American Journal of Plant Sciences
Vol.3 No.4(2012), Article ID:18689,7 pages DOI:10.4236/ajps.2012.34052
Biological Control Potential of Colletotrichum gloeosporioides for Coffee Senna (Cassia occidentalis)
![]()
1United States Department of Agriculture-Agricultural Research Service (USDA-ARS), Biological Control of Pests Research Unit, Stoneville, USA; 2USDA-ARS, Crop Production Systems Research Unit, Stoneville, USA.
Email: doug.boyette@ars.usda.gov
Received September 30th, 2011; revised October 14th, 2011; accepted December 5th, 2011
Keywords: Bioherbicide; Mycoherbicide; Coffee Senna; Cassia occidentalis; Colletotrichum gloeosporioides
ABSTRACT
A fungal pathogen, Colletotrichum gloeosporioides was isolated from a greenhouse-grown seedling of coffee senna (Cassia occidentalis) and evaluated as a mycoherbicide for that weed. Host range tests revealed that coffee senna, wild senna (C. marilandica), and sicklepod (C. obtusifolia) were also affected by this pathogen, but 35 other crop and weed species, representing 8 botanical families were not affected. The fungus sporulated prolifically on solid and liquid media with maximum spore germination and growth occurring at 20˚C - 30˚C. Optimal environmental conditions included at least 12 h of free moisture (dew) at 20˚C - 30˚C. Spray mixtures containing approximately 1.0 × 105 or more conidia∙ml–1 gave maximum control when coffee senna seedlings were sprayed until runoff occurred. Coffee senna seedlings that were in the cotyledon to first-leaf growth stage were most susceptible to this pathogen. Weed control efficacy studies under field conditions demonstrated that control of coffee senna was directly proportional to the inoculum concentration applied. Results of these tests suggest that this fungus has potential as a mycoherbicide to control coffee senna, a serious weed in the southeastern U.S.
1. Introduction
Coffee senna (Cassia occidentalis L.) is a non-nodulating legume that was originally introduced as a potential crop plant [1,2]. It has escaped cultivation and has become widely distributed in the south-central and southeastern regions of the United States [3]. It is a very troublesome weed in soybean [Glycine max (L.) Merr.], cotton (Gossypium hirsutum L.), and peanut (Arachis hypogaea L.) fields in much of the southeastern U.S. [4-8].
Coffee senna is economically important because it causes yield loss, seed quality degradation, and difficulty with harvest [9]. Higgins et al. [10] reported that one coffee senna plant 7.5 m–1 of row can reduce seed cotton yields by 117 kg∙ha–1. Coffee senna seeds can germinate and emerge throughout the growing season and its germination and emergence have been recently assessed [11]. Its seeds have impermeable seed coats and scarification is required to allow imbibition of water, and the breaking of dormancy [3].
Control of this weed is difficult because of its tolerance to many commonly used herbicides, its prolific growth habit, and emergence throughout the growing season [4,9,12-14]. Alternative controls are needed to replace or supplement existing methods. The technical and biological feasibility of mycoherbicides for controlling various weeds has been established [15], and this method warrants consideration for controlling coffee senna.
An anthracnose disease was observed on greenhousegrown seedlings of coffee senna and the fungus Colletotrichum gloeosporioides (Penz.) Penz. & Sacc, an anomorph of Glomerella cingulata (Stoneman) Spauld. and Schrenk, was isolated from infected leaf and stem tissue. Coffee senna seedlings sprayed with conidial suspensions of this fungus developed severe disease symptoms under controlled conditions (suggesting potential bioherbicidal activity) and a patent was issued for the use of this fungus as a biological control agent (16). Later studies indicated that this fungus was also useful in the biological control of sicklepod, a closely related weed of several crops [17,18]. More extensive studies on the potential of using this fungus as a mycoherbicide for coffee senna comprise the subject of this report. C. gloeosporioides was evaluated in laboratory, growth chamber, and field studies to determine the effects of temperature on germination and growth of the fungus in vitro, the effect of dew period, dew temperature, inoculum concentration, and plant growth stage on biocontrol efficacy, and the host range of this pathogen. Determination of these parameters is essential for evaluating this fungus as a mycoherbicide.
2. Materials and Methods
2.1. Isolation and Culture of Colletotrichum gloeosporioides
The fungus was isolated from diseased coffee senna tissue by surface sterilizing sections of diseased tissue in 0.05% NaOC1 for 1 min, then placing the sections on potato-dextrose agar (PDA) amended with the antibiotics chloramphenicol (0.75 mg∙ml–1) and streptomycin sulfate (1.25 mg∙ml–1). The plates were incubated for 48 h at 25˚C. Advancing edges of fungal colonies were transferred to PDA and incubated for 5 days at 25˚C under alternating 12-h light/12-h dark regimens, provided by cool white fluorescent lights. The fungus was sub-cultured on PDA without antibiotics, and preserved under refrigeration in sterilized sandy loam soil (25% water holding capacity), and on sterile silica gel containing skim milk [19]. Conidia of C. gloeosporioides were produced in a 30-L fermenter (B. Braun, Model C-30, Bethlehem, PA, USA) at a 20-L working volume containing 20-L of modified Richards’ medium [20] plus V-8 vegetable juice (15%; v/v) (Campbell’s Soup Co., Camden, NJ, USA), with an aeration rate of 10 L air min–1 and an agitation of 250 rpm using 2 Rushton-style impellers. A silicon-based antifoam (HODAG FD-62, Lambent Technologies, Gurnee, IL, USA) was used to control foaming, as required. Inoculum for the fermentations consisted of 250 ml of conidia and mycelium grown in identical medium that was produced in 500 ml Erlenmeyer shakeflasks, incubated at 25˚C and 250 rpm for 5 days. Fermenter batch fermentations were maintained at 25˚C for 5 days. Spores were separated from the spent medium by filtering through double-layered cheesecloth. The spore suspension was centrifuged at 2500 x g for 15 min at 15˚C. The medium had an initial pH of 5.5, and pH was not controlled or monitored during culture growth. Conidial concentrations used in the tests were determined with hemacytometers.
