American Journal of Plant Sciences
Vol.4 No.3(2013), Article ID:28685,4 pages DOI:10.4236/ajps.2013.43062
Karyological and Electrophoretic Distinction between Sexes of Trichosanthes bracteata
![]()
Department of Botany, Tripura University, Suryamaninagar, India.
Email: *sangram.sinha@gmail.com
Received December 29th, 2012; revised February 5th, 2013; accepted February 16th, 2013
Keywords: Trichosanthes bracteata; Dioecious; Reducing and Non-Reducing SDS-PAGE
ABSTRACT
Chromosome studies and soluble protein profiles, fractionated by reducing and non-reducing SDS-PAGE, were carried out in dioecious Trichosanthes bracteata. Somatic chromosome no. 2n = 22 was recorded in both sexes. The karyotype of male and female plant shows high homogeneity and the absence of any heteromorphic pair of chromosomes negates the possibility of XY mechanism. Soluble protein profiles from the tuberous roots of the male and female plants, fractionated by reducing SDS-PAGE, did not show any qualitative distinction. Whereas the protein profile in non-reducing SDS-PAGE reveals a clear distinction when compared on a single gel. The difference is marked by the presence of a disulphide linked tertiary or folded protein at 19 k D region detected in male sex. However, at the level of primary structure the qualitative expression is similar indicating a common ancestry.
1. Introduction
Most of the flowering plants are bisexual having flowers with both male and female reproductive organs and only less then 4% plant species are dioecious in nature i.e., strictly maintain their sexual phenotypes [1]. The vast majority of the dioecious plant species have no visibly different sex chromosomes and only some species show distinct X and Y chromosomes in relation to sex [2-5]. Chromosomal sex determination system in flowering plants also indicates that the plant sex chromosomes have evolved recently through replicated independent events [6]. Trichosanthes bracteata (Lamk.) Voigt, a vegetatively propagated dioecious perennial species of cucurbitaceae, commonly grows in moist thickets and is distributed in the Eastern Himalayas in India, Bangladesh, Southern China, Southern Japan, Malayasia and tropical Australia [7,8]. T. bracteata is an important medicinal plant in several traditional systems [9]. The seeds of T. bracteata also contains high amount of punicic acid [10].
In Trichosanthes bracteata, male and female plants strictly maintain their respective sexual phenotypes and every year sprouting occurs, from their respective vegetative reproductive structure i.e., the tuberous root, without failing to reproduce their own kind. Limited cytogenetic studies had so far been made in this taxon and there is a record of polyploid series from diploid to hexaploid [11-15]. However, comparative analysis of the detailed karyotype of the sex forms of T. bracteata was not carried out till date probably due to its ecological distribution and extreme difficulty in obtaining suitable chromosome spreads needed for thorough analysis.
In the absence of information on chromosomal basis of sex determination, electrophoretic study of soluble protein profile of the vegetative propagules could be useful in understanding the genic expression of sexual phenotypes. The present investigation has, therefore, been aimed at karyotype and SDS-PAGE analysis with or without 2-mercaptoethanol to resolve the differences, if any, between the sexes of T. bracteata.
2. Materials and Method
The tuberous roots of the sex forms of T. bracteata growing in wild condition collected from west Tripura were grown in the experimental garden of the Department of Botany, Tripura University.
2.1. Study of Somatic Chromosome
Young leaf tips of the sex forms of Trichosanthes bracteata were pre-treated in saturated solution of para-dichlorobenzene at 10˚C - 15˚C for 4 hours followed by overnight fixation in 1:3 acetic-ethanol mixture. The leaf tips were then stained overnight in 2% aceto-orcein after hydrolysis in 5(N) HCl at cold for 20 minutes and finally squashed in 45% acetic acid. While preparing the karyotype, five well spread metaphase plates were compared and in cases where the length and arm ratio varied the mean was taken to calculate the F%.
2.2. Sodium Dodecyl Sulphate Gel Electrophoresis
2.2.1. Quantitative Estimation of Protein
Two grams of fresh tuberous tissue were homogenized in 4 ml of extraction buffer containing 0.25 M sucrose and 1mM EDTA in 0.1 M Tris-HCl buffer (pH 6.8). The homogenates were then centrifuged at 12,000 rpm for 45 minutes at cold. The supernatants were collected and immediately used for electrophoresis. The protein concentration was estimated by the method of Lowry et al. [16] using BSA as a standard.
