Modern Chemotherapy
Vol.2 No.2(2013), Article ID:29740,14 pages DOI:10.4236/mc.2013.22004
Safety and efficacy of pemetrexed in gynecologic cancers: A systematic literature review
![]()
1University of Texas Southwestern Medical Center, Dallas, USA; *Corresponding Author: David.Miller@utsouthwestern.edu
2Eli Lilly and Company, Indianapolis, USA
3University Hospital Leuven, Leuven, Belgium
Copyright © 2013 David Scott Miller et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 23 February 2013; revised 22 March 2013; accepted 2 April 2013
Keywords: Pemetrexed; Ovarian; Cervical; Endometrial
ABSTRACT
Gynecologic cancers represent a significant problem worldwide. Advanced, recurrent gynecologic cancers are often refractory to chemotherapy, so new treatment regimens are needed. Pemetrexed is a third-generation, multi-targeted antifolate that has been approved for use in nonsquamous non-small cell lung cancer and malignant pleural mesothelioma in both the United States and European Union. This paper reviews the safety and efficacy of pemetrexed in gynecologic cancers, which were defined as malignancies of the ovaries (including fallopian tubes and primary peritoneum), uterine endometrium, and uterine cervix. A search of English-language literature via PubMed and American Society of Clinical Oncology proceedings was performed from database inception to June 2012. Thirteen clinical trials involving the use of pemetrexed (alone and in combination with other agents) in gynecologic cancers were identified. These were phase I and phase II trials; there were 9 studies in ovarian cancer, 1 study in endometrial cancer, and 3 studies in cervical cancer. Pemetrexed with vitamin supplementation was tolerable in all clinical trials and had activity in ovarian and cervical cancers. Therefore, it may be reasonable to further explore the use of pemetrexed in ovarian and cervical malignancies.
1. INTRODUCTION
Antimetabolites act by disrupting cell replication and division [1]. This disruption can occur directly through incorporation of analogues into cellular DNA or indirectly through interference with pathways involved in DNA synthesis. Eukaryotic cells require folates, which transfer 1-carbon units needed for the biosynthesis of pyrimidines, purines, and some amino acids, for growth [2,3]. Diet-derived folic acid must be reduced to tetrahydrofolate (THF), which then serves as the 1-carbon (methyl group) donor in biosynthetic processes. Deletion of intracellular THF co-factors inhibits the biosynthesis of purine nucleotides and thymidine, thereby inhibiting DNA synthesis [4]. Consequently, antifolates have the greatest effect on rapidly growing and dividing cells, but also affect rapidly growing normal cells, such as those in bone marrow and the gastrointestinal tract, thereby explaining toxicities of this compound class [5].
Pemetrexed (Alimta®; LY231514) is a third-generation antifolate [6]. Unlike older agents that target a single enzyme, pemetrexed targets multiple enzymes: thymidylate synthase (TS), glycinamide ribonucleotide formyltransferase (GARFT), 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase (AICARFT), and dihydrofolate reductase (DHFR) [3,7-9]. Pemetrexed enters the cell through the reduced folate carrier (RFC), which is the major transporter for folates, and is also a substrate for the folate receptor-alpha (FR-α) [10,11]. A low pH transporter may also be involved in pemetrexed internalization [12,13]. Pemetrexed is activated intracellularly to a polyglutamated form, with the pentaglutamated form being most active [8]. Pentaglutamated pemetrexed potently inhibits TS and also inhibits GARFT and AICARFT, but has a low affinity for DHFR [6,8,14,15].
Currently, pemetrexed with vitamin supplementation is approved in the United States (US) for use in locally advanced or metastatic nonsquamous non-small cell lung cancer (NSCLC) as initial treatment in combination with cisplatin, as maintenance treatment for patients whose disease has not progressed after 4 cycles of platinumbased first-line chemotherapy, and after prior therapy as a single agent; pemetrexed is also approved for use in combination with cisplatin for malignant pleural mesothelioma [16-22]. Pemetrexed has similar indications in the European Union [23]. Use of pemetrexed in patients with nonsquamous NSCLC is based on subset analyses (prespecified for the first-line and maintenance studies) of phase III trials showing that pemetrexed relative to a comparator confers overall survival (OS) and progression-free survival (PFS) advantages in patients with nonsquamous tumors relative to squamous tumors [16,17,20, 24,25].
Herein, the results from clinical trials involving the use of pemetrexed in gynecologic cancers are reviewed. For the purpose of this article, gynecologic cancers include malignancies of the ovaries (including fallopian tubes and primary peritoneum), uterine endometrium, and uterine cervix. The purpose of this review was to examine the efficacy and safety of pemetrexed, alone and in combination with other agents, in patients with these cancers.
2. METHODS
English-language literature was identified through searches of PubMed (database inception through mid June 2012) and Proceedings of the American Society of Clinical Oncology (ASCO) (database inception through ASCO June 2012). Title search terms included combinations of pemetrexed, MTA, multi-targeted antifolate, LY231514, ovarian, cervical, and endometrial. References within identified articles were also reviewed. Review articles and retrospective analyses were excluded from this review, as was one report involving compassionate use.
3. RESULTS
Thirteen phase I and phase II clinical trials met the search criteria. There were 9 studies in ovarian cancer (Tables 1 and 2), 1 study in endometrial cancer (Table 3), and 3 studies in cervical cancer (Table 4).
3.1. Pemetrexed in Ovarian Cancer
Among woman, ovarian cancer comprises about 3% of all cancers and causes more deaths than any other cancer of the female reproductive system [26]. In 2012, an estimated 22,280 new cases of ovarian cancer are expected in the US, with an estimated 15,500 deaths. Worldwide, 225,000 new cases of ovarian cancer and 140,200 deaths were expected in 2008 [27]. Because there is no good screening test, the majority of cases (63%) are diagnosed at an advanced stage. For women with distant diseaseTable 1. Phase I trials of pemetrexed in ovarian cancer.
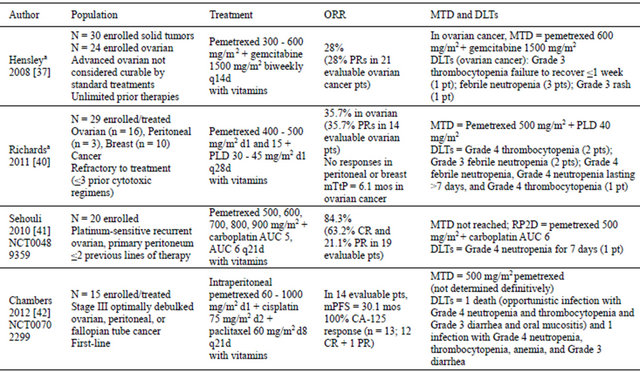
aClinical trial registration number not available. AUC: area under curve; CR: complete response; d: day; DLT: dose-limiting toxicity; mos: months; mPFS: median progression-free survival; MTD: maximum-tolerated dose; N: population size; ORR: overall response rate; mTtPD: median time to progression; PLD: pegylated liposomal doxorubicin; PR: partial response; pt: patient; q: every; RP2D: recommended Phase II dose.
Table 2. Phase II trials of pemetrexed in ovarian cancer.

aFive most common (by incidence) grades 3 and 4 AEs. AEs: adverse events; AUC: area under curve; chemo: chemotherapy; CR: complete response; d: day; ITT: intent-to-treat; mOS: median overall survival; mos: months; mPFS: median progression-free survival; N: population size; ORR: overall response rate; pem: pemetrexed; PR: partial response; pt(s): patient(s); q: every.
Table 3. Phase II trial of pemetrexed in endometrial cancer.
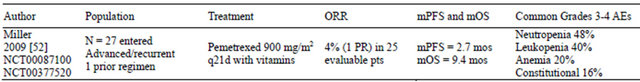
AEs: adverse events; d: day; mOS: median overall survival; mos: months; mPFS: median progression-free survival; N: population size; PR: partial response; pt(s): patient(s); ORR: overall response rate.
the 5-year survival rate is only 27% [26].
Ovarian cancer treatment involves cytoreductive surgery followed by chemotherapy using platinum-based, multi-agent regimens [28]. Most patients respond to treatment initially; however, the disease often recurs, and responses to subsequent therapy are not durable [29]. The 4th Ovarian Cancer Consensus Conference of The Gynecologic InterGroup recently defined subgroups of ovarian cancer patients as those with: 1) progression while receiving the last line of platinum-based therapy, or within 4 weeks of the last platinum dose; 2) progression-free interval since last platinum line of >1 month and <6 months; 3) progression-free interval since the last platinum line of 6 to 12 months; and 4) progression-free interval since the last platinum line of >12 months [30].