2.2. Effect of Temperature on Germination and Radial Growth Rate
Conidial germination was measured by spreading 0.1 ml of a suspension (1.0 × 106 conidia∙ml–1) on PDA plates, and incubating them at 10˚C, 15˚C, 20˚C, 25˚C, 30˚C, or 35˚C on open-mesh wire shelves of an incubator (Precision Scientific Inc., Chicago, IL, USA). Twelve-hour photoperiods were provided by two-20 W, cool-white fluorescent lamps positioned in the incubator door. Light intensity at plate level was 200 µE∙m–2∙s–1 photosynthetically active radiation (PAR) as measured with a light meter (LI-COR Inc., Lincoln, NE, USA). Germinated conidia (500 plate–1) were counted after 16 h.
For radial growth studies, 5-mm plugs were taken from advancing margins of a 7-day old colony of the fungus and placed in the centers of PDA plates. The plates were incubated at temperatures of 10˚C, 15˚C, 20˚C, 25˚C, 30˚C, or 35˚C as described above for the conidial germination studies.
Colony diameters were measured after 7 days of incubation. In each experiment, five replicate plates for each temperature were utilized. Both experiments were conducted twice, and the results of each experiment were pooled following testing for homogeneity.
2.3. Plant Production
In all experiments coffee senna plants were grown from seed in a commercial potting mix contained in peat strips. Each strip contained 12 plants. The potting mix was supplemented with a slow-release fertilizer (14:14:14, NPK). The plants were placed in sub-irrigated trays on benches in the greenhouse. Greenhouse temperatures ranged from 25˚C to 30˚C with 40% to 60% relative humidity (RH). The photoperiod was 12 h with 1650 µE∙m–2∙s–1 (PAR) measured at midday.
2.4. Effect of Air and Dew Temperature
Five to seven-day old seedlings (cotyledonary to early first-leaf stage) of coffee senna were sprayed until runoff with 1.0 × 107 conidia∙ml–1 in water. Control plants were sprayed with distilled water only. The plants were placed in darkened dew chambers at 100% RH at temperatures of 15˚C, 20˚C, 25˚C, 30˚C, or 35˚C. The plants were then transferred to environmental growth chambers (Conviron, Model E-7, Pembina, ND) with day/night air temperatures of either 20˚C/10˚C, 25˚C/15˚C, 30˚C/15˚C, 35˚C/ 25˚C, or 40˚C/30˚C. Photoperiods were 14 h with 820 to 840 µE∙m–2∙s–1 PAR and a RH of 65%.
2.5. Effect of Dew Period Duration
Coffee senna seedlings in the cotyledonary to early first leaf stage of growth were sprayed until runoff occurred with a spray mix containing 1.0 × 107 conidia∙ml–1. Control plants were sprayed with distilled water. The inoculated plants were then placed in darkened dew chambers at 25˚C and 100% RH for periods of 4, 8, 12, 16, 20, or 24 h. Following the dew period inoculation, the plants were placed on sub-irrigated trays in the greenhouse and monitored for 14 days for disease development and mortality. Greenhouse temperatures were 28˚C to 32˚C, with 40% to 60% RH at day lengths of 12 h with an average of 1650 mol∙m–2∙s–1 photosynthetically active radiation at mid-day. Mortality and dry weight reductions were recorded after 14 days after treatment. The experiment was conducted twice with 3 sets of 12 plants for each experiment.
2.6. Effect of Inoculum Concentration and Plant Growth Stage
Coffee senna plants in the cotyledonary, 1 to 2 true-leaf, 3 to 4 true-leaf and 5 to 7 true-leaf stages of growth were sprayed with conidial suspensions of 1.0 × 103 to 1.0 × 107 conidia∙ml–1 and held in a dew chamber for 16 h at 25˚C. Control plants were sprayed with distilled water only. Plants were moved to the greenhouse, and mortality and dry weight reductions were recorded after 14 days after treatment. Experiments were conducted twice with 3 sets of 12 plants for each experiment.