2.2.2. Molecular Analysis of Extracted Samples
The soluble protein obtained in extraction buffer was boiled with equal amount of 1 X strength electrophoresis sample buffer (12.5% glycerol, 1.25% SDS, 0.005% bromophenol blue, 62.5% Tris-HCl, pH 6.8) in presence and absence of 178 mM 2-mercaptoethanol for 5 minutes & allowed to cool at room temperature before proceeding to the next step. All the reagents used were of electrophoresis grade (SRL & MERCK). Electrophoresis in 12% polyacrylamide slab gel containing 0.1% SDS [17] using a discontinuous system [18] was carried out and approximately 15 µg or more than 15 µg protein was loaded onto each lane as per experimental design. Protein patterns were visualized by staining the gel for overnight with 0.2% Coomassie Brilliant Blue R-250 in methanol: glacial acetic acid: DDH2O (9:2:9) mixture followed by destaining repeatedly with mixture of iso-propanol: acetic acid: DDH2O (2:1:7).
The position of the bands was expressed as relative mobility (Rm) and was determined by measuring the ratio of the distances traveled by a particular band and the indicator Bromophenol Blue. The different Rm values of the protein bands were numbered serially. Using five cycle semi log graph paper their molecular weights were determined from the standard curve.
3. Results
The sexual phenotypes of T. bracteata differ and it has been observed that the male flowers of T. bracteata are in racemes and having bracts 3 - 4 cm long whereas female flowers are solitary and without any bract (Figures 1(a) and (b)).
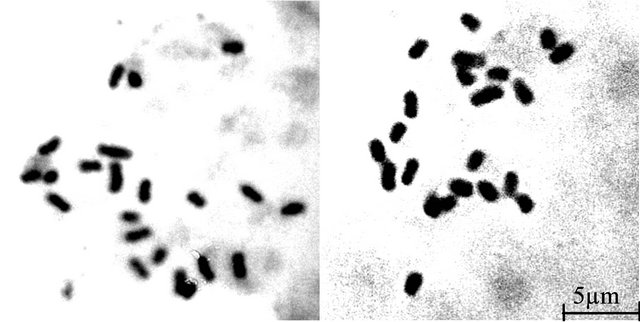
The somatic chromosome number 2n = 22 (Figures 2(a) and (b)) was found in both sexes of T. bracteata having two pairs of chromosomes bearing secondary constrictions. In general, chromosomes are short to medium in size and could be classified [19] into 4 distinct morphological types:
Type A: Short chromosomes (1.68 µm) bear 2 constrictions, primary and secondary, one is nearly sub-median and the other is sub-terminal in position;
Type B: Chromosomes are short (2.13 µm) having 2 constrictions, primary and secondary, both are nearly sub-median in position;
Type C: Short chromosomes (1.06 µm - 1.60 µm), the constriction of chromosomes are median and or nearly median in position;
Type D: The chromosomes (2.96 µm) are medium in size and the constrictions are nearly sub-terminal in position.
The biggest chromosome of the somatic chromosome complements is a medium sized acrocentric chromosome with sub-terminal constriction and does not bear any secondary constriction. The TF% of male and female plants is 36.04 and 35.93 respectively. According to Stebbin’s categorization, the karyotype of both male and female plants falls under category 2B.
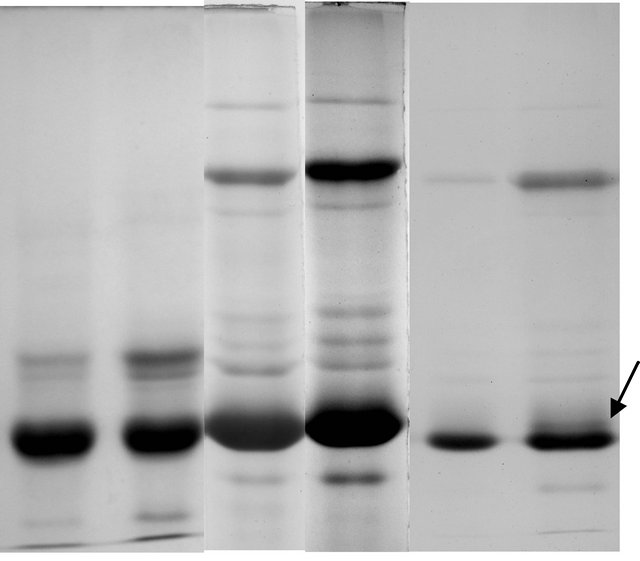
Soluble protein profile of tuberous roots of male and female plants showed differential expression in reducing SDS-PAGE and non-reducing SDS-PAGE (Figures 3(a) and (b)) profiles. The reducing SDS-PAGE of both the sex forms showed 25 common bands in all the experimental set studied. In the non-reducing SDSPAGE protein profile the female sex showed 28 bands
 (a)
(a) (b)
(b)
Figure 1. T. bracteata twigs with female and male flowers respectively.