In preclinical models of ovarian cancer, pemetrexed demonstrated activity in combination with cisplatin [31]. In ovarian epithelial cells undergoing malignant transformation, the FR-α, a potential pemetrexed transporter, is overexpressed [32]. In ovarian cancer cells, the RFC
Table 4. Phase II trials of pemetrexed in cervical cancer.

aClinical trial registration number for clinical trial not available. AEs: adverse events; mOS: median overall survival; mos: months; mPFS: median progression-free survival; mTtTF: median time to treatment failure; N: population size; PR: partial response; q: every; ORR: overall response rate; SCC: squamous cell carcinoma.
may have a more important role than the FR-α in transporting 5-methyltetrahydrate folate into ovarian cancer cell lines [33]. It is hypothesized that cancer cells that overexpress folate analogue transporters may exhibit preferential uptake of pemetrexed [34]. These observations provided the rationale for testing pemetrexed alone and in combination with other agents in ovarian cancer (Tables 1 and 2).
3.1.1. Phase I Trials
Combined, pemetrexed and gemcitabine were found to have synergistic activity in preclinical models [35,36]. In a phase I trial, Hensley and colleagues reported the results of combined bi-weekly gemcitabine (1500 mg/m2 every 14 days) and pemetrexed (escalated from 300 mg/m2 every 14 days) in patients with solid tumor malignancies or advanced epithelial ovarian cancer [37]. The trial was designed to determine whether bi-weekly dosing with vitamin support would allow efficacious doses of pemetrexed to be delivered. Twenty-four patients with unlimited prior cytotoxic regimens were enrolled in the ovarian cancer cohort. In this cohort, the maximum-tolerated dose (MTD) occurred at 600 mg/m2 pemetrexed, with 2 of 9 patients experiencing a doselimiting toxicity (DLT). The most common grades 3 and 4 adverse events (AEs) were neutropenia (83%), leukopenia (67%), lymphopenia (73%), and febrile neutronpenia (12.0%). Six of 21 evaluable patients (28%) had a confirmed partial response (PRs) (Table 1).
In preclinical models, pemetrexed and doxorubicin were found to have additive cytotoxicity [38]. Pegylated liposomal doxorubicin (PLD) is a formulation that improves the pharmacodynamic, pharmacokinetic, and toxicity profiles relative to doxorubicin [39]. This provided a rationale for testing pemetrexed and PLD in refractory breast, ovarian, primary peritoneal, or fallopian tube cancer [40].
A phase I dose-escalation trial tested pemetrexed (400 - 500 mg/m2 on days 1 and 15) and PLD (30 - 45 mg/m2 on day 1) in a 3 + 3 design [40]. Cycles were 28 days, and all patients received folic acid and vitamin B12. Twenty-nine patients were enrolled with pathologically confirmed, locally advanced, unresectable breast, ovarian, or primary peritoneal cancer that was refractory to conventional treatment. The median age was 60.6 (range: 47.5 - 80.1) years. The distribution of cancers was: ovarian (n = 16), breast (n = 10), and peritoneal (n = 3). Most patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1 (72%). Ovarian/ peritoneal cancer patients had received a mean of 2.3 (range: 1 - 4) prior chemotherapy regimens, and 17 had platinum-refractory disease.
In this trial, the MTD was pemetrexed 500 mg/m2 and PLD 40 mg/m2 (Table 1) [40]. Because the day 15 pemetrexed dose could not be administered in 66% of patients, the recommended phase II dose was pemetrexed 500 mg/m2 and PLD 40 mg/m2, both on day 1 only. The most common drug-related grades 3 and 4 AEs were neutropenia (86%), leukopenia (59%), thrombocytopenia (48%), anemia (41%), mucosal inflammation (24%), febrile neutropenia (24%), hand-foot syndrome (14%), and hypokalemia (10%). Of the 24 evaluable patients (breast + ovarian + peritoneal), 5 (21%) ovarian cancer patients achieved a PR, and the median time to progression (TtP) was 6.1 months. In evaluable platinum-refractory ovarian/peritoneal cancer patients (89% of ovarian/peritoneal cancer patients), the overall response rate (ORR) was 36% with a median TtP of 6.3 months. Because this TtP appeared superior to published data for this patient population, the authors suggested consideration of a randomized phase II trial comparing pemetrexed + PLD to PLD alone [40].
Sehouli and colleagues performed a phase I-II trial testing the combination of carboplatin and pemetrexed. In 2010, the authors reported the results of the phase I portion, which consisted of a standard 3-patient dose escalation scheme in 20 patients with platinum-sensitive recurrent ovarian cancer or primary peritoneal (Table 1) [41]. The dose-escalation trial started at carboplatin area under curve (AUC) 5 and pemetrexed 500 mg/m2. Patients also received folic acid and vitamin B12. The maximal dose was carboplatin AUC 6 and pemetrexed 900 mg/m2 every 21 days; the MTD was not reached. There was 1 DLT (grade 4 neutropenia for 7 days) in dose level 3 (600 mg/m2 pemetrexed + carboplatin AUC 6). In 19 evaluable patients, the complete response (CR), PR, and stable disease (SD) rates were 63.2%, 21.1%, and 5.3%, respectively. The committee chose 500 mg/m2 pemetrexed plus carboplatin AUC 6 for the phase II portion of the trial [41].
Chambers and colleagues recently reported the first front-line trial in stage III optimally debulked ovarian, peritoneal, or fallopian tube cancer in which 3 drugs were administered intraperitoneally (IP) (Table 1) [42]. Fifteen patients with a median age of 65 (range: 46 - 76) years and an ECOG PS of 0 (47%) or 1 (53%) were enrolled. The primary sites were ovarian (67%), fallopian tube (20%), and peritoneal (13%). Most patients had serous tumors (87%), grade 3 tumors (93%), and residual disease <1 cm (67%). Only 20% had received neoadjuvant chemotherapy.
In this trial, IP pemetrexed (60 - 1000 mg/m2 on day 1) was dose-escalated in combination with IP cisplatin (75 mg/m2 on day 2) and IP paclitaxel (60 mg/m2 on day 8) [42]. Patients received folic acid and vitamin B12. Three patients accrued to each of 5 dose levels. Cycles were repeated every 21 days for 6 cycles. Fifteen patients were enrolled and treated, with a 6-cycle completion rate of 80%. One patient at dose level 2 (120 mg/m2) experienced a seizure of unclear etiology in cycle 5 and discontinued, and 2 patients at dose level 5 (1000 mg/m2) experienced ≥grade 3 DLTs (hematologic, infection, gastrointestinal) and discontinued. One of these patients died from an opportunistic infection, having grade 4 neutropenia and thrombocytopenia and grade 3 diarrhea and oral mucositis; the second patient survived an infection. Due to the severity of the toxicities at the pemetrexed dose of 1000 mg/m2, the trial was put on hold pending pharmacokinetic analyses. These toxicities remain unexplained by pharmacokinetic analyses or homocysteine levels.
At the lower pemetrexed doses (60 - 750 mg/m2), there were no grade 4 AEs; the most common grade 3 AE was fatigue (25%) [42]. At these dose levels, the incidences of alopecia (17% grade 1) and sensory neuropathy (8% grade 1) were low. Fourteen patients were evaluable for efficacy. Of the 13 patients evaluable by CA-125, 12 experienced CR and 1 had a PR. The median PFS was 30.1 months, with an 18-month PFS rate of 78.6% (median follow-up, 22.4 months). The authors recommended proceeding to phase II trials of this regimen with IP pemetrexed at 500 mg/m2, which appeared to be the MTD.
3.1.2. Phase II Trials Involving Platinum-Resistant Disease
The Gynecologic Oncology Group (GOG) evaluated pemetrexed (900 mg/m2 every 21 days) with folic acid and vitamin B12 in a single-arm phase II trial involving patients with recurrent or metastatic ovarian or primary peritoneal carcinoma (Table 2) [43]. Patients must have had no more than 1 prior platinum-based regimen for treatment of primary disease. Fifty-one patients were entered, and 48 were treated. Most patients were Caucasian (n = 47), and had a GOG performance status (PS) of 0 (n = 30), serous histology (n = 34), and grade 3 tumors (n = 31). The median TtP or recurrence following initial platinum-based therapy was 9 months. The median platinum-free interval was 3 (range: 0 - 6) months. Patients received a median of 4 (range: 1 - 19) cycles, with 40% receiving ≥6 cycles. The ORR was 21% (95% CI: 10.5 - 35.0); there was 1 CR. The median response duration was 8.4 (range: 2.0 - 45.1+) months. The disease stabilization rate was 35%, with a median duration of 4.1 months. Eighteen patients (38%) progressed during therapy. The median OS was 11.4 (range: 1.6 - 34.4) months and the median PFS was 2.9 (range: 1.0 - 33.1) months. Grades 3 and 4 AEs occurring in ≥10% of patients were: neutropenia (42%), leukopenia (25%), thrombocytopenia (13%), anemia (15%), constitutional (15%), infection (10%), and neurologic (10%). Seven patients were withdrawn due to toxicity, but there were no treatment-related deaths.