2.7. Host Range
2.7.1. Crop Plants
A variety of crop plants were inoculated with the fungus in order to evaluate its host range: pumpkin (Cucurbita pepo L.) cv. Jack-o-Lantern; squash [Cucurbita pepo var. melopepo (L.) Alef.] cv. Golden Summer Crookneck; watermelon (Citrullus vulgaris Schr.) Charleston Grey; sweet corn (Zea mays L.) cvs. Truckers Favorite and Honey Butter; rice (Oryza sativa L.) cvs. Labelle and Starbonnett; grain sorghum [Sorghum bicolor (L.) Moench] cv. Texas C-424; alfalfa (Medicago sativa L.) cv. Delta; soybean cvs. Bedford, Bragg, Davis, Dare, Centennial, Forrest, Hill, Hood, and Tracey; peanut (Arachis hypogaea L.) cv. Tennessee Reds; garden bean (Phaseolus vulgaris L.) cvs. Kentucky Wonder, Romano Pole, Ohio Pole, Jackson Wonder, and Henderson Bush; blackeyed pea [Vigna unguiculata (L.) Wolf.] cvs. Lady Cowpea, and White Crowder Pea; cotton cvs. Stoneville 506 and Deltapine 61; tomato (Lycopersicon esculentum Mill.) cvs. Beefsteak and Marion. Seeds of these plant species were planted and grown under greenhouse conditions to various growth stages when they were subjected to the experimental procedures related to the objectives of these studies.
2.7.2. Weeds
Various grass and dicotyledonous weeds were inoculated to determine this pathogens host range as follows: common cocklebur (Xanthium strumarium L.); tall morningglory (Ipomoea purpurea (L.) Roth. and pitted morningglory (Ipomoea lacunosa L.); johnsongrass [Sorghum halepense (L.) Pers.)]; coffee senna (Cassia occidentalis L.); sicklepod (C. obtusifolia L.), partridge pea (C. fasciculata Michx.), wild senna (C. marilandica L.), hairy sensitive pea (C. pilosa L.), and round-leaf cassia (C. rotundifolia L.); Florida beggarweed (Desmodium tortuosum L.); showy crotalaria (Crotalaria spectabilis Roth); hemp sesbania (Sesbania exaltata Rydb. ex. A.W. Hill; rattlebox (S. drummondii L.), northern jointvetch [Aeschynomene virginica (L.) B.S.P.], prickly sida (Sida spinosa L.); velvetleaf (Abutilon theophrasti Medic); and jimsonweed (Datura stramonium L.). Seedlings ranging from the cotyledonary to the third true-leaf stage were sprayed until runoff with conidial suspension of 1.0 × 107 ml–1 and incubated in dew chambers for 24 h at 25˚C. Non-inoculated strips containing 12 plants of each species were included as a control. Coffee senna seedlings were included in all experiments as susceptible controls. Plants were considered either resistant (no visible reaction) or susceptible (necrosis, flecking) by visual observation after 10 days of incubation in the greenhouse. Seeds of these weeds were grown under greenhouse conditions and tested as described above for the crop species.
2.8. Field Experiments
Two field experiments were conducted on a Dundee very fine sandy loam (Aeric Ochraqualf) soil at the USDAARS, Southern Weed Science Experimental Farm at Stoneville, MS. Plots consisted of four rows of soybeans (cv. “Centennial”), 12.2 m long and 1 m apart, with the two center rows receiving treatment. All rows were planted with scarified coffee senna seed at a density of about 100 seeds∙m–1 of row. Treatments consisted of: 1) 1.0 × 105 conidia∙ml–1 in distilled water and 0.2% Silwet L-77 surfactant; 2) 1.0 × 106 conidia∙ml–1 in distilled water and 0.2% Silwet L-77 surfactant; 3) 1.0 × 107 conidia∙ ml–1 in distilled water and 0.2% Silwet L-77 surfactant; 4) 0.2% Silwet L-77 only; and 5) distilled water only. The seedlings were in the cotyledonary to first-leaf growth stage, and were sprayed until fully wetted (approximately 450 l∙ha–1), resulting in inoculum densities of 9.0 × 1012 conidia∙ha–1 in those plots that received fungal treatments. Applications were made at midday with a hand-held pressurized sprayer. For the first experiment, environmental conditions at the time of inoculation and for 24 h following treatment were: temperature at inoculation, 29˚C with a RH of 69%. The high temperature for the 24 h period was 31˚C and the low temperature was 25˚C. Maximum RH was 94%, with a short dew period of 3 h. For the second experiment, environmental conditions at the time of inoculation and for 24 h following treatment were: temperature at inoculation, 34˚C with a RH of 58%. The high temperature for the 24 h period was 34˚C, and the low temperature was 24˚C. Maximum RH was 92%, with a short dew period of 4 h. The plants were monitored for disease development at 7 day intervals for 21 days. A randomized complete block design was utilized, and the treatments were replicated four times. Data over the 2 years from the two experiments were pooled following subjection to Bartlett’s test for homogeneity, and analyzed using analysis of variance. Percentage data of coffee senna control were determined in two randomly selected 1-m2 subplots from each treated plot 1, 2, and 3 weeks after treatment. The experimental plots were furrow-irrigated as required to maintain healthy coffee senna and soybean plants throughout the growing season. Significant differences were determined using Fisher’s Protected Least Significant Difference (FLSD) at P = 0.05.
