was performed by mixing both inoculants with the seeds and allowing them to dry in the shade for 1 h. Seeds were sown in 10 m-long rows, with eight rows per replicate and distance between rows was of 50 cm. Plots were separated by 1.5 m-wide terraces to avoid cross contamination.
The experimental design was the same of the greenhouse experiment, but with six replicates. Five plant samples were taken from each of the six replicates at 15, 18, 21, 24 and 30 DAE, avoiding the two external lines.
Average maximum and minimum temperatures during the experiment were 28.8˚C and 19.2˚C, respectively, and accumulated rainfall was 202.4 mm well distributed in the 30 days of the experiment.
Table 1. Soil chemical properties, granulometry and population of soybean bradyrhizobia and free-living diazotrophic bacteria at the 0 - 20 cm layer of the field site in Londrina. Analyses performed before sowing.
aSB, sum of bases; bBS, bases saturation = [(K + Ca + Mg)/Tcec] × 100, where Tcec = K + Ca + Mg + total acidity at pH 7.0 (H + Al); cEstimated by the most probable number (MPN) method [30] using soybean as trap plants; CFU, colony forming units; dEstimated by dilutions and counts in semi- solid NFb medium [32] .
2.5. Evaluation of Nodulation, Dry Matter Accumulation and N Content in the Shoots
From the greenhouse experiment, at each sampling date, all plants from four replicates were cut at the cotiledonary node, separating roots and shoots. Shoots were stored in paper bags and dried in the oven (approximately 50˚C, 72 h) until constant weight was obtained. Roots were carefully removed from the jars to avoid nodule loss and were rinsed over a sieve. The roots and all the nodules collected were dried until constant weight was obtained. After drying, nodules were removed from the roots and allowed to dry further. Nodules were then counted and dry nodules and shoots were weighed.
From the field, five plants were randomly taken from each plot, digging carefully around the roots with a straight shovel. Shoots were separated from the roots at the cotiledonary node. Excess soil was removed from the roots over a sieve in order to avoid nodule loss. All other procedures were performed as in the greenhouse experiment.
After weighing the material from the greenhouse and the field, shoots were ground (18 mesh) and employed for determination of N content by the salicylate green spectrophotometric method [33] , with readings taken at 697 nm.
2.6. Statistical Analyses
Data were analyzed with SISVAR [34] statistical package. Data were also statistically analyzed by analysis of variance (ANOVA) preceded by verification of normality of residues and variance homogeneity [35] .
3. Results and Discussion
In the greenhouse, plants responded positively but not significantly to the dual inoculation with Bradyrhizobium and Azospirillum when nodule number (NN), root (RDW) and shoot (SDW) dry weight were analyzed (Table 2). However, a significant increase (p < 0.05) in nodule dry weight (NDW) and precocity of nodulation was observed in response to co-inoculation at 21 DAE, as well as total N in shoots (TNS) at 24 DAE (Table 2).
Correlation coefficients among all variables analyzed are presented in Table 3. Although a highly significant (r = 0.92, p < 0.001) correlation between NN and NDW was observed, NDW was more strongly correlated with SDW (r = 0.95, p < 0.001). Positive and highly significant (r = 0.95, p < 0.001) correlation between SDW and TNS was also observed.
Highly significant correlation between NDW and TNS has been reported previously on soybean both under greenhouse and field conditions [36] -[40] . Correlation coefficients between NDW and TNS obtained in this study are similar to those reported from soybean experiments grown in sterile substrate under greenhouse controlled conditions (means of three strains of Bradyrhizobium on 152 cultivars, r = 0.80, p < 0.001 [36] ) and in pots containing non-sterile soil [means of 152 cultivars, r = 0.697, p < 0.001 [38] ]. Furthermore, it has been demonstrated that NDW is the best variable to evaluate biological N2 fixation in the field [40] , since it is highly correlated with SDW and TNS, which was confirmed in this study. It is also important to mention the high correlation between SDW and TNS obtained in our study, emphasizing that under N-limiting conditions the simple measurement of SDW could be used to assess nitrogen fixation, eliminating the need to determine TNS [36] [38] -[41] .
Differences due to the inoculation treatments were more noticeable in the field (Table 4) than in the greenhouse (Table 2), where growth conditions were controlled and optimized. Significant (p < 0.05) differences in NN in response to co-inoculation could be observed at 21 and 24 DAE, whereas differences in NDW could be observed as early as 18 DAE till the last evaluation (Table 4). At this early sampling time, the benefits due to the presence of Azospirillum under field conditions, where environmental stresses are frequent, were translated into an expressive 90% increase in NDW very early in the growth stage (18 DAE) (Table 4).