Vergote and colleagues performed a randomized, double-blind, phase II trial of 2 pemetrexed doses in patients with platinum-resistant epithelial ovarian or primary peritoneal cancer [34]. Patients having received >2 prior chemotherapy regimens were excluded. Patients received either pemetrexed 500 mg/m2 (Pem500) or pemetrexed 900 mg/m2 (Pem900), both on 21-day cycles with folic acid and vitamin B12. The primary endpoint was comparison of response rates. This study also included assessment of potential biomarkers.
Of 102 patients randomized, 98 were evaluable for safety (47 Pem500; 51 Pem900), and 91 were evaluable for efficacy (43 Pem500; 48 Pem900) [34]. The treatment arms were well-balanced, except more patients in the Pem900 arm progressed on prior platinum (12% vs 0%) than did those in the Pem500 arm, and the Pem900 arm contained fewer stage IV patients than the Pem500 arm (6% vs 19%). Compared with the Pem500 arm, a higher percentage of evaluable patients on the Pem900 arm had a platinum-free interval of <3 months (30% vs 44%), although this difference was not statistically significant. The median number of delivered cycles was 4 (range: 1 - 11) for Pem500 and 3 (range: 1 - 8) for Pem900. The combined ORR was 9.9% (95% CI: 4.6 - 18.0); four patients (9.3%; 95% CI: 2.6 - 22.1) on the Pem500 arm and 5 (10.4%; 95% CI: 3.5 - 22.7) on the Pem900 arm had a best response of PR. There were no between-arm differences in any of the time-to-event parameters (Pem500 vs Pem900): median PFS, 2.8 (95% CI: 2.6 - 4.2) months vs 2.8 (95% CI: 2.1 - 4.2) months; median OS 11.9 (95% CI: 7.9 - 14.8) months vs 10.3 (95% CI: 7.7 - 14.8) months; median time to response, 2.1 (95% CI: 1.4 - 3.4) months vs 1.5 (95% CI: 1.1 - 2.3) months; and median response duration, 4.0 (95% CI: 3.1 - 6.0) months vs 4.3 (95% CI: 3.2 - 6.1) months (Table 2).
In the Vergote trial, higher percentages of Pem900- treated patients experienced drug-related serious AEs (28% vs 17%) and discontinuations (10% vs 2%) than Pem500-treated patients [34]. There were 2 potentially drug-related deaths on the Pem900 arm resulting from sepsis and neutropenic sepsis. In general, there were lower rates of grades 3 and 4 hematologic AEs in the Pem500 arm (Pem500 grade 3%/Pem500 grade 4% vs Pem900 grade 3%/Pem900 grade 4%): anemia, 6/4 vs 12/2; leukopenia, 4/2 vs 6/4; neutropenia, 2/11 vs 8/6; febrile neutropenia, 2/4 vs 4/0; and thrombocytopenia, 2/2 vs 6/6.
In the translational research component of the Vergote trial, low excision repair cross-complementation group 1 messenger RNA (mRNA) was significantly associated with longer PFS, time to progressive disease (TtPD), and time to treatment failure (TtTF) [34]. Lower levels of reduced folate carrier mRNA were associated with improved best ORR and TtTF. Additionally, high folylpolyglutamate synthase and low glutathione-S-transferase pi mRNA were significantly associated with response, and low glycinamide ribonucleotide reductase formyl transferase expression was significantly associated with TtTF.
3.1.3. Phase II Trials Involving Platinum-Sensitive Disease
The standard first-line treatment of advanced ovarian cancer is paclitaxel and carboplatin, but cumulative neurotoxicity of this combination compromises re-treatment with these agents [44-46]. This, in combination with the preference of patients to avoid alopecia, prompted the exploration of non-taxane-containing platinum combinations, such as pemetrexed and carboplatin [41,47,48].
Matulonis and colleagues reported the results of a phase II trial testing pemetrexed (500 mg/m2) plus carboplatin (AUC 5), both administered on day 1 every 21 days for 6 or up to 8 cycles, if clinical benefit occurred (Table 2) [47]. Eligible patients had platinum-sensitive recurrent epithelial ovarian cancer, peritoneal serous cancer, or fallopian tube cancer. Patients received folic acid and vitamin B12. Forty-five patients were enrolled, and 44 were treated. The median age was 57.5 (range: 36 - 78) years. Most patients had ovarian cancer (93%), papillary serous histology (68%), and ECOG PS of 0 (57%). Half of the patients had no prior treatment for recurrence, 36% received 1 prior regimen, and 14% received 2 prior regimens for recurrent cancer. The median platinum-free interval was 19 (range: 6 - 52) months. In the intentto-treat population (n = 45), the ORR was 51.1% (95% CI: 35.8 - 66.3); there were no CRs. The SD rate was 31.1%. Most responses were recorded after 2 therapy cycles (n = 13), and the mean response duration was 6.7 (±4.2) months. Seven patients completed the 8-cycle maximum; of these, there were 6 PRs and 1 SD. The median PFS was 7.6 (95% CI: 6.4 - 10.2) months, and the mean OS was 20.3 months, whereas the median OS had not been reached. The mean number of cycles delivered was 5.3 (range: 1 - 8). Twenty-eight patients received ≥6 cycles. Grades 3 and 4 AEs occurring in ≥10% of patients were: neutropenia (41%), carboplatin allergic reaction (36%), thrombocytopenia (23%), leukopenia (16%), and fatigue (11%) [47].
The results of the phase II component of the trial by Sehouli and colleagues were reported in 2012 (Table 2) [48]. Patients must have had recurrent, platinum-sensitive ovarian or primary peritoneal cancer and could have received up to 2 courses of prior platinum-based therapy. Patients received pemetrexed (500 mg/m2) in combination with carboplatin (AUC 6), both administered every 21 days in combination with folic acid and vitamin B12 [48]. Sixty-six patients were treated. The mean age was 58 (range: 27 - 79) years. Most patients were Caucasian (89%), had an ECOG PS of 0 (70%) and had epithelial ovarian cancer (92%). The platinum-free intervals were: 5%, <6 months; 35%, 6 to 12 months, and 61%, >12 months.
In the intent-to-treat population, the ORR was 31.8% (95% CI: 20.9 - 44.4); there was 1 CR [48]. The SD rate was also 31.8%. No patient had progressive disease, but 36.4% of patients had unknown responses because no post-baseline assessment was performed or assessments were incomplete. The median time to response was 1.8 (95% CI: 1.4 - 2.8) months and the median response duration was 8.3 (95% CI: 6.7 - 10.2) months. At a median follow-up time of 15.4 months, the median PFS was 9.4 (95% CI: 8.3 - 11.1) months. Due to a high censoring rate, an analysis of OS was not performed. Grades 3 and 4 AEs were: neutropenia (39%), thrombocytopenia (24%), leukopenia (9%), hypersensitivity to carboplatin (9%), nausea (6%), vomiting (6%), and anemia (5%). There was 1 death due a possibly drug-related AE (multiple organ failure). These AEs are consistent with the phase I portion of the trial [41], as well as the unrelated trial reported by Matulonis [47]. According to the authors, the clinical activity of the pemetrexed-carboplatin combination and the low incidence of serious toxicities suggest that a randomized phase III trial may be warranted [48].
At the 2012 ASCO Annual Meeting, Hageman and colleagues presented the results of a phase II trial testing the combination of bevacizumab and pemetrexed (Table 2) [49]. Enrolled were patients with measurable disease and recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer after at least 1 prior platinumor taxane-containing regimen. Patients may have received <2 prior cytotoxic chemotherapy regimens but no prior bevacizumab. Patients were treated with pemetrexed 500 mg/m2 (with vitamins) and bevacizumab 15 mg/kg every 3 weeks until progression, unacceptable AEs, or patient/physician choice. Thirty-four patients were enrolled; of these, 28 were platinum-sensitive. Patients received a median of 7 (range: 2 - 26) cycles and the median follow-up time was 17.1 (range: 2.7 - 31.2) months. The 6-month PFS rate, which was the primary endpoint, was 58.2% (95% CI: 40 - 73) in all patients and 50% (95% CI: 19 - 81) in platinum-resistant patients. The median PFS was 7.8 (95% CI: 4.7 - 10.7) months, and 12-month OS rate was 88% (95% CI: 71 - 95). The response rate was 41% (no complete responses) and the SD rate was 53%. Among CA-125 evaluable patients (n = 27), 62% and 30% of patients experienced CA-125 declines of ≥50% and 75%, respectively. Grades 3 and 4 AEs included neutropenia (50%), metabolic (29%), leukopenia (26%), constitutional (18%), pain (18%), gastrointestinal (15%), thrombocytopenia (12%), and anemia (9%). There were no bowel perforations.