2.9. Statistical Procedures
All experiments utilized a randomized complete block experimental design, with a minimum of three replications of 12 plants for each experiment. All experiments were repeated. Data were subjected to the analysis of variance and values are presented as the means of replicated experiments. Duncan’s multiple range test at the 0.05 level of probability was used to separate means in the dew and air temperature experiment. Confidence limits (95% level) were used to analyze the data in remaining experiments.
3. Results
The fungus was identified as Colletotrichum gloeosporioides based on microscopic examination of diseased tissue and cultural characteristics. The morphology of the isolate is identical to that reported previously for this species [21]. The acervuli are glabrous (non-setose) and are slightly sunken in the host tissue. Under moist conditions, slimy masses of conidia accumulate on the upper surface of the acervulus and cause breaking of the epidermal layer and cuticle. Identification was confirmed by B. C. Sutton at the International Mycological Institute (IMI) in Kew, England (the fungus is an anomorph of the ascomycetous fungus Glomerella cingulata). Cultures of this pathogen have been deposited in the International Mycological Institute as No. IMI 325028 and with the Agricultural Research Culture Collection (NRRL) as accession No. NRRL 21046.
3.1. Isolation and Culture
The fungus was readily isolated from diseased tissue and it sporulated abundantly on PDA. When reinoculated onto healthy seedlings, the fungus was highly virulent and killed all inoculated plants within 5 days, while the controls remained healthy (data not shown). The organism produced typical anthracnose lesions on leaves and stems, with acervuli scattered throughout the lesions. The acervuli were glabrous (non-setose) and were slightly sunken in the host tissue. Conidia were elliptical, with rounded ends, and range in size from 15 to 19 µm by 4 to 6 µm and averaged 16 by 6 µm.
3.2. Effect of Temperature on Germination and Radial Growth
Maximal germination of conidia on PDA occurred at 25˚C (86%) and 30˚C (79%), with significant decreases in germination at the other temperatures (Figure 1(A)). The fungus grew well at 20˚C to 30˚C, but growth was significantly reduced when incubated at 10˚C or 15˚C and slow growth occurred at incubation temperatures of 35˚C or higher (Figure 1(B)). The fungus also sporulated prolifically under submerged culture in modified Richard’s
 (A)
(A)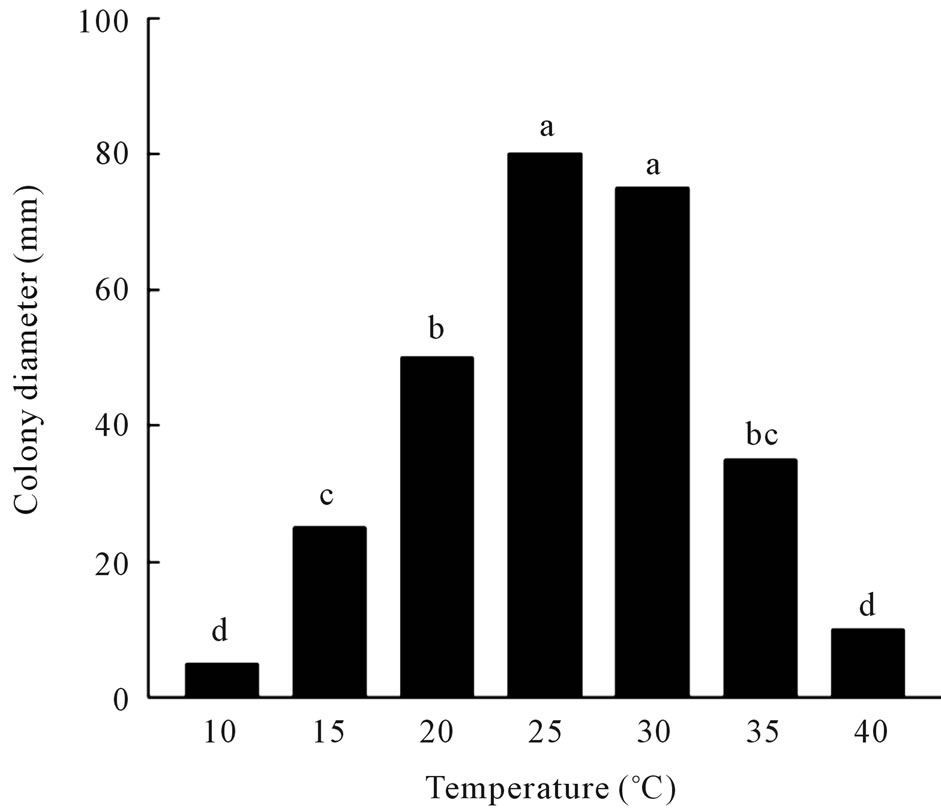 (B)
(B)
Figure 1. (A) Effect of temperature on germination of conidia of C. gloeosporioides on potato dextrose agar. a: germination; b: colony diameter. Values of bars denoted by the same letter are not significantly different at the 95% confidence level; (B) Effect of temperature on radial growth of C. gloeosporioides colonies on potato dextrose agar. Values of bars denoted by the same letter are not significantly different at the 95% confidence level.
medium. Conidial yields averaged 3.0 × 108 conidia∙ml–1 after 7 days at 25˚C and 250 rpm (data not shown). This growth is comparable to conidial yields produced by other C. gloeosporioides formae speciales that have been evaluated as bioherbicides against other weeds [22,23].