The increased nodulation due to co-inoculation reflected in larger (p < 0.05) plant biomass (SDW) from 18 to 21 DAE and higher nitrogen fixation, indicated by a greater (p < 0.05) TNS from 18 DAE to the last evaluation, at 30 DAE. Considering all five evaluations, co-inoculated plants were 18% superior to those that received only Bradyrhizobium inoculation when SDW was analyzed, and 32% superior when TNS was considered (Table 4).
The results from this study are in line with those from previous studies on the benefits of co-inoculation of soybeans with Bradyrhizobium spp. and A. brasilense on nodule numbers [26] [42] -[44] , nodule dry matter [24] [42] , and root dry matter [25] [43] [45] . It is possible that the increased nodulation of co-inoculated plants is a
Table 2. Nodule number (NN) and dry weight (NDW), root (RDW) and shoot (SDW) dry weight and total N accumulated in the shoots (TNS) of soybean plants, cultivar BRS 295RR, inoculated with Bradyrhizobium alone or co-inoculated with Bradyrhizobium and Azospirillum, and grown in sterile substrate under controlled greenhouse conditions. Plant samples were harvested at 15, 18, 21, 24 and 30 days after emergence (DAE).
1Brady = B. japonicum strain SEMIA 5079 and B. diazoefficiens strain SEMIA 5080; Azo = A. brasilense strains Ab-V5 e Ab-V6; 2Data are means of four replicates and when followed by distinct lower case letters on the same line are significantly different; when followed by different uppercase letters on the column, data are significantly different for the variable they represent, within the same sampling time. Mean comparisons performed by Tukey’s test at p < 0.05.
Table 3. Spearman rank correlation matrix1 among variables nodule number (NN) and dry weight (NDW), root (RDW) and shoot (SDW) dry weight and total N accumulated in the shoots (TNS) of soybean plants, cultivar BRS 295RR analyzed in the greenhouse experiment.
1p < 0.001 for all variables.
response to the alterations caused by Azospirillum in root morphology [18] [46] , resulting in more nodulation sites. Interestingly, there are also reports that co-inoculation increases nodulation of the root crown and of the tap root [25] [43] [47] [48] forming nodules that are critical to seedling establishment and higher rates of nitrogen fixation [38] [41] [49] . The fact that co-inoculation of legumes with rhizobia and Azospirillum results in increased nodulation of the root crown, when compared to the inoculation of rhizobia alone [50] suggests that Azospirillum may induce earlier nodulation, since the nodules present in the root crown are the first to be formed and are directly related to the inoculated strains of rhizobia [36] [41] [49] [50] . Therefore, it is possible that the
Table 4. Nodule number (NN) and dry weight (NDW), shoot dry weight (SDW) and total N accumulated in shoots (TNS) of soybean plants, cultivar BRS 295RR, inoculated with Bradyrhizobium alone or co-inoculated with Bradyrhizobium and Azospirillum, and grown under field conditions. Plant samples were taken at 15, 18, 21, 24, and 30 days after emergence (DAE).
1Brady = Bradyrhizobium japonicum strain SEMIA 5079 and Bradyrhizobium diazoefficiens strain SEMIA 5080; Azo = Azospirillum brasilense strains Ab-V5 and Ab-V6; 2Data are means of six replicates and when followed by distinct lowercase letters on the same line are significantly different; when followed by different uppercase letters on the column, data are significantly different for the variables they represent, within the same sampling time. Mean comparisons performed by Tukey’s test at p < 0.05.
increased nodulation of co-inoculated plants is a response to the alterations caused by Azospirillum in root morphology [18] [46] , resulting in more nodulation sites.
It was very important to observe that co-inoculation resulted in earlier nodulation. Precocious nodule formation is very critical for the establishment of the symbiosis and onset of N2 fixation, particularly in the case of crops with a short growth cycle, as is the case with soybean. The positive effects of co-inoculation were even more evident in the field, where limiting factors that affect nodulation, such as temperature and rainfall frequently result in poor establishment of the symbiosis [51] [52] .
Increased plant growth observed in this study in response to inoculation with Azospirillum is in agreement with previously reported results [25] [26] [43] [47] and may be attributed to increased availability of N, due to nitrogen fixation [18] and to the presence of hormones that stimulate plant root growth [25] [43] [48] [50] among other factors.