Further supporting the addition of bevacizumab to chemotherapy are the results from AURELIA, a randomized phase III trial of bevacizumab plus chemotherapy in platinum-resistant ovarian cancer [50]. These data were also presented at ASCO 2012. In AURELIA, the addition of bevacizumab to chemotherapy (PLD or topotecan or paclitaxel) provided a statistically significant improvement in PFS (6.7 vs 3.4 months; p < 0.001) and ORR (RECIST and CA-125) (30.9% vs 12.6%; p = 0.001) relative to chemotherapy alone.
3.2. Pemetrexed in Endometrial Cancer
In the European Union, the estimated number of new corpus uteri cancer cases in 2008 was 55,900, and 12,900 women died of the disease [51]. In the US, an estimated 47,130 cases of corpus uteri cancer will have been diagnosed in 2012, with 8010 deaths reported [26]. Most of these cancers originate in the endometrium. The 1- and 5-year relative survival rates for uterine corpus cancer are 92% and 82%, respectively. The 5-year survival rates decline if the cancer is diagnosed at the regional (67%) or metastatic (16%) stages. At every diagnostic stage, the relative survival of Caucasians exceeds that of African Americans by greater than 7 percentage points.
Few chemotherapeutic agents have activity in metastatic or recurrent endometrial cancer that is not amenable to treatment by radiotherapy or surgery [52]. To date, agents having definite activity are cisplatin [53,54], doxorubicin [55,56], and paclitaxel [57]. The agents 5-fluorouracil, ifosfamide, ixabepilone, and vincristine have possible activity in this setting [58-61]. More recently, pemetrexed has been evaluated in this setting [52]. A rationale for using pemetrexed comes from the observation that some endometrial tumors lack expression of the enzyme, methylthioadenosine phosphorylase (MTAP) [62]. Cells deficient in MTAP are potentially more sensitive than MTAP-positive cells to inhibitors of de novo purine synthesis, such as pemetrexed. Additionally, folate receptors are overexpressed in endometrial cancers [63], which may facilitate the transport of pemetrexed into the cell [34].
The GOG evaluated single-agent pemetrexed (900 mg/m2 every 21 days with folic acid and vitamin B12) in a phase II trial in women with recurrent or persistent endometrial carcinoma after 1 prior chemotherapy regimen (Table 3) [52]. Of the 27 entered patients, 25 were eligible and evaluable. Most patients had a GOG PS of 0 (52%), were Caucasian (84%), and had grade 3 tumors (52%). Forty-four percent had received prior radiotherapy, and 24 patients received prior combination therapy containing platinum and a taxane. In this trial, patients received a median of 2 (range: 1 - 16) cycles, with 28% of patients receiving ≥5 cycles. There was 1 PR (4%) lasting 4.5 months. Eleven patients (44%) experienced SD for a median duration of 5.3 months. Eleven patients experienced progressive disease. The estimated median PFS was 2.7 (range: 0.7 - 13.3) months, and the estimated median OS was 9.4 (range: 1.1 - 24.1) months. Grades 3 and 4 AEs included neutropenia (48%), leukopenia (40%), anemia (20%), and constitutional (16%). There were no treatment-related deaths. The authors concluded that pemetrexed was well tolerated but had minimal activity in this setting.
3.3. Pemetrexed in Cervical Cancer
In the US, 12,170 new cases of invasive cervical cancer were expected to be diagnosed in 2012, with an estimated 4200 deaths [26]. Due to prevention and early detection, mortality rates declined rapidly in past decades, but this decline has slowed in recent years. In contrast, cervical cancer is the third most commonly diagnosed cancer and the fourth leading cause of cancer death in women worldwide, accounting for 529,800 new cases and 275,100 cancer deaths among women in 2008 [27].
The high number of cervical cancer cases in developing countries and medically underserved populations is largely due to a lack of screening for precancerous and early-stage lesions. The prognosis for patients with advanced and recurrent cervical cancer that is not amenable to surgery or radiotherapy is poor, with a 1-year survival rate of 15% to 20% [64].
To date, pemetrexed has been tested in 3 single-arm phase II trials in patients with cervical cancer (Table 4). One trial involved chemonaïve patients [65], and 2 trials investigated the agent as second-line therapy [64,66]. The primary objective was measurement of antitumor activity in all 3 trials [64-66].
Goedhals and colleagues treated 35 chemonaive patients with stages III and IV cervical squamous cell carcinoma (SCC) [65]. Patients treated with prior chemotherapy or radiotherapy were excluded, but patients with stage IV disease who underwent prior pelvic radiotherapy and then developed measurable lesions outside the pelvis were allowed to have undergone additional radiotherapy if they had completed their last session at least 6 weeks prior to enrollment. Thirty-five patients were enrolled with a median age of 47 (range: 26 - 76) years; most patients had stage IIIB disease (63%) and were of African descent (71%). The initial 24 patients received pemetrexed 600 mg/m2 every 21 days, but the dose was subsequently reduced to 500 mg/m2. Patients received a median of 3 (range: 1 - 8) cycles. For patients receiving 600 mg/m2, the median number of received cycles was 3 (range: 2 - 8), whereas patients receiving 500 mg/m2 received a median of 4 cycles (range not reported).
Of the 34 evaluable patients, 6 (18%) achieved a PR, 24 patients (71%) had SD, 1 patient had progressive disease (3%), and 3 were not evaluated for response [65]. The median response duration was 3.8 (95% CI: 3.3 - 3.9) months, the median TtTF was 7.5 (95% CI: 5.2 - 9.6) months, and the median OS was 15.2 (95% CI: 11.2 - 21.4) months. The response rates were similar in both dose groups. Grade 4 toxicities were: neutropenia (37%), leukopenia (9%), anemia (6%), cutaneous rash (6%), thrombocytopenia (3%), stomatitis (3%), and vomiting (3%). There was 1 drug-related death; a patient in the 600 mg/m2 group died of hypotensive shock associated with a rectal hemorrhage. Transient, nonsymptomatic grade 3 increases in liver enzymes were observed, and 2 patients developed grade 3 kidney dysfunction (low creatinine clearance). In patients receiving 500 mg/m2 plus folic acid, the only grade 4 AEs were neutropenia (27%), leukopenia (9%), and lymphopenia (9%).
The GOG evaluated pemetrexed (900 mg/m2 every 21 days) with folic acid and vitamin B12 as second-line therapy in patients with persistent or recurrent carcinoma of the cervix [66]. Twenty-nine patients were entered, and 27 received treatment and were evaluable. The median age was 49. Most patients were Caucasian (88%), had Grade II tumors (56%), SCC histology (70%), and a PS of 0 (60%). Prior platinum-based chemotherapy was received by all, and prior radiotherapy was received by most patients (85%). Patients received a median of 4 (range: 1 - 10) cycles, with 37% of patients receiving ≥6 cycles. Four patients (15%; 95% CI: 4.2 - 33.7) experienced a PR with a median duration of 4.4 months. The SD rate was 59%. The response rates for irradiated and nonirradiated disease sites were 7% and 25%, respectively. The median PFS was 3.1 (range: 0.9 - 23.7) months, and the median OS was 7.4 (range: 1.4 - 23.7) months. Grades 3 and 4 AEs included anemia (41%), leukopenia (30%), neutropenia (26%), constitutional (26%), and infection (22%). There were no treatment-related deaths.
