Open Journal of Regenerative Medicine
Vol.2 No.2(2013), Article ID:32109,8 pages DOI:10.4236/ojrm.2013.22004
Clinical translation of neuro-regenerative medicine in India: A study on barriers and enabling strategies
![]()
1McLaughin-Rotman Centre for Global Health, University Health Network & University of Toronto, Toronto, Canada; *Corresponding Author: mark.messih@utoronto.ca, claudia.emerson@utoronto.ca, halla.thorsteinsdottir@utoronto.ca, a.daar@utoronto.ca
2Toronto Western General Hospital, University Health Network, University of Toronto, Toronto, Canada; Michael.Fehlings@uhn.ca
3Stellenbosch Institute for Advanced Study, Wallenberg Research Centre at Stellenbosch University, Stellenbosch, South Africa
Copyright © 2013 Mark J. Messih et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 23 March 2013; revised 24 April 2013; accepted 16 May 2013
Keywords: Regenerative Medicine; Translational Research; Stem Cell Policy; Indian Biotechnology; Neuro-Regeneration
ABSTRACT
We present the findings of a study of barriers and enabling strategies to clinical translation of Neuro-Regenerative Medicine (Neuro-RM) technologies in India. Twenty-three people were included in this qualitative study, including researchers, clinicians, firm representatives and policy makers working in Neuro-RM. The study has identified barriers that may arise at each stage of translation and how these are being addressed. Understanding of the molecular and cellular basis of Neuro-RM is being supported through government investment in existing neuroscience centres and the creation of new centres with regenerative medicine expertise. Clinical trials benefit from the support of clinicians who partner with researchers in study design and data collection. Government agencies have developed guidelines to inform best practices in preclinical and clinical studies. Addressing the barriers to Neuro-RM translation identified in this study can be achieved through continued support for capacity building and priority setting in preclinical studies, international efforts to achieve clinical trial protocol standardization, and multidisciplinary collaborations between clinicians, researchers, government and industry.
1. INTRODUCTION
We present here the findings of a study of barriers and enabling strategies to clinical translation of neuro-regenerative medicine technologies in India. India was chosen as the country of study as it is one of the first to move towards clinical translation in this field [1]. Furthermore, the burden of neuro-degenerative conditions is rising rapidly in India according to a review of existing neuro-epidemiological reports [2] (Table 1). Neuro-Regenerative Medicine (Neuro-RM) refers to the application of regenerative medicine approaches, such as stem cell technologies; tissue engineering and gene therapy to nervous system disorders to slow or reverse the deterioration associated with debilitating neurodegenerative disorders [3]. Clinical translation refers to the application of research discoveries from the laboratory to patient care or as part of human studies [4]. Regional studies on the prevalence of certain neurodegenerative disorders [5], when extrapolated to the total population suggest that the number of people living with stroke, peripheral neuropathy or Parkinson’s Disease is 1.76 million, 1.50 million and 3.87 million respectively. Globally, The World Health Organization anticipates that the number of persons living with Alzheimer’s and other dementias, Parkinson’s disease or neurological injuries will rise by 46.7%, 12.3% and 15.9% by 2030 [6]. Disorders of the peripheral and central nervous system (including the optic nerve) are included in this study. Previous research has identified an emerging regenerative medicine sector in India for conditions such as cardiovascular disease and diabetes [7]. What had not yet been studied is how the translation of Neuro-RM technologies in India is taking place. In this study we identify challenges, and strategies that support, clinical translation of Neuro-RM technologies; we believe the findings may be helpful in the translation of regenerative medicine more broadly, in India
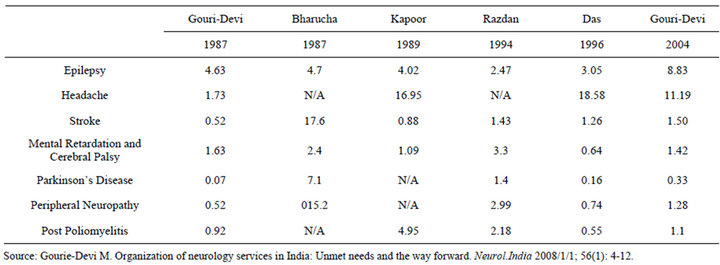
Table 1. Epidemiology of neurodegenerative disease per 1000 persons within India 1987-2004.
and in other countries. A qualitative case study was conducted with four groups of stakeholders: Clinicians, researchers, representatives of private firms, and government representatives to identify barriers to translation and the strategies being used to overcome them. Here we present the findings of our study, implications for the field, and considerations for future direction in India’s emerging Neuro-RM sector.