In previous studies done by our research group, we observed that co-inoculation of soybean with Bradyrhizobium and Azospirillum has resulted in increased grain yield [26] [53] , and the technology has been approved and registered for utilization with soybean by the Brazilian regulatory agency. In the present study, we observed that co-inoculation is beneficial throughout the plant’s growth cycle, since it favors the precocity of nodulation, a critical step for the establishment of the symbiotic relationship between soybean plants and N2-fixing bacteria. Earlier nodulation may be particularly important for crops with short growth cycles such as soybean, thus extending and increasing the benefits of inoculation and N2 fixation.
4. Conclusion
Co-inoculation of soybean with Bradyrhizobium spp. and Azospirillum brasilense results in earlier nodulation of soybean plants.
Acknowledgements
A. M. Chibeba acknowledges a PhD fellowship from Wageningen University under the N2Africa Project. Study partially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil), CNPq- Repensa (562008/2010-1) and Total Biotecnologia Indústria e Comércio S/A. M. F. Guimarães and M. Hungria are also research fellows from CNPq. Approved for publication by the Editorial Board of Embrapa Soja as manuscript number 312/2014.
References
- Chang, W.-S., Lee, H.-I. and Hungria, M. (2015) Soybean Production in the Americas (Chapter 41). In: Lugtenberg, B., Ed., Principles of Plant-Microbe Interactions, Springer, Switzerland, 393-400. http://dx.doi.org/10.1007/978-3-319-08575-3_41
- Hungria, M. and Campo, R. (2007) Inoculantes Microbianos: Situação no Brasil. In: Izaguirre-Mayoral, M.L., La- bandera, C. and Sanjuan, J., Eds., Biofertilizantes en Iberoamérica: Visión Técnica, Científica y Empresarial, Cyted/ Biofag, Montevideo, 22-31.
- Hungria, M., Barradas, C. and Wallsgrove, R. (1991) Nitrogen Fixation, Assimilation and Transport during the Initial Growth Stage of Phaseolus vulgaris L. Journal of Experimental Botany, 42, 839-844. http://dx.doi.org/10.1093/jxb/42.7.839
- Abendroth, L.J., Elmore, R.W. and Ferguson, R.B. (2006) G06-1621 Soybean Inoculation: Understanding the Soil and Plant Mechanisms Involved (Part One of a Two-Part Series). Historical Materials from University of Nebraska-Lincoln Extension. http://digitalcommons.unl.edu/extensionhist/2078
- Atkins, C.A., Pate, J.S., Sanford, P.J., Dakora, F.D. and Matthews, I. (1989) Nitrogen Nutrition of Nodules in Relation to “N-Hunger” in Cowpea (Vigna unguiculata L. Walp). Plant Physiology, 90, 1644-1649. http://dx.doi.org/10.1104/pp.90.4.1644
- Zilli, J., Valicheski, R.R., Rumjanek, N.G., Simões-Araújo, J.L., Freire Filho, F.R. and Neves, M.C.P. (2006) Efi- ciência Simbiótica de Estirpes de Bradyrhizobium Isoladas de Solo do Cerrado em Caupi. Pesquisa Agropecuária Bra- sileira, 41, 811-818. http://dx.doi.org/10.1590/S0100-204X2006000500013
- Marschner, H. (1995) Nitrogen Fixation. In: Marschner, H., Ed., Mineral Nutrition of Higher Plants, 2nd Edition, Aca- demic Press, London, 201-228. http://dx.doi.org/10.1016/b978-012473542-2/50009-2
- Saharan, B. and Nehra, V. (2011) Plant Growth Promoting Rhizobacteria: A Critical Review. Life Science and Medical Research, 21, 1-30.
- Hirsch, A., Fang, Y., Asad, S. and Kapulnik, Y. (1997) The Role of Phytohormones in Plant-Microbe Symbioses. Plant and Soil, 194, 171-184. http://dx.doi.org/10.1023/A:1004292020902
- Ashraf, M.A., Rasool, M. and Mirza, M.S. (2011) Nitrogen Fixation and Indole Acetic Acid Production Potential of Pacteria Isolated from Rhizosphere of Sugarcane (Saccharum officinarum L.). Advances in Biological Research, 5, 348-355.
- Tien, T., Gaskins, M. and Hubbell, D. (1979) Plant Growth Substances Produced by Azospirillum brasilense and Their Effect on the Growth of Pearl Millet (Pennisetum americanum L.). Applied and Environmental Microbiology, 37, 1016-1024.