The Multicentre Italian Trials in Ovarian Cancer and Gynecologic Malignancies Group evaluated second-line pemetrexed 500 mg/m2 every 21 days with folic acid and vitamin B12 in patients with advanced or recurrent squamous or nonsquamous cervical carcinoma [64]. Fortythree patients were entered and were evaluable. The median age was 61 (range: 30 - 84) years. Most patients had SCC histology (67%), ECOG PS of 1 (49%), and grade 3 tumors (49%). All patients received prior platinumbased chemotherapy, and most received prior radiotherapy (63%). Patients received a median of 2 (range: 1 - 9) cycles of pemetrexed. Six patients (14%) experienced a PR with a median duration of 7 (range: 3 - 27) weeks, and 23 patients (53%) experienced disease stabilization with a median duration of approximately 14.5 (range: 8 - 56) weeks. Fourteen patients (33%) had disease progression. The median PFS and median OS were 10 weeks and 35 weeks, respectively. Eighteen patients had measurable lesions in previously irradiated fields; of these, the response rate in previously irradiated fields was 7%. Hematologic grades 3 and 4 AEs included anemia (12%), leukopenia (28%), neutropenia (30%), and thrombocytopenia (9%). Grades 3 and 4 asthenia, anorexia, and diarrhea were recorded in 4% of cycles; grade 3 alkaline phosphatase and transaminase increases occurred in 7% of cycles. Three patients developed allergic reactions, which typically manifested as a cutaneous pruriginous rash that resolved with local corticosteroids. There were no treatment-related deaths.
According to Miller and colleagues, in second-line cervical cancer, the activity of pemetrexed is similar to that of other agents that have been combined with cisplatin in first-line cervical carcinoma trials [66]. Thus, further study of pemetrexed with cisplatin may be warranted in patients with advanced or recurrent cervical cancer not previously treated with chemotherapy.
Accordingly, the GOG undertook and recently completed accrual to GOG0076-GG, “A Limited Access Phase II Trial of Pemetrexed (Alimta, LY231514) (NSC #698037) in Combination with Cisplatin (NSC #119875) in the Treatment of Advanced, Persistent, or Recurrent Carcinoma of the Cervix.” The results are not yet available.
4. FUTURE DIRECTIONS IN RESEARCH
4.1. Ovarian Cancer
Pemetrexed as single agent in recurrent ovarian cancer had similar or superior activity (ORR = 21%) to that seen with other agents studied in first-line combination in GOG 182 [43]. Among the agents selected for induction in GOG 182, response rates were topotecan 6.5%, pegylated liposomal doxorubicin 12%, and gemcitabine 13% - 17% in patients with platinum-resistant disease [67-69]. Therefore, pemetrexed could be considered in future trials for combination with other agents, particularly carboplatin, in first line therapy.
Two studies have evaluated the role of bevacizumab in combination with paclitaxel and carboplatin in the firstline setting of ovarian cancer treatment (GOG 218 and ICON 7) [70,71]. Bevacizumab is an antitumor agent targeting VEGF [72] and for which there are phase II trials demonstrating efficacy in recurrent ovarian cancer [73]. GOG 218 showed a median PFS benefit of 4 months in the arm containing up-front bevacizumab and continued as single-agent maintenance [70]. The activity and tolerability of pemetrexed in combination with bevacizumab in recurrent ovarian cancer [49] may also suggest future trials that include these 2 drugs in the frontline or recurrent setting.
In the maintenance setting, pemetrexed provided PFS and OS benefits following induction chemotherapy with pemetrexed and cisplatin in nonsquamous NSCLC [18, 19]. Maintenance pemetrexed also provided a PFS and OS benefit in advanced nonsquamous NSCLC following 4 cycles of platinum-based chemotherapy [16]. The role of maintenance chemotherapy with the intent of improving disease-free survival or OS has been investigated in ovarian cancer patients with a response after primary therapy [74,75]. A meta-analysis that included randomized controlled trials published through part of 2009 concluded that there was no evidence to suggest that maintenance platinum or doxorubicin was more effective than observation alone [75]. Likewise, in randomized trials, maintenance erlotinib or abagovomab did not improve outcomes [76,77]. However, a phase III study by GOG/Southwest Oncology Group compared 3 cycles with 12 cycles of paclitaxel after a complete clinical response to platinum-paclitaxel [78]. Patients in the 12- cycle arm showed a significant PFS benefit (22 months vs 14 months; p = 0.006) but had higher incidences of neuropathy and pain and no difference in OS. The role of maintenance chemotherapy continues to be studied by GOG (protocol 212) [79] by randomizing patients to monthly paclitaxel, paclitaxel poliglumex, or observation alone for 12 months following completion of primary chemotherapy. Due to its tolerability and efficacy in the maintenance setting of nonsquamous NSCLC, pemetrexed may have utility in maintenance therapy of ovarian cancer; however, this indication has not yet been investigated.
The clinical activity of pemetrexed shown in phase II trials in the setting of platinum-resistant and platinumsensitive recurrent ovarian cancer (Table 2) may warrant its further testing in comparison with standard chemotherapy approved for recurrent ovarian cancer. This may offer another treatment option for women with this difficult-to-treat cancer.
Several randomized trials and meta-analyses of intraperitoneal (IP) versus intravenous (IV) chemotherapy for stage III minimal residue ovarian cancer have shown an OS advantage for IP chemotherapy [80-86]. The survival benefit of the IP approach led to a 2006 NCI clinical alert that women with optimally debulked stage III ovarian cancer should be informed about the benefits of IP therapy [87]. However, significant toxicity has been a barrier to universal acceptance of the IP approach [88]. The results of the phase I study of an all IP therapy including pemetrexed showed remarkable tolerance of the combination with no alopecia and peripheral neuropathy at the first 4 dose levels [42]. The PFS seen in this phase 1 study was is in line with other studies involving patients with stage III ovarian cancer who receive frontline therapy with IV/IP cisplatin or carboplatin/paclitaxel [80, 89-93]. The results of this phase I study support further research of this approach, including IP pemetrexed.
4.2. Cervical Cancer
A meta-analysis of several studies has shown that adding chemotherapy to radiotherapy in the treatment of cervical cancer improves both OS and disease-free survival [94]. This meta-analysis showed the benefit of platinum-based chemoradiotherapy as well as nonplatinum regimens. Recent randomized phase II trials support a potential role of pemetrexed as a radiation sensitizer in the treatment of locally advanced NSCLC [95,96]. Other nonrandomized clinical trials are supportive of pemetrexed and radiotherapy in malignant pleural mesothelioma [97,98], and this combination is being studied in head and neck cancer [99]. However, the potential role of pemetrexed in addition to radiotherapy for cervical cancer has not yet been investigated.
The final results from the phase II GOG 76 GG trial (combined pemetrexed and cisplatin) will inform efficacy and tolerability of this combination in advanced or recurrent cervical cancer. As a potential follow-up, a comparison of this combination with other active cisplatin combinations in recurrent cervix cancer may be warranted.
A phase II study of bevacizumab monotherapy in patients with ≤2 prior cytotoxic regimens had encouraging results yielding a median overall survival of 7.3 months and acceptable toxicity [100]. Standard chemotherapy regimens with and without bevacizumab are being studied in GOG 240 [79]. Future studies could also include pemetrexed in combination with bevacizumab or other targeted agents.
4.3. Endometrial Cancer
In spite of promising pre-clinical rationale for potential activity of pemetrexed in endometrial cancer, GOG evaluation of single agent pemetrexed in recurrent or persistent endometrial cancer demonstrated minimal activity in this disease setting [52]. However, identification of predictive biomarkers in future research may identify patients with endometrial cancer who may potentially benefit from this drug.
4.4. Biomarkers
Thymidylate synthase and dihydrofolate reductase are inhibited by pemetrexed [7-9,14]. Retrospective analyses of thymidylate synthase and dihydrofolate reductase expression in NSCLC and mesothelioma suggest potential predictive role of these biomarkers in NSCLC and MPM [101-103], thereby suggesting that prospective trials are warranted. Retrospective studies of other biomarkers, such as genetic polymorphisms in folate metabolic pathway genes and expression of thyroid transcription factor 1 in NSCLC, also suggest a potential predictive role of these biomarkers [102,104,105]. Similarly, identification of biomarkers that allow selection of gynecologic cancer patients most likely to benefit from pemetrexed would be important for individualization of therapy.
Pemetrexed with vitamin supplementation was tolerable in all clinical trials to date and had activity in ovarian and cervical cancers. Its exact role in treatment of gynecologic malignancies, however, remains to be defined through future research.
5. ACKNOWLEDGEMENT
Medical writing support was provided on behalf of Eli Lilly and Company by Lori Kornberg, PhD, who is a full-time employee of PharmaNet-i3, an inVentiv Health company.
REFERENCES
- Karnofsky, D.A. (1968) Mechanism of action of anticancer drugs at a cellular level. CA: A Cancer Journal for Clinicians, 18, 232-234. doi:10.3322/canjclin.18.4.232
- Bailey, L.B. and Gregory, J.F. III (1999) Folate metabolism and requirements. Journal of Nutrition, 129, 779- 782.