2. METHODOLOGY
A qualitative case study approach was used to study the barriers and strategies that impact clinical translation of neuro-regenerative medicine in India. Twenty-three face-to-face interviews were undertaken with key experts in this field. Persons of interest were identified through a search of publication databases including PubMed, Web of Knowledge, Web of Science, Medline and Scholar’s Portal. Three search criteria were cross-referenced for the search: 1) type of technology, 2) condition studied, 3) location of study. Participants also referred colleagues with expertise in this field that were also interviewed. Data was supplemented with secondary data sources including mission statements, annual reports and regulations on regenerative medicine development from the Indian Council of Medical Research and Department of Biotechnology. In order to obtain a well-rounded understanding of Neuro-RM translation, a diverse range of stakeholders (scientists, clinicians, government representatives and private firms representatives) were interviewed. Several interviewees bridged more than one category of stakeholder type; for example, one interviewee was working as both a clinician and a scientist (Table 2). The inclusion of a stakeholder in each group reflects the participant’s work experience and self-identification as a scientist, clinician, policy maker and/or private firm employee. One semi-structured, open-ended interview guide was developed by incorporating questions querying basic re-
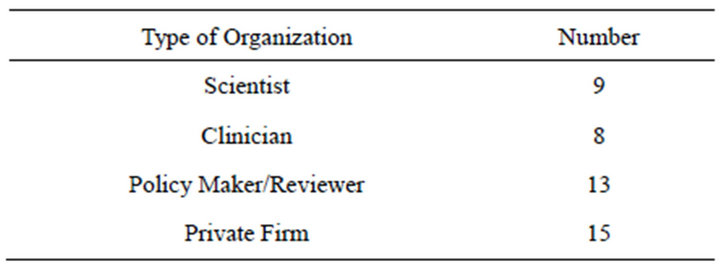
Table 2. Number of experts interviewed in each organization type.
search, clinical research, and policy facets of translation. Each interviewee was primarily asked questions pertaining to their own work and experience.
All interviews were transcribed and verified, then coded using Atlas-Ti version 5.2, a qualitative data analysis software package. Field notes were similarly coded, using the same steps as interview transcripts. The analysis was verified through triangulation with existing literature and member checks with participants upon completion of fieldwork. All participants received information about the project in advance and completed informed consent forms before starting the interview. The University of Toronto Health Sciences II Research Ethics Board approved the study.
3. RESULTS
3.1. The Role of Researchers in Clinical Translation
We found that clinicians and researchers consider it important to set research goals with clinical endpoints in mind [8-10]. According to the International Society for Stem Cell Research—Guidelines for the Clinical Translation of Stem Cells [11]:
The purpose of preclinical studies is to (a) provide evidence of product safety and (b) establish proof-ofprinciple for the desired therapeutic effect. Before initiation of clinical studies with stem cells in humans, persuasive evidence in an appropriate in vitro and/or animal model must support the likelihood of a relevant positive clinical outcome.
Indian agencies such as the Department of Biotechnology and its Centres of Excellence Program [12] support multidisciplinary centres that have clinical and research departments to focus preclinical studies on potential therapeutics. According to one statement from the department [13]:
In order to formulate road map in the area of stem cell research, a series of disease specific meetings were organized. Based on the consensus, road map for stem cell research has been categorized into basic research, translational research; human resource development; creation of infrastructure facilities; establishment of Centre of Excellence, etc.
We identified a number of neuroregenerative research studies that may inform clinical research. These include a study at the National Institute of Mental Health and Neurosciences that is investigating the differentiation and survival of implanted neuro-stem cells in rat models [14]. Recently completed stem cell research facilities at the All India Institute of Medical Sciences encompass groups researching bone marrow derived pluripotent cells for peripheral nerve repair in adult rats. That centre is also investigating bone marrow stromal cell transplantation and magnetic stimulation in sensory motor recovery in rat models of spinal cord injury [15]. At the National Centre for Biological Sciences, scientists are examining the role of serotonin on the protection and proliferation of embryonic neural stem cell populations, while at the Rajiv Ghandi Centre for Biotechnology, scientists have generated transgenic embryonic stem cells for studies on rat models of epilepsy. Each of these studies was designed with a focus on future clinical treatments in mind. Most researchers interviewed in this study believe an understanding of basic science of neurodegeneration and neuro-regenerative approaches is critical before moving into clinical research. As one participant in the study noted:
We have to have basic science research institutes too because it was recognized that brain, by itself, brain was and (is) going to be a black box for quite a number of years… if you wanted to have effective treatment for any of these complex neurological illnesses you have to understand more about the biology.