- Bottini, R., Fulchieri, M., Pearce, D. and Pharis, R.P. (1989) Identification of Gibberellins A1, A3, and Iso-A3 in Cultures of Azospirillum lipoferum. Plant Physiology, 90, 45-47. http://dx.doi.org/10.1104/pp.90.1.45
- Strzelczyk, E., Kampert, M. and Li, C. (1994) Cytokinin-Like Substances and Ethylene Production by Azospirillum in Media with Different Carbon Sources. Microbiological Research, 149, 55-60. http://dx.doi.org/10.1016/S0944-5013(11)80136-9
- Gurska, J., Wang, W., Gerhardt, K.E., Khalid, A.M., Isherwood, D.M., Huang, X.-D., Glick, B.R. and Greenberg, B.M. (2009) Three Year Field Test of a Plant Growth Promoting Rhizobacteria Enhanced Phytoremediation System at a Land Farm for Treatment of Hydrocarbon Waste. Environmental Science and Technology, 43, 4472-4479. http://dx.doi.org/10.1021/es801540h
- Bashan, Y. and Holguin, G. (2002) Plant Growth-Promoting Bacteria: A Potential Tool for arid Mangrove Reforestation. Trees, 16, 159-166. http://dx.doi.org/10.1007/s00468-001-0152-4
- Bhattacharyya, P.N. and Jha, D.K. (2012) Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World Journal of Microbiology and Biotechnology, 28, 1327-1350. http://dx.doi.org/10.1007/s11274-011-0979-9
- Glick, B.R. (2012) Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica, 2012, Article ID: 963401. http://dx.doi.org/10.6064/2012/963401
- Saikia, S.P., Dutta, S.P., Goswami, A., Bhau, B.S. and Kanjilal, P.B. (2010) Role of Azospirillum in the Improvement of Legumes. In: Khan, M.S., Zaidi, A. and Musarrat, J., Eds., Microbes for Legume Improvement, Springer-Verlag, Wien, 389-408. http://dx.doi.org/10.1007/978-3-211-99753-6_16
- Dobereiner, J. and Pedrosa, F.O. (1987) Nitrogen-Fixing Bacteria in Nonleguminous Crop Plants. Springer, Madison.
- Hungria, M., Campo, R.J., Souza, E.M. and Pedrosa, F.O. (2010) Inoculation with Selected Strains of Azospirillum brasilense and A. lipoferum Improves Yields of Maize and Wheat in Brazil. Plant and Soil, 331, 413-425. http://dx.doi.org/10.1007/s11104-009-0262-0
- Hadas, R. and Okon, Y. (1987) Effect of Azospirillum brasilense Inoculation on Root Morphology and Respiration in Tomato Seedlings. Biology and Fertility of Soils, 5, 241-247. http://dx.doi.org/10.1007/bf00256908
- Ribaudo, C.M., Krumpholz, E.M., Cassán, F.D., Bottini, R., Cantore, M.L. and Curá, J.A. (2006) Azospirillum sp. Promotes Root Hair Development in Tomato Plants through a Mechanism That Involves Ethylene. Journal of Plant Growth Regulation, 25, 175-185. http://dx.doi.org/10.1007/s00344-005-0128-5
- Bashan, Y., and Levanony, H. (1990) Current Status of Azospirillum Inoculation Technology: Azospirillum as a Challenge for Agriculture. Canadian Journal of Microbiology, 36, 591-608. http://dx.doi.org/10.1139/m90-105
- Benintende, S., Uhrich, W., Herrera, M., Gangge, F., Sterren, M. and Benintende, M. (2010) Comparación entre Coinoculación con Bradyrhizobium japonicum y Azospirillum brasilense e Inoculación simple con Bradyrhizobium japonicum en la Nodulación, Crecimiento y Acumulación de N en el Cultivo de Soja. Agriscientia, 27, 71-77.