- Goldman, I.D. and Matherly, L.H. (1985) The cellular pharmacology of methotrexate. Pharmacology & Therapeutics, 28, 77-102. doi:10.1016/0163-7258(85)90083-X
- Bertino, J.R. (1993) Karnofsky memorial lecture. Ode to methotrexate. Journal of Clinical Oncology, 11, 5-14.
- Calvert, H. (1999) An overview of folate metabolism: Features relevant to the action and toxicities of antifolate anticancer agents. Seminars in Oncology, 26, 3-10.
- Rollins, K.D. and Lindley, C. (2005) Pemetrexed: A multitargeted antifolate. Clinical Therapeutics, 27, 1343- 1382.
- Rustum, Y.M., Harstrick, A., Cao, S., et al. (1997) Thymidylate synthase inhibitors in cancer therapy: Direct and indirect inhibitors. Journal of Clinical Oncology, 15, 389- 400.
- Shih, C., Chen, V.J., Gossett, L.S., et al. (1997) LY- 231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Research, 57, 1116-1123.
- Taylor, E.C., Kuhnt, D., Shih, C., et al. (1992) A dideazatetrahydrofolate analogue lacking a chiral center at C-6,N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3d] pyrimidin-5-yl)ethyl]benzoyl]-l-glutamic acid, is an inhibitor of thymidylate synthase. Journal of Medicinal Chemistry, 35, 4450-4454. doi:10.1021/jm00101a023
- Theti, D.S. and Jackman, A.L. (2004) The role of alphafolate receptor-mediated transport in the antitumor activity of antifolate drugs. Clinical Cancer Research, 10, 1080-1089.
- Zhao, R., Babani, S., Gao, F., Liu, L. and Goldman, I.D. (2000) The mechanism of transport of the multitargeted antifolate (MTA) and its cross-resistance pattern in cells with markedly impaired transport of methotrexate. Clinical Cancer Research, 6, 3687-3695.
- Sierra, E.E. and Goldman, I.D. (1998) Characterization of folate transport mediated by a low pH route in mouse L1210 leukemia cells with defective reduced folate carrier function. Biochemical Pharmacology, 55, 1505-1512. doi:10.1016/S0006-2952(97)00673-4
- Wang, Y., Zhao, R. and Goldman, I.D. (2004) Characterization of a folate transporter in hela cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clinical Cancer Research, 10, 6256-6264. doi:10.1158/1078-0432.CCR-04-0645
- Goldman, I.D. and Zhao, R. (2002) Molecular, biochemical, and cellular pharmacology of pemetrexed. Seminars in Oncology, 29, 3-17. doi:10.1016/S0093-7754(02)70040-7
- Zhao, R. and Goldman, I.D. (2004) Enter Alimta: A new generation antifolate. The Oncologist, 9, 242-244. doi:10.1634/theoncologist.9-3-242
- Ciuleanu, T., Brodowicz, T., Zielinski, C., et al. (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase III study. Lancet, 374, 1432-1440.
- Hanna, N., Shepherd, F.A., Fossella, F.V., et al. (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of Clinical Oncology, 22, 1589-1597. doi:10.1200/JCO.2004.08.163
- Paz-Ares, L., De Marinis, F., Dediu, M., et al. (2012) PARAMOUNT: Final overall survival (OS) results of the phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo (plb) plus BSC immediately following induction treatment with pem plus cisplatin (cis) for advanced nonsquamous (NS) non-small cell lung cancer (NSCLC). Journal of Clinical Oncology, 30, Abstract LBA7507.
- Paz-Ares, L., De Marinis, F., Dediu, M., et al. (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): A double-blind, phase III, randomised controlled trial. Lancet Oncology, 13, 247-255. doi:10.1016/S1470-2045(12)70063-3
- Scagliotti, G.V., Parikh, P., von Pawel, J., et al. (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. Journal of Clinical Oncology, 26, 3543-3551. doi:10.1200/JCO.2007.15.0375
- Alimta [package insert] (2012) Eli Lilly and Company, Indianapolis.
- Vogelzang, N.J., Rusthoven, J.J., Symanowski, J., et al. (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. Journal of Clinical Oncology, 21, 2636-2644. doi:10.1200/JCO.2003.11.136
- European Medicines Agency (2011) Alimta. http://www.ema.europa.eu/ema/index.jsp?curl=pages/home/Home_Page.jsp&mid= . 2011
- Scagliotti, G., Hanna, N., Fossella, F., et al. (2009) The differential efficacy of pemetrexed according to NSCLC histology: A review of two phase III studies. The Oncologist, 14, 253-263. doi:10.1634/theoncologist.2008-0232
- Scagliotti, G., Brodowicz, T., Shepherd, F.A., et al. (2011) Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. Journal of Thoracic Oncology, 6, 64-70. doi:10.1097/JTO.0b013e3181f7c6d4
- American Cancer Society (2012) Cancer facts & figures 2012. American Cancer Society, Atlanta.
- Jemal, A., Bray, F., Center, M.M., et al. (2011) Global cancer statistics. CA: A Cancer Journal for Clinicians, 61, 69-90. doi:10.3322/caac.20107
- Bookman, M.A. (2003) Developmental chemotherapy and management of recurrent ovarian cancer. Journal of Clinical Oncology, 21, 149s-167s. doi:10.1200/JCO.2003.02.553
- Modesitt, S.C. and Jazaeri, A.A. (2007) Recurrent epithelial ovarian cancer: Pharmacotherapy and novel therapeutics. Expert Opinion on Pharmacotherapy, 8, 2293-2305. doi:10.1517/14656566.8.14.2293
- Friedlander, M., Trimble, E., Tinker, A., et al. (2011) Clinical trials in recurrent ovarian cancer. International Journal of Gynecological Cancer, 21, 771-775. doi:10.1097/IGC.0b013e31821bb8aa
- Kano, Y., Akutsu, M., Tsunoda, S., et al. (2006) Schedule-dependent interactions between pemetrexed and cisplatin in human carcinoma cell lines in vitro. Oncology Research, 16, 85-95.
- Tomassetti, A., Mangiarotti, F., Mazzi, M., et al. (2003) The variant hepatocyte nuclear factor 1 activates the P1 promoter of the human alpha-folate receptor gene in ovarian carcinoma. Cancer Research, 63, 696-704.
- Corona, G., Giannini, F., Fabris, M., et al. (1998) Role of folate receptor and reduced folate carrier in the transport of 5-methyltetrahydrofolic acid in human ovarian carcinoma cells. International Journal of Cancer, 75, 125-133. doi:10.1002/(SICI)1097-0215(19980105)75:1<125::AID-IJC19>3.0.CO;2-F
- Vergote, I., Calvert, H., Kania, M., et al. (2009) A randomised, double-blind, phase II study of two doses of pemetrexed in the treatment of platinum-resistant, epithelial ovarian or primary peritoneal cancer. European Journal of Cancer, 45, 1415-1423. doi:10.1016/j.ejca.2008.12.013
- Giovannetti, E., Mey, V., Nannizzi, S., et al. (2005) Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human nonsmall-cell lung cancer cells. Molecular Pharmacology, 68, 110-118. doi:10.1124/mol.104.009373
- Tonkinson, J.L., Worzalla, J.F., Teng, C.H., et al. (1999) Cell cycle modulation by a multitargeted antifolate, LY- 231514, increases the cytotoxicity and antitumor activity of gemcitabine in HT29 colon carcinoma. Cancer Research, 59, 3671-3676.
- Hensley, M.L., Larkin, J., Fury, M., et al. (2008) A phase I trial of pemetrexed plus gemcitabine given biweekly with B-vitamin support in solid tumor malignancies or advanced epithelial ovarian cancer. Clinical Cancer Research, 14, 6310-6316. doi:10.1158/1078-0432.CCR-08-0338
- Schultz, R.M. and Dempsey, J.A. (2001) Sequence dependence of Alimta (LY231514, MTA) combined with doxorubicin in ZR-75-1 human breast carcinoma cells. Anticancer Research, 21, 3209-3214.