 (a) (b)
(a) (b)
Figure 2. Somatic metaphase plates of male and female plants of T. bracteata respectively showing 2n = 22 chromosomes.
and the male plant showed an additional band when lesser amount of protein (15 µg/lane) protein was loaded on to gel (Figures 3(e) and (f)).
4. Discussion
Somatic chromosome count 2n = 22 are found constant in both sexes of Trichosanthes bracteata which corroborates the findings of Verghese [17]. The biggest chromosome of the somatic chromosome complements is a medium sized acrocentric chromosome with sub-terminal constriction and does not bear any secondary constriction as was reported by Verghese [18]. The karyotype of male and female plants exhibits a gross similarity in the types of chromosome present, number of chromosomes with secondary constriction, TF%, chromosome arm symmetry index and total chromosome length (Table 1). The karyotype formula of both sexes is also identical (A2B2- C16D2). The diploid males are homogametic and no heteromorphic pair of chromosome is recorded in relation to sex. The chromosome complements, therefore, do not show XY mechanism in relation to sex. Obviously, the sex expression in T. bracteata is under genic control. Stebbins [20] analysed the degree of asymmetry of the flowering plants recognizing three degrees of difference between the largest and smallest chromosome complements and four degrees with respect to the proportion of chromosome which are acroor telocentric. In the light of this knowledge, the karyotype of both male and female plants falls under Category 2B (Table 1) and a progressive asymmetry is, therefore, noticed.
The soluble protein profiles of the sex forms of T. bracteata fractionated by reducing SDS-PAGE did not show any marked distinction and only a variation in the intensity of staining pattern was observed in all experimental set (Figures 3(a) and (b)). A different result in protein profile was however obtained when subjected to non-reducing SDS-PAGE analysis. A total of 28 bands were found to be common in male and female plants in non-reducing profile when ~30 µg proteins were loaded (Figures 3(c) and (d)). On the contrary when ~15 µg proteins were loaded onto each lane a single band difference was observed along with a significant variation in the intensity of the band patterns between the sexes. The electrophoretic distinction between two sexes is, therefore, marked by the presence of disulphide linked tertiary or folded protein at the 19 K D region in male sex. The variability thus obtained suggests that such tertiary or folded proteins are formed in the vegetative propagules of male sex which is not found in female plant. But eventually in the primary structure the qualitative expression is similar (Figures 3(e) and (f)) in both sexes indicating a common ancestry.
5. Conclusion
The karyotype of male and female plants of dioecious T. bracteata, analysed separately for the first time, is identical and no heteromorphicity is recorded in relation to sex. Obviously, the sex expression is under genic control. The present study also suggests that non-reducing SDSPAGE profile could be used to resolve the distinction between the sexes of dioecious Trichosanthes bracteata.
 (a) (b) (c) (d) (e) (f)
(a) (b) (c) (d) (e) (f)
Figure 3. (a)-(d) Electrophoregram of reducing and nonreducing protein profiles from tuberous roots of female and male plants of T. bracteata (a-female, b-male). (e), (f) Electrophoregram of non reducing protein profile (with lesser amount of proteins) of female and male plants of T. bracteata respectively (Arrow indicates a band at 19 K D region).
Table 1. Comparative analysis of karyotype of male and female T. bracteata.

*Mean of 5 plates.
6. Acknowledgements
Financial assistance by UGC to the first author for NonNET Ph.D. scholarship is duly acknowledged.
REFERENCES
- D. S. Guttman and D. Charlesworth, “An X-Linked Gene with a Degenerate Y-Linked Homologue in a Dioecious Plant,” Nature, Vol. 396, No. 6682, 1998, pp. 263-266. doi:10.1038/30492
- D. Chattopadhyay and A. K. Sharma, “Sex Determination in Dioecious Species of Plants,” Feddes Repertorium, Vol. 102, No. 1-2, 1991, pp. 29-55.