3.3. Effect of Dew and Air Temperatures
Optimal day, night, and dew temperatures for maximum weed control (100%) were 30˚C/25˚C/20˚C (Figure 2). Both weed mortality and biomass reduction were significantly reduced when the day/dew/night temperatures were lowered to 20˚C/15˚C/10˚C. No pathogenesis or mortality occurred on coffee senna seedlings under a day/dew/night temperature regime of 40˚C/35˚C/30˚C (Figure 2).
3.4. Effect of Dew Period Duration
The fungus killed coffee senna over a broad range of dew period durations conducted at 25˚C (Figure 3). Although some infection occurred at all dew periods tested, a dew period of at least 12 h was required to kill about 80% of fungus-inoculated plants. Complete mortality was not achieved at any dew period, but many plants were severely stunted, which resulted in greatly reduced biomass.
3.5. Host Range
In greenhouse tests the fungus had no effect on other weed and crop plants tested except for sicklepod, which was slightly affected by the pathogen, and wild senna, which was nearly as susceptible as coffee senna (data not
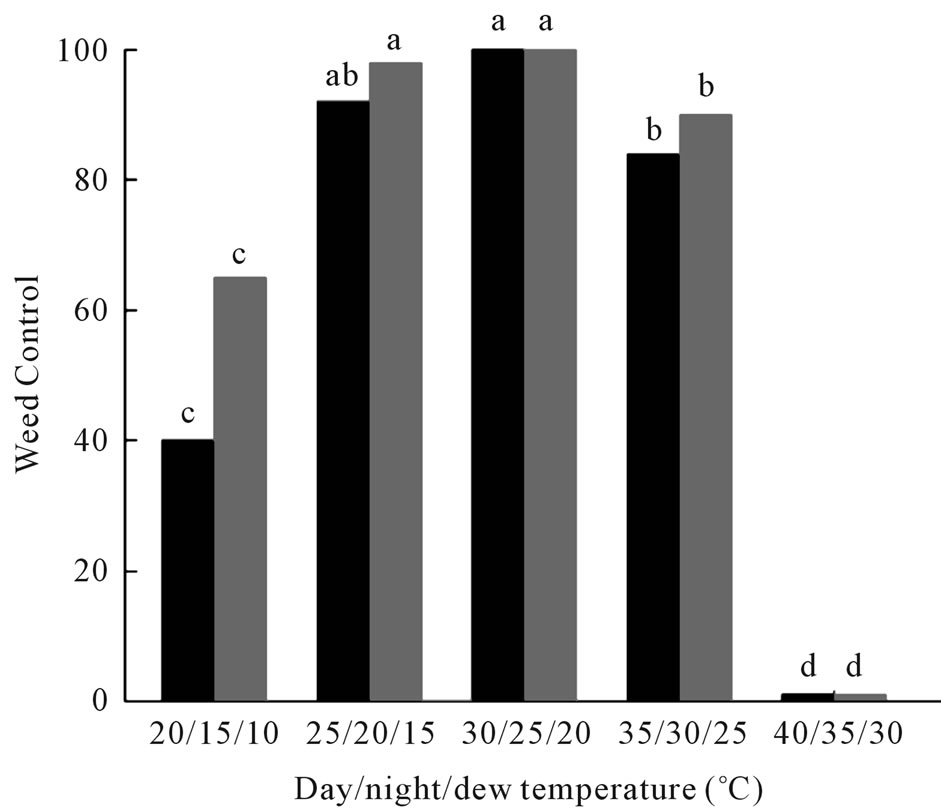
Figure 2. Effect of temperatures of day/night/dew periods on weed control of coffee senna under growth chamber conditions. Black bars = mortality; gray bars = reduction in biomass. Values of bars denoted by the same letter are not significantly different at the 95% confidence level.
shown). Plants were grown to the cotyledonary to thirdleaf growth stage and tested as described in the material and methods. Severe disease developed on coffee senna and wild senna within 2 to 3 days of inoculation, and seedlings were killed within 4 to 6 days.