- Galal, Y.G.M. (1997) Dual Inoculation with Strains of Bradyrhizobium japonicum and Azospirillum brasilense to Improve Growth and Biological Nitrogen Fixation of Soybean (Glycine max L.). Biology and Fertility of Soils, 24, 317- 322. http://dx.doi.org/10.1007/s003740050250
- Hungria, M., Nogueira, M.A. and Araujo, R.S. (2013) Co-Inoculation of Soybeans and Common Beans with Rhizobia and Azospirilla: Strategies to Improve Sustainability. Biology and Fertility of Soils, 49, 791-801. http://dx.doi.org/10.1007/s00374-012-0771-5
- Okon, Y. and Labandera-Gonzalez, C.A. (1994) Agronomic Applications of Azospirillum: An Evaluation of 20 Years Worldwide Field Inoculation. Soil Biology and Biochemistry, 26, 1591-1601. http://dx.doi.org/10.1016/0038-0717(94)90311-5
- Badenoch-Jones, J., Flanders, D.J. and Rolfe, B.G. (1985) Association of Rhizobium Strains with Roots of Trifolium repens. Applied and Environmental Microbiology, 49, 1511-1520.
- Hungria, M., Franchini, J.C., Campo, R.J., Crispino, C.C., Moraes, J.Z., Sibaldelli, R.N.R., Mendes, I.C. and Arihara, J. (2006) Nitrogen Nutrition of Soybean in Brazil: Contributions of Biological N2 Fixation and N Fertilizer to Grain Yield. Canadian Journal of Plant Science, 86, 927-939. http://dx.doi.org/10.4141/P05-098
- Vincent, J.M. (1970) A Manual for the Practical Study of the Root-Nodule Bacteria. Blackwell, Oxford.
- Andrade, D.S. and Hamakawa, P.J. (1994) Estimativa do Número de Células Viáveis de Rizóbio no Solo e em Inoculantes por Infecção em Plantas. In: Hungria, M. and Araujo, R.S., Eds., Manual de Métodos Empregados em Estudos de Microbiologia Agrícola, EMBRAPA-SPI, Brasilia, 63-94.
- Döbereiner, J., Baldani, V.L.D. and Baldani, J.I. (1995) Como Isolar e Identificar Bactérias Diazotróficas de Plantas não-Leguminosas. Embrapa-SPI, Itaguaí.
- Feije, F. and Anger, V. (1972) Spot Tests in Inorganic Analyses. Analytica Chimica Acta, 149, 363-367.
- Ferreira, D.F. (2011) Sisvar: A Computer Statistical Analysis System. Ciência e Agrotecnologia (UFLA), 35, 1039- 1042.
- Barbin, D. (2003) Planejamento e Análise Estatística de Experimentos Agronômicos. 2nd Edition, Mecenas, Londrina.
- Bohrer, T.R.J. and Hungria, M. (1998) Avaliação de Cultivares de Soja quanto à Fixação Biológica do Nitrogênio. Pesquisa Agropecuária Brasileira, 33, 937-952.
- Döbereiner, J. (1966) Evaluation of Nitrogen Fixation in Legumes by the Regression of Total Plant Nitrogen with Nodule Weight. Nature, 210, 850-852. http://dx.doi.org/10.1038/210850a0
- Hungria, M. and Bohrer, T. (2000) Variability of Nodulation and Dinitrogen Fixation Capacity among Soybean Cultivars. Biology and Fertility of Soils, 31, 45-52. http://dx.doi.org/10.1007/s003740050622
- Souza, R.A., Hungria, M., Franchini, J.C., Maciel, C.D., Campo, R.J. and Zaia, D.A.M. (2008) Conjunto Mínimo de Parâmetros para Avaliação da Microbiota do Solo e da Fixação Biológica do Nitrogênio pela Soja. Pesquisa Agro- pecuária Brasileira, 43, 83-91. http://dx.doi.org/10.1590/S0100-204X2008000100011
- Souza, R.A, Hungria, M., Franchini, J.C., Chueire, L.M.O., Barcellos, F.G. and Campo, R.J. (2008) Avaliação Quali- tativa e Quantitativa da Microbiota do Solo e da Fixação Biológica do Nitrogênio pela Soja. Pesquisa Agropecuária Brasileira, 43, 71-82. http://dx.doi.org/10.1590/s0100-204x2008000100010
- Cardoso, J.D., Gomes, D.F., Goes, K.C., Fonseca, N. S., Dorigo, O.F., Hungria, M. and Andrade, D.S. (2009) Relationship between Total Nodulation and Nodulation at the Root Crown of Peanut, Soybean and Common Bean Plants. Soil Biology and Biochemistry, 41, 1760-1763. http://dx.doi.org/10.1016/j.soilbio.2009.05.008
- Aung, T.T., Tittabutr, P., Boonkerd, N., Herridge, D. and Teaumroong, N. (2013) Co-Inoculation Effects of Bradyrhizobium japonicum and Azospirillum sp. on Competitive Nodulation and Rhizosphere Eubacterial Community Structures of Soybean under Rhizobia-Established Eoil Conditions. African Journal of Biotechnology, 12, 2850-2862.