- S. Grant, A. Houber, B. Vyskot, J. Siroky, W. H. Pan and J. Macas, “Genetics of Sex Determination in Flowering Plants,” Developmental Genetics, Vol. 15, No. 3, 1994, pp. 214-230. doi:10.1002/dvg.1020150304
- S. Sinha, B. Debnath and R. K. Sinha, “Differential Condensation of Chromosome Complements of Dioecious Momordica dioca Roxb. in Relation to DNA Content,” Indian Journal of Experimental Biology, Vol. 35, No. 11, 1997, pp. 1246-1248.
- M. Westergaard, “The Mechanism of Sex Determination in Dioecious Flowering Plants,” Advances in Genetics, vol. 9, 1958, pp. 217-281. doi:10.1016/S0065-2660(08)60163-7
- S. S. Mayer and D. Charlesworth, “Genetic Evidence for Multiple Origins of Dioecy in Hawaiian Shrub Wikstroemia (Thymelaeaceae),” Evolution, Vol. 46, No. 1, 1992, pp. 207-215.
- S. Bhandari, U. Dobhal, M. Sajwan and N. S, Bisht, “Trichosanthes tricuspidata: A Medicinally Important Plant,” Trees for Life Journal, Vol. 3, No. 5, 2008, p. 1.
- A. H. M. M. Rahman, M. Anisuzzaman, M. Ainsuzzaman, M. Z. Alam, A. K. M. R Islam and A. T. M. N. Zaman, “Taxonomic Studies of the Cucurbits Grown in the Northern Parts of Bangladesh,” Research Journal of Agriculture and Biological Science, Vol. 2, No. 6, 2006, pp. 299- 302.
- B. K. Duvey, R. Goyel, B. Parashar, D. Verma, H. Dhameja and D. Sharma, “Trichosanthes Tricuspidata: Exploration of It’s Medicinal Value,” Asian Journal of Pharmacy and Technology, Vol. 2, No. 1, 2011, pp. 26- 28.
- G. Lakshminarayana, K. Sundar Rao, M. H. Klttur and C. S. Mahajanshetty, “Occurrence of Punicic Acid in Trichosanthes bracteata and Trichosanthes nervifolia Seed Oils,” Journal of the American Oil Chemist’s Society, Vol. 65, No. 3, 1988, pp. 347-348. doi:10.1007/BF02663074
- B. M. Verghese, “Cytology of Trichosanthes palmata Roxb.,” Cytologia, Vol. 36, No. 2, 1971, pp. 205-209. doi:10.1508/cytologia.36.205
- B. M. Verghese, “Cytology and Origin of a Tetraploid Trichosanthes palmata Roxb.,” Genetica, Vol. 43, No. 2, 1972, pp. 292-301. doi:10.1007/BF00123636
- A. K. Rangaswami, “Sex Chromosome of Trichosanthes palmata Roxb.,” Proceedings of 36th Indian Science Congress, Allahabad, Vol. 3, 1949, p. 137.
- A. K. Singh and R. P. Roy, “Cytological Studies in Trichosanthes palmata Roxb. A Natural Hexaploid,” Science & Culture, Vol. 39, No. 11, 1973, pp. 505-506.
- G. K. Thakur, “A Natural Tetraploid in the Genus Trichosanthes from Bihar,” Proceedings of 60th Indian Science Congress, Chandigarh, Vol. 3, 1973, p. 324.
- O. H. Lowry, A. L. Rosebrough Farr and R. J. Randall, “Protein Measurement with the Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, No. 1, 1951, pp. 265-275.
- K. Weber and M. Osborn, “Proteins and Sodium Dodecyl Sulfate: Molecular Weight Determination on Polyacrylamide Gels and Related Procedures. In: H. Neurath and R. L. Hill, Eds., The Proteins, Academic Press, New York, 1975, pp. 179-223.
- U. K. Laemmli, “Clevage of Structural Proteins during the Assembly of the Head of Bacteriophage T4,” Nature, Vol. 227, 1970, pp. 680-685. doi:10.1038/227680a0
- A. Levan, K. Fredga and A. A. Sandbery, “Nomenclature for Centromeric Position on Chromosomes,” Heriditas, Vol. 52, No. 2, 1964, pp. 201-220. doi:10.1111/j.1601-5223.1964.tb01953.x
- G. L. Stebbins, “Chromosomal Evolution in Higher Plants,” Addison-Wesley Publishing Co., Melno Park, 1971.
NOTES
*Corresponding author.