3.6. Effect of Plant Growth Stage and Inoculum Concentration
Weed control (mortality) under greenhouse conditions was significantly increased at all growth stages by increasing the fungal inoculum concentration (Figure 4). Coffee senna seedlings in the 5 to 7 leaf stage were more resistant to infection than younger plants. Weed control was significantly less than that achieved with plants at
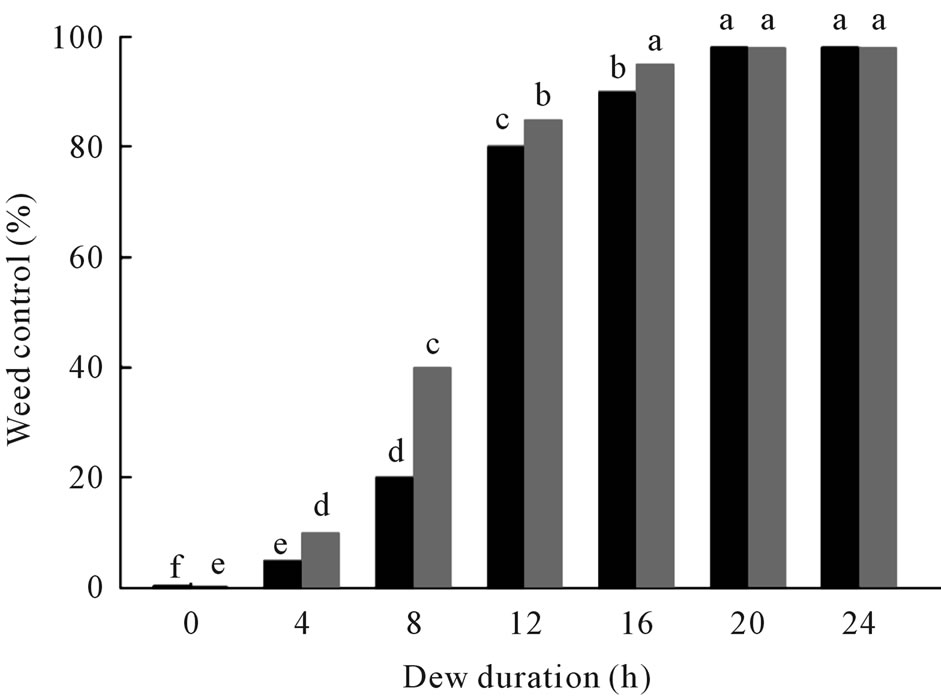
Figure 3. Effect of dew period duration on weed control of coffee senna inoculated with C. gloeosporioides at 1.0 × 107 under greenhouse conditions. Black bars = mortality; gray bars = reduction in biomass. Values of bars denoted by the same letter are not significantly different at the 95% confidence level.

Figure 4. Effect of plant growth stage on weed control (mortality) of coffee senna inoculated with C. gloeosporioides at various inoculums concentrations under greenhouse conditions. Growth stages: cotyledon-to-1 leaf stage (○), 2 to 4 leaves (▲); 5 to 7 leaves (▽); 8 to 10 leaves (□); 11 to 13 leaves (●). Error bars are ±1 SEM.
earlier growth stages at the inoculum concentrations tested. Similar results were found on the dry weight reductions of plants at these growth stages and conidia concentrations (data not shown).
3.7. Field Tests
In field experiments, coffee senna plants treated with the highest inoculum concentration (1.0 × 107 conidia∙ml–1) were controlled over 80% at 21 days after treatment, while only 40% and 20% weed control was achieved with 1.0 × 106 conidia∙ml–1 and 1.0 × 105 conidia∙ml–1, respectively, following this time period (Figure 5). The fungus sporulated profusely on infected tissue, and killed greenhouse-grown coffee senna seedlings when re-inoculated onto them, thus fulfilling Koch’s Postulates. No disease or mortality occurred to untreated control plants, or to plants treated with surfactant only (data not shown).
4. Discussions
These studies show that this isolate of C. gloeosporioides satisfies the requirements of a successful mycoherbicide as established by Daniel et al. [22]. It produces abundant inoculum in culture, is highly specific and virulent, and is pathogenic over a reasonably wide temperature range. The optimum temperatures for growth, germination, and disease development are similar to those reported for other Colletotrichum spp. evaluated as mycoherbicides [24-28]. These temperatures are typical of those that occur in much of the southeastern U. S., where coffee senna is a serious weed problem [4]. This isolate of C. gloeosporioides did not affect the soybean cultivars or any other leguminous crop species tested. However, in order to further define the host range, more research should be conducted with this pathogen to include other soybean cultivars and other leguminous crop species that encompass a more diverse genetic background [29].
A major constraint of most fungi that have been evaluated as mycoherbicides, is the requirement for a lengthy period of free moisture (dew) following inoculation. However our finding that generally, better control was achieved under field versus greenhouse conditions was somewhat contrary to our expectations. This may have been caused by a more rapid drying of the dew and inoculum on plants soon after placement in the greenhouse compared to conditions in the field. This could be attributed to lower RH and the drying effects of air movement by fans. The temperature in the greenhouse tests were also generally lower than those in the field studies. The higher RH in the field could have prolonged favorable conditions for infectivity even though there the dew period in the field was shorter than for the greenhouse studies.