- Papahadjopoulos, D., Allen, T.M., Gabizon, A., et al. (1991) Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proceedings of the National Academy of Sciences USA, 88, 11460-11464. doi:10.1073/pnas.88.24.11460
- Richards, D.A., Loesch, D., Vukelja, S.J., et al. (2011) Phase I study of pemetrexed and pegylated liposomal doxorubicin in patients with refractory breast, ovarian, primary peritoneal, or fallopian tube cancer. Investigational New Drugs, 29, 963-970. doi:10.1007/s10637-010-9414-6
- Sehouli, J., Camara, O., Mahner, S., et al. (2010) A phase-I trial of pemetrexed plus carboplatin in recurrent ovarian cancer. Cancer Chemotherapy and Pharmacology, 66, 861-868. doi:10.1007/s00280-009-1230-3
- Chambers, S.K., Chow, H.H., Janicek, M., et al. (2012) Phase I trial of intraperitoneal pemetrexed, cisplatin, and paclitaxel in optimally debulked ovarian cancer. Clinical Cancer Research, 18, 2668-2678. doi:10.1158/1078-0432.CCR-12-0261
- Miller, D.S., Blessing, J.A., Krasner, C.N., et al. (2009) Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: A study of the Gynecologic Oncology Group. Journal of Clinical Oncology, 27, 2686- 2691. doi:10.1200/JCO.2008.19.2963
- Pfisterer, J., Plante, M., Vergote, I., et al. (2006) Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. Journal of Clinical Oncology, 24, 4699-4707. doi:10.1200/JCO.2006.06.0913
- Pignata, S., De Placido, S., Biamonte, R., et al. (2006) Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: The Multicenter Italian Trial in Ovarian cancer (MITO-4) retrospective study. BMC Cancer, 6, 5. doi:10.1186/1471-2407-6-5
- Bacon, M., Kitchener, H., Stuart, G.C., et al. (2011) The global impact of the Gynecologic Cancer InterGroup in enhancing clinical trials in ovarian cancer. International Journal of Gynecological Cancer, 21, 746-749. doi:10.1097/IGC.0b013e31821bb446
- Matulonis, U.A., Horowitz, N.S., Campos, S.M., et al. (2008) Phase II study of carboplatin and pemetrexed for the treatment of platinum-sensitive recurrent ovarian cancer. Journal of Clinical Oncology, 26, 5761-5766. doi:10.1200/JCO.2008.17.0282
- Sehouli, J., Alvarez, A.M., Manouchehrpour, S., et al. (2012) A phase II trial of pemetrexed in combination with carboplatin in patients with recurrent ovarian or primary peritoneal cancer. Gynecologic Oncology, 124, 205-209. doi:10.1016/j.ygyno.2011.09.007
- Hagemann, A.R., Zighelboim, I., Novetsky, A.P., et al. (2012) Phase II trial of bevacizumab and pemetrexed for recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer. Journal of Clinical Oncology, 30, Abstract 5013.
- Pujade-Lauraine, E., Hilpert, F., Weber, B., et al. (2012) AURELIA: A randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). Journal of Clinical Oncology, 30, Abstract LBA5002.
- Ferlay, J., Parkin, D.M. and Steliarova-Foucher, E. (2010) Estimates of cancer incidence and mortality in Europe in 2008. European Journal of Cancer, 46, 765-781. doi:10.1016/j.ejca.2009.12.014
- Miller, D.S., Blessing, J.A., Drake, R.D., et al. (2009) A phase II evaluation of pemetrexed (Alimta, LY231514, IND #40061) in the treatment of recurrent or persistent endometrial carcinoma: A phase II study of the gynecologic oncology. Gynecologic Oncology, 115, 443-446. doi:10.1016/j.ygyno.2009.09.004
- Barrett, R.J., Blessing, J.A., Homesley, H.D., et al. (1993) Circadian-timed combination doxorubicin-cisplatin chemotherapy for advanced endometrial carcinoma. A phase II study of the Gynecologic Oncology Group. American Journal of Clinical Oncology, 16, 494-496. doi:10.1097/00000421-199312000-00007
- Thigpen, J.T., Blessing, J.A., Homesley, H., et al. (1989) Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecologic Oncology, 33, 68-70. doi:10.1016/0090-8258(89)90605-7
- Thigpen, J.T., Buchsbaum, H.J., Mangan, C., et al. (1979) Phase II trial of adriamycin in the treatment of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Cancer Treatment Reports, 63, 21- 27.
- Thigpen, J.T., Blessing, J.A., DiSaia, P.J., et al. (1994) A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Journal of Clinical Oncology, 12, 1408-1414.
- Lincoln, S., Blessing, J.A., Lee, R.B., et al. (2003) Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: A Gynecologic Oncology Group study. Gynecologic Oncology, 88, 277-281. doi:10.1016/S0090-8258(02)00068-9
- Dizon, D.S., Blessing, J.A., McMeekin, D.S., et al. (2009) Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: Gynecologic oncology group trial 129-P. Journal of Clinical Oncology, 27, 3104-3108. doi:10.1200/JCO.2008.20.6995
- Sutton, G.P., Blessing, J.A., Homesley, H.D., et al. (1994) Phase II study of ifosfamide and mesna in refractory adenocarcinoma of the endometrium. A Gynecologic Oncology Group study. Cancer, 73, 1453-1455. doi:10.1002/1097-0142(19940301)73:5<1453::AID-CNCR2820730521>3.0.CO;2-X
- [61] Thigpen, T., Vance, R.B., Balducci, L., et al. (1981) Chemotherapy in the management of advanced or recurrent cervical and endometrial carcinoma. Cancer, 48, 658- 665. doi:10.1002/1097-0142(19810715)48:1+<658::AID-CNCR2820481334>3.0.CO;2-R
- [62] Broun, G.O., Blessing, J.A., Eddy, G.L., et al. (1993) A phase II trial of vincristine in advanced or recurrent endometrial carcinoma. A Gynecologic Oncology Group study. American Journal of Clinical Oncology, 16, 18-21. doi:10.1097/00000421-199302000-00005
- [63] Bertino, J.R., Waud, W.R., Parker, W.B., et al. (2011) Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: Current strategies. Cancer Biology & Therapy, 11, 627-632. doi:10.4161/cbt.11.7.14948
- [64] Lu, Y. and Low, P.S. (2003) Immunotherapy of folate receptor-expressing tumors: Review of recent advances and future prospects. Journal of Controlled Release, 91, 17-29. doi:10.1016/S0168-3659(03)00215-3
- [65] Lorusso, D., Ferrandina, G., Pignata, S., et al. (2010) Evaluation of pemetrexed (Alimta, LY231514) as secondline chemotherapy in persistent or recurrent carcinoma of the cervix: The CERVIX 1 study of the MITO (Multicentre Italian Trials in Ovarian Cancer and Gynecologic Malignancies) Group. Annals of Oncology, 21, 61-66. doi:10.1093/annonc/mdp266
- [66] Goedhals, L., van Wiyk, A.L., Smith, B.L., et al. (2006) Pemetrexed (Alimta, LY231514) demonstrates clinical activity in chemonaive patients with cervical cancer in a phase II single-agent trial. International Journal of Gynecological Cancer, 16, 1172-1178. doi:10.1111/j.1525-1438.2006.00451.x
- [67] Miller, D.S., Blessing, J.A., Bodurka, D.C., et al. (2008) Evaluation of pemetrexed (Alimta, LY231514) as second line chemotherapy in persistent or recurrent carcinoma of the cervix: A phase II study of the Gynecologic Oncology Group. Gynecologic Oncology, 110, 65-70. doi:10.1016/j.ygyno.2008.03.009
- [68] Gordon, A.N., Fleagle, J.T., Guthrie, D., et al. (2001) Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. Journal of Clinical Oncology, 19, 3312-3322.
- [69] Lund, B., Hansen, O.P., Theilade, K., et al. (1994) Phase II study of gemcitabine (2’,2’-difluorodeoxycytidine) in previously treated ovarian cancer patients. Journal of the National Cancer Institute, 86, 1530-1533. doi:10.1093/jnci/86.20.1530
- [70] Shapiro, J.D., Millward, M.J., Rischin, D., et al. (1996) Activity of gemcitabine in patients with advanced ovarian cancer: responses seen following platinum and paclitaxel. Gynecologic Oncology, 63, 89-93. doi:10.1006/gyno.1996.0284
- [71] Burger, R.A., Brady, M.F., Bookman, M.A., et al. (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. New England Journal of Medicine, 365, 2473-2483. doi:10.1056/NEJMoa1104390
- [72] Perren, T.J., Swart, A.M., Pfisterer, J., et al. (2011) A phase 3 trial of bevacizumab in ovarian cancer. New England Journal of Medicine, 365, 2484-2496. doi:10.1056/NEJMoa1103799
- [73] Ferrara, N., Hillan, K.J., Gerber, H.P., et al. (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature Reviews Drug Discovery, 3, 391-400. doi:10.1038/nrd1381
- [74] Sato, S. and Itamochi, H. (2012) Bevacizumab and ovarian cancer. Current Opinion in Obstetrics and Gynecology, 24, 8-13. doi:10.1097/GCO.0b013e32834daeed
- [75] Eisenhauer, E.L., Salani, R. and Copeland, L.J. (2012) Epithelial ovarian cancer. In: Di Saia, P.J. and Creasman, W.T., Eds., Clinical Gynecologic Oncology, 8th Edition, Elsevier Saunders, Philadelphia, 285-328. doi:10.1016/B978-0-323-07419-3.00011-4
- [76] Mei, L., Chen, H., Wei, D.M., et al. (2010) Maintenance chemotherapy for ovarian cancer. Cochrane Database of Systematic Reviews, CD007414.