- Cassán, F., Perrig, D., Sgroy, V., Masciarelli, O., Penna, C., and Luna, V. (2009) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, Inoculated Singly or in Combination, Promote Seed Germination and Early Seedling Growth in corn (Zea mays L.) and Soybean (Glycine max L.). European Journal of Soil Biology, 45, 28-35. http://dx.doi.org/10.1016/j.ejsobi.2008.08.005
- Singh, C. and Rao, N.S. (1979) Associative Effect of Azospirilium brasilense with Rhizobium japonicum on Nodulation and Yield of Soybean (Glycine max). Plant and Soil, 53, 387-392. http://dx.doi.org/10.1007/BF02277872
- Molla, A.H., Shamsuddin, Z.H., Halimi, M.S., Morziah, M. and Puteh, A.B. (2001) Potential for Enhancement of Root Growth and Nodulation of Soybean Co-Inoculated with Azospirillum and Bradyrhizobium in Laboratory Systems. Soil Biology & Biochemistry, 33, 457-463. http://dx.doi.org/10.1016/S0038-0717(00)00186-3
- Kloepper, J.W., Lifshitz, R. and Zablotowicz, R.M. (1989) Free-Living Bacterial Inocula for Enhancing Crop Productivity. Trends in Biotechnology, 7, 39-44. http://dx.doi.org/10.1016/0167-7799(89)90057-7
- Itzigsohn, R., Kapulnik, Y., Okon, Y. and Dovrat, A. (1993) Physiological and Morphological Aspects of Interactions Between Rhizobium meliloti and Alfalfa (Medicago sativa) in Association with Azospirillum brasilense. Canadian Journal of Microbiology, 39, 610-615. http://dx.doi.org/10.1139/m93-088
- Groppa, M.D., Zawoznik, M.S. and Tomaro, M.L. (1998) Effect of Co-Inoculation with Bradyrhizobium japonicum and Azospirillum brasilense on Soybean Plants. European Journal of Soil Biology, 34, 75-80. http://dx.doi.org/10.1016/S1164-5563(99)90004-3
- Bashan, Y. and Holguin, G. (1997) Azospirillum-Plant Relationships: Environmental and Physiological Advances (1990-1996). Canadian Journal of Microbiology, 43, 103-121. http://dx.doi.org/10.1139/m97-015
- Yahalom, E., Okon, Y. and Dovrat, A. (1990) Possible Mode of Action of Azospirillum brasilense Strain Cd on the Root Morphology and Nodule Formation in Burr Medic (Medicago polymorpha). Canadian Journal of Microbiology, 36, 10-14. http://dx.doi.org/10.1139/m90-003
- Hungria, M. and Vargas, M.A.T. (2000) Environmental Factors Affecting N2 Fixation in Grain Legumes in the Tropics, with an Emphasis on Brazil. Field Crops Research, 65, 151-164. http://dx.doi.org/10.1016/S0378-4290(99)00084-2
- Hungria, M. and Kaschuk, G. (2014) Regulation of N2 Fixation and
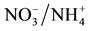 Assimilation in Nodulated and N-Fertilized Phaseolus vulgaris L. Exposed to High-Temperature Stress. Environmental and Experimental Botany, 98, 32-39. >http://html.scirp.org/file/2-2602048x6.png" class="200" /> Assimilation in Nodulated and N-Fertilized Phaseolus vulgaris L. Exposed to High-Temperature Stress. Environmental and Experimental Botany, 98, 32-39. http://dx.doi.org/10.1016/j.envexpbot.2013.10.010
Assimilation in Nodulated and N-Fertilized Phaseolus vulgaris L. Exposed to High-Temperature Stress. Environmental and Experimental Botany, 98, 32-39. >http://html.scirp.org/file/2-2602048x6.png" class="200" /> Assimilation in Nodulated and N-Fertilized Phaseolus vulgaris L. Exposed to High-Temperature Stress. Environmental and Experimental Botany, 98, 32-39. http://dx.doi.org/10.1016/j.envexpbot.2013.10.010 - Hungria, M., Nogueira, M.A. and Araujo, R.S. (2015) Soybean Seed Co-Inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A New Biotechnological Tool to Improve Yield and Sustainability. American Journal of Plant Science, 6, 811-817. http://dx.doi.org/10.4236/ajps.2015.66087
NOTES

*Corresponding author.