Proper timing of application to weeds in the most susceptible growth stages (cotyledonary to first-leaf stage)
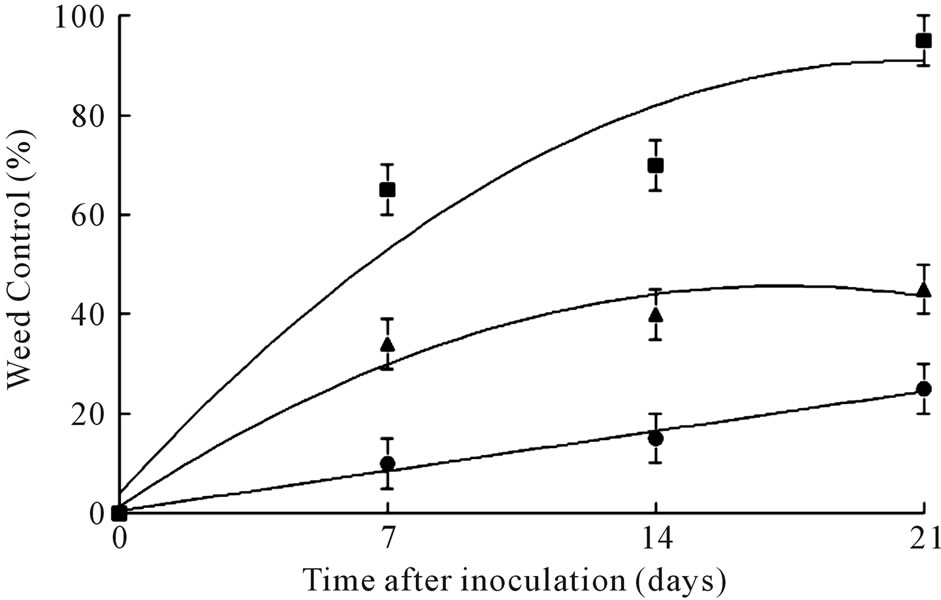
Figure 5. Effects of C. gloeosporioides conidia at several concentrations on control of coffee senna under field conditions. Concentrations are: (●) = 105, (▲) = 106 and (■) = 107 conidia∙ml–1. Error bars are ± 1SEM.
can optimize the chances for successful control. Waterin-oil formulations have shown promise in reducing the dew period requirements of some mycoherbicidal fungi [18,24,30] that control various weeds. More specifically with coffee senna, granulated formulations [31,32] and an emulsified (water-in oil) formulation [16] exhibited greater bioherbicidal efficacy and stabilization of this pathogen.
Although sicklepod was only slightly affected by this pathogen under the conditions tested here, it is possible that through pathogen selection and formulation improvements, such as the use of crop oils, invert emulsions and/or surfactants, this weed could also be controlled by this isolate of C. gloeosporioides. Additional studies under field conditions will be required to further evaluate the potential for this pathogen as a mycoherbicide for coffee senna control.
REFERENCES
- P. Bruere, “A Coffee Substitute, Cassia occidentalis, That Is Toxic before Roasting,” Pharmaceutical Chemistry Journal, Vol. 9, No. 2, 1942, pp. 321-324.
- H. L. Tookey and Q. Jones, “New Sources of WaterSoluble Seed Germs,” Economic Botany, Vol. 10, No. 2, 1965 pp. 165-174. doi:10.1007/BF02862828
- D. H. Teem, C. S. Hoverland and G. A. Buchanan, “Sicklepod (Cassia obtusifolia) and Coffee Senna (Cassia occidentalis): Geographic Distribution, Germination, and Emergence,” Weed Science, Vol. 28, No. 1, 1980, pp. 68- 71.
- C. D. Elmore, “Weed Survey: Southern States,” Proceedings of the Southern Weed Science Society, Vol. 42, 1989, pp. 408-420.
- T. M. Webster, “Weed Survey—Southern States: Broadleaf Crops Subsection,” Proceedings of the Southern Weed Science Society, Vol. 54, 2001, pp. 244-259.
- T. M. Webster and G. E. MacDonald, “A Survey of Weeds in Various Crops in Georgia,” Weed Technology, Vol. 15, No. 4, 2001, pp. 771-790. doi:10.1614/0890-037X(2001)015[0771:ASOWIV]2.0.CO;2
- D. W. Hall, V. V. Vandiver and J. A. Ferrell, “Weeds in Florida (SP 37), Coffee Senna, Senna occidentalis L.,” Institute of Food and Agricultural Sciences, University of Florida, 2009. http://edis.ifas.ufl.edu/ref.html
- J. Grichar, “Weed Control Issues and Update,” Texas Cooperative Extension Service, Peanut Progress, Vol. 1, No. 4, 2007, p. 2.
- S. M. Brown and D. C. Bridges, “Comparative Biology and Control of Sicklepod and Coffee Senna,” Proceedings of the Southern Weed Science Society, Vol. 42, 1989, p. 111.
- J. M. Higgins, R. H. Walker and T. Whitwell, “Coffee Senna (Cassia occidentalis) Competition with Cotton (Gossypium hirsutum),” Weed Science, Vol. 34, No. 1, 1986, pp. 52-56.
- J. K. Norsworthy and M. J. Oliveira, “Coffee Senna (Cassia occidentalis) Germination and Emergence Is Affected by Environmental Factors and Seeding Depth,” Weed Science, Vol. 53, No. 5, 2005, pp. 657-662. doi:10.1614/WS-04-209R.1
- F. E. Dayan, J. D. Weete and H. G. Hancock, “Physiological Basis for Differential Sensitivity to Sulfentrazone by Sicklepod (Senna obtusifolia) and Coffee Senna (Cassia occidentalis),” Weed Science, Vol. 44, No. 1, 1996, pp. 12-17.