- [77] Pfisterer, J., Berek, J.S., Casado, A., et al. (2011) Randomized double-blind placebo-controlled international trial of abago-vomab maintenance therapy in patients with advanced ovarian cancer after complete response to first-line chemotherapy: The Monoclonal Antibody Immunotherapy for Malignancies of the Ovary by Subcutaneous Abago-vomab (MIMOSA) trial. Journal of Clinical Oncology, 29, Abstract LBA5002.
- [78] Vergote, I.B., Joly, F., Katsaros, D., et al. (2012) Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: A GCIG and EORTC-GCG study. Journal of Clinical Oncology, 30, Abstract LBA5000.
- [79] Markman, M., Liu, P.Y., Moon, J., et al. (2009) Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: Follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase III trial. Gynecologic Oncology, 114, 195-198. doi:10.1016/j.ygyno.2009.04.012
- [80] Women’s Cancer Network (2013) Gynecologic Oncology Group (GOG) trials. http://www.wcn.org/research/ gog.html
- [81] Markman, M., Bundy, B.N., Alberts, D.S., et al. (2001) Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. Journal of Clinical Oncology, 19, 1001-1007.
- [82] Alberts, D.S., Liu, P.Y., Hannigan, E.V., et al. (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. New England Journal of Medicine, 335, 1950-1955. doi:10.1056/NEJM199612263352603
- [83] Armstrong, D.K., Bundy, B., Wenzel, L., et al. (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New England Journal of Medicine, 354, 34-43. doi:10.1056/NEJMoa052985
- [84] Gadducci, A., Carnino, F., Chiara, S., et al. (2000) Intraperitoneal versus intravenous cisplatin in combination with intravenous cyclophosphamide and epidoxorubicin in optimally cytoreduced advanced epithelial ovarian cancer: A randomized trial of the Gruppo Oncologico NordOvest. Gynecologic Oncology, 76, 157-162. doi:10.1006/gyno.1999.5677
- [85] Hess, L.M., Ham-Hutchins, M., Herzog, T.J., et al. (2007) A meta-analysis of the efficacy of intraperitoneal cisplatin for the front-line treatment of ovarian cancer. International Journal of Gynecological Cancer, 17, 561-570. doi:10.1111/j.1525-1438.2006.00846.x
- [86] Elit, L., Oliver, T.K., Covens, A., et al. (2007) Intraperitoneal chemotherapy in the first-line treatment of women with stage III epithelial ovarian cancer: A systematic review with metaanalyses. Cancer, 109, 692-702. doi:10.1002/cncr.22466
- [87] Jaaback, K. and Johnson, N. (2006) Intraperitoneal chemotherapy for the initial management of primary epithetlial ovarian cancer. Cochrane Database of Systematic Reviews, CD005340.
- [88] National Cancer Institute (2006) Cancer Therapy Evaluation Program. NCI Clinical announcement on intraperitoneal chemotherapy in ovarian cancer. http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf
- [89] Tummala, M.K., Alagarsamy, S. and McGuire, W.P. (2008) Intraperitoneal chemotherapy: Standard of care for patients with minimal residual stage III ovarian cancer? Expert Review of Anticancer Therapy, 8, 1135-1147. doi:10.1586/14737140.8.7.1135
- [90] Kim, S.W., Paek, J., Nam, E.J., et al. (2010) The feasibility of carboplatin-based intraperitoneal chemotherapy in ovarian cancer. European Journal of Obstetrics & Gynecology and Reproductive Biology, 152, 195-199. doi:10.1016/j.ejogrb.2010.05.033
- [91] Konner, J.A., Grabon, D.M., Gerst, S.R., et al. (2011) Phase II study of intraperitoneal paclitaxel plus cisplatin and intravenous paclitaxel plus bevacizumab as adjuvant treatment of optimal stage II/III epithelial ovarian cancer. Journal of Clinical Oncology, 29, 4662-4668. doi:10.1200/JCO.2011.36.1352
- [92] Seamon, L.G., Carlson, M.J., Richardson, D.L., et al. (2009) Outpatient platinum-taxane intraperitoneal chemotherapy regimen for ovarian cancer. International Journal of Gynecological Cancer, 19, 1195-1198. doi:10.1111/IGC.0b013e3181b33d5b
- [93] Smith, H.O., Moon, J., Wilczynski, S.P., et al. (2009) Southwest Oncology Group trial S9912: Intraperitoneal cisplatin and paclitaxel plus intravenous paclitaxel and pegylated liposomal doxorubicin as primary chemotherapy of small-volume residual stage III ovarian cancer. Gynecologic Oncology, 114, 206-209. doi:10.1016/j.ygyno.2009.04.023
- [94] Tiersten, A.D., Liu, P.Y., Smith, H.O., et al. (2009) Phase II evaluation of neoadjuvant chemotherapy and debulking followed by intraperitoneal chemotherapy in women with stage III and IV epithelial ovarian, fallopian tube or primary peritoneal cancer: Southwest Oncology Group study S0009. Gynecologic Oncology, 112, 444-449. doi:10.1016/j.ygyno.2008.10.028
- [95] Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration (2008) Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. Journal of Clinical Oncology, 26, 5802-5812. doi:10.1200/JCO.2008.16.4368
- [96] Choy, H., Schwartzberg, L.S., Dakhil, S.R., et al. (2012) Phase II study of pemetrexed (P) plus carboplatin (Cb) or cisplatin (C) with concurrent radiation therapy followed by pemetrexed consolidation in patients (pts) with favorable-prognosis inoperable stage IIIA/B non-small cell lung cancer (NSCLC). Journal of Clinical Oncology, 30, Abstract 7002.
- [97] Govindan, R., Bogart, J., Stinchcombe, T., et al. (2011) Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. Journal of Clinical Oncology, 29, 3120-3125. doi:10.1200/JCO.2010.33.4979
- [98] Krug, L.M., Pass, H.I., Rusch, V.W., et al. (2009) Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. Journal of Clinical Oncology, 27, 3007-3013. doi:10.1200/JCO.2008.20.3943
- [99] Van Schil, P.E., Baas, P., Gaafar, R., et al. (2010) Trimodality therapy for malignant pleural mesothelioma: Results from an EORTC phase II multicentre trial. European Respiratory Journal, 36, 1362-1369. doi:10.1183/09031936.00039510
- [100] Kotsakis, A.P., Heron, D.E., Kubicek, G.J., et al. (2010) Phase II randomized trial of radiotherapy (RT), cetuximab (E), and pemetrexed (Pem) with or without bevacizumab (B) in locally advanced squamous cell carcinoma of the head and neck (SCCHN). Journal of Clinical Oncology, 28, Abstract TPS264.
- [101] Monk, B.J., Sill, M.W., Burger, R.A., et al. (2009) Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: A gynecologic oncology group study. Journal of Clinical Oncology, 27, 1069-1074. doi:10.1200/JCO.2008.18.9043
- [102] Chen, C.Y., Chang, Y.L., Shih, J.Y., et al. (2011) Thymidylate synthase and dihydrofolate reductase expression in non-small cell lung carcinoma: The association with treatment efficacy of pemetrexed. Lung Cancer, 74, 132- 138. doi:10.1016/j.lungcan.2011.01.024
- [103] Sun, J.M., Han, J., Ahn, J.S., et al. (2011) Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. Journal of Thoracic Oncology, 6, 1392-1399. doi:10.1097/JTO.0b013e3182208ea8
- [104] Zucali, P.A., Giovannetti, E., Destro, A., et al. (2011) Thymidylate synthase and excision repair cross-complementing group-1 as predictors of responsiveness in mesothelioma patients treated with pemetrexed/carboplatin. Clinical Cancer Research, 17, 2581-2590. doi:10.1158/1078-0432.CCR-10-2873
- [105] Kim, J.A., Sun, J.M., Lee, S.Y., et al. (2010) Genetic polymorphisms in folate metabolic pathway genes correlate with clinical outcomes in pemetrexed-treated patients. Journal of Thoracic Oncology, 5, S399.
- [106] Pastoelli, D., Farina, M., Diamanti, O., et al. (2010) Germline polymorhisms of methylenetetrahydrafolate reductase (MTHFR C677T) and thymidylate synthase (TS VNTR) chemotherapy-related enzymes in patients treated with pemetrexed. Annals of Oncology, 21, 152.