- D. L. Jordan, J. W. Wilcut and J. S. Richburg III, “DPXPE350 for Weed Control in Peanut (Arachis hypogaea L.),” Peanut Science, Vol. 20, No. 2, 1993, pp. 97-101. doi:10.3146/i0095-3679-20-2-8
- A. Keeton, E. C. Murdock, G. S. Stapleton and J. E. Toler, “Chemical Control Systems for Coffee Senna (Cassia occidentalis) in Cotton (Gossypium hirsutum),” Weed Technology, Vol. 10, No. 3, 1996, pp. 550-555.
- R. Charudattan, “The Mycoherbicide Approach with Plant Pathogens,” In: D. O. TeBeest, Ed., Microbial Control of Weeds, Chapman and Hall, New York, 1991, pp. 24-57. doi:10.1007/978-1-4615-9680-6_2
- C. D. Boyette and J. R. McAlpine, “Herbicidal Control of Sicklepod and Coffee Senna with Colletotrichum gloeosporioides,” US Patent No. 5529773, 1996.
- C. D. Boyette, “Adjuvants Enhance the Biological Control Potential of an Isolate of Colletotrichum gloeosporioides for Biological Control of Sicklepod (Senna obtusifolia),” Biocontrol Science and Technology, Vol. 16, No. 10, 2006, pp. 1057-1066. doi:10.1080/09583150600828692
- C. D. Boyette, R. E. Hoagland and M. A. Weaver, “Effect of Row Spacing on Biological Control of Sicklepod (Senna obtusifolia) with Colletotrichum gloeosporioides,” Biocontrol Science and Technology, Vol. 17, No. 9, 2007, pp. 957-967. doi:10.1080/09583150701553157
- C. E. Windels, P. M. Burnes and T. Kommendahl, “FiveYear Preservation of Fusarium Species in Silica Gel and Soil,” Phytopathology, Vol. 78, No. 1, 1988, pp. 107-109. doi:10.1094/Phyto-78-107
- J. Tuite, “Plant Pathological Methods: Fungi and Bacteria,” Burgess Press, Minneapolis, 1969, 239 Pages.
- J. E. M. Mordue, “CMI Descriptions of Pathogenic Fungi and Bacteria,” No. 315, 1971.
- J. T. Daniel, G. E. Templeton, R. J. Smith Jr. and W. T. Fox, “Biological Control of Northern Jointvetch in Rice with an Endemic Fungal Disease,” Weed Science, Vol. 21, No. 4, 1973, pp. 303-307.
- C. D. Boyette, G. E. Templeton and R. J. Smith, “Control of Winged Water Primrose (Jussiae decurrens) and Northern Jointvetch (Aeshynomene virginica) with Fungal Pathogens,” Weed Science, Vol. 27, No. 2, 1979, pp. 497- 501.
- C. D. Boyette, “Control of Hemp Sesbania with a Fungal Pathogen, Colletotrichum truncatum,” US Patent No. 5304328, 1991.
- C. D. Boyette, P C Quimb, Jr., C. T. Bryson, G. H. Egley and F. E. Fulgham, “Biological Control of Hemp Sesbania (Sesbania exaltata) under Field Conditions with Colletotrichum truncatum Formulated in an Emulsifiable Invert,” Weed Science, Vol. 41, No. 3, 1993, pp. 496-499.
- J. Cardina, R. H. Littrell and R. T. Hanlin, “Anthracnose of Florida Beggarweed (Desmodium tortussum) by (Colletotrichum truncatum),” Weed Science, Vol. 36, No. 3, 1988, pp. 329-334.
- T. L. Kirkpatrick and G. E. Templeton, “Potential of Colletotrichum malvarum for Biological Control of Prickly Sida,” Plant Disease, Vol. 66, No. 4, 1982, pp. 323-325. doi:10.1094/PD-66-323
- L. A. Wymore, C. Poirier, A. K. Watson and A. R. Gotlieb, “Colletotrichum coccodes, a Potential Bioherbicide for Control of Velvetleaf (Abutilon theophrasti),” Plant Disease, Vol. 72, No. 6, 1988, pp. 534-538. doi:10.1094/PD-72-0534
- A. J. Wapshire, “A Strategy for Evaluating the Safety of Organisms for Biological Weed Control,” Annals of Applied Biology, Vol. 77, No. 2, 1974, pp. 201-211. doi:10.1111/j.1744-7348.1974.tb06886.x
- P. C. Quimby Jr., F. E. Fulgham, C. D. Boyette and W. J. Connick Jr., “An Invert Emulsion Replaces Dew in Biocontrol of Sicklepod—A Preliminary Study,” In: D. A. Hovde and G. B. Beestman, Eds., Pesticide Formulations and Application System, Vol. 8, 1989, ASTM-STP 980. American Society for Testing Materials, Philadelphia, pp. 264-270.
- P. C. Quimby Jr., N. K. Zidak, C. D. Boyette and W. E. Grey, “A Simple Method for Stabilizing and Granulating Fungi,” Biocontrol Science and Technology, Vol. 9, No. 1, 1999, pp. 5-8. doi:10.1080/09583159929857
- P.C. Quimby Jr., A. J. Caesar, J. L. Birdsall, W. J. Connick Jr., C. D. Boyette, N. K. Zidack and W. E. Grey, “Granulated Formulation and Method for Stabilizing Biocontrol Agents,” US Patent No. 6455036, 2002.