3.2. Initiating Clinical Studies: Physicians’ Role in Translation
Clinicians are collaborating with researchers in developing clinical studies and generating early neuroregenerative therapies. Physicians interviewed in this study are applying clinical knowledge to generate protocols, design robust clinical studies and perform clinical follow up with human subjects of the research. We interviewed scientists with the Nichi-In Centre for Regenerative Medicine (Nichi-In) in Chennai, India and their three collaborating physicians from two different health centres. In this collaboration, each clinician receives autologous stem cells for spinal cord injury patients and is responsible for administration and follow-up with the patient. Clinicians are located in multidisciplinary institutions that have both clinical and research departments. Examples include the National Institute of Mental Health and Neurosciences, Lakshmi Varaprasad Eye Institute (LVPEI) and Stempeutics based at Manipal University and Manipal hospital. This arrangement facilitates discussion and collaboration, and transitions technologies from the bench into the clinical setting.
Our study participants reported that there is limited opportunity for partnering between clinical trial leaders. One reported barrier was the difference in protocols between studies concerning patient selection criteria and ones concerning administration procedures. Despite the reported lack of collaboration, participants believe that these collaborations would be important in clinical translation of Neuro-RM, as pooling data from multiple sources would allow for standardization of good practices. Physicians interviewed in this study reported two barriers to clinical translation: patient follow-up and outcome measurement. Physicians report that many patients seeking Neuro-RM treatment travel from rural towns, sometimes travelling several days; as such, some of these patients do not return for follow up. Additionally, economic constraints and lack of family support also hinder access.
You have… patients coming from the Northeast and they travel for 2 days before they get here and you know, there is no follow up possible there.
For patients who live right next door to the hospital who are still not coming in for follow up, it’s [an] attitude thing, it’s an absence of awareness, you know, and the people in the family who are working, there’s nobody who is available to bring the patient to the hospital.
Furthermore, when assessing patient outcomes, clinicians reported limited access to equipment, lack of patient compliance and insufficient funding for long-term assessment. This limits the amount of quantitative data coming out of early Neuro-RM studies. Consequently, clinicians face skepticism from researchers in the West surrounding Neuro-RM clinical studies in India.
We say that we would be happy if you have evoked potentials, somatosensory potentials, neuro-conduction studies, urodynamic studies to look at how the bladder function happens. But the vast majority of these patients, these patients don’t get them done… and they don’t do it… So what we are left with is, just looking at subjective improvements of patients.
Anyone who is asking me, why do you say this patient has improved and the answer is, I could tell them that the patient says that he has improved and there is nothing more that I can tell them. So, no journal will accept that. So yes, there is a science to it but we are unable to do the science the way it should be done because of a lot of factors, patient compliance being one of the factors.
3.3. Commercial Development in Neuro-RM: Private Firms’ Role in Translation
What is presented here is a sampling of the products in the pipeline. Three firms were identified, through online resources, government documentation such as ICMR submissions and referrals from study participants. Stempeutics and Reliance Life Sciences have already developed a commercial product. The Nichi-In Centre for Regenerative Medicine offers cell culture services and has commercialized the process of generating autologous stem cells for patient use. Two commercialized products from Stempeutics and Reliance Life Sciences are described below. These examples illustrate the types of commercial products that are coming to market now and the regulatory approvals that were needed to move forward.
3.3.1. Stempeutics
Stempeutics has completed pre-clinical studies for the use of human bone marrow-derived ex vivo cultured adult Mesenchymal stem cells in allogenic settings. With approval of the Drug Controller General of India (DCGI) and Indian Council of Medical Research (ICMR), they have completed multi-centric phase I/II combined double blind randomized allogenic clinical trials on acute myocardial infarction and critical limb ischemia. Stempeutics recently published results of a clinical study on autologous bone marrow-derived Mesenchymal stem cell transplantation in Parkinson’s disease. Following data collection, Stempeutics announced plans to collaborate with Cipla, an Indian pharmaceutical firm, to market stem cell therapies for critical limb ischemia [16].
3.3.2. ReliNethra©
ReliNethra© is an autologous bio-engineered composite limbal epithelial cell graft for corneal disorders. This product is marketed to treat conditions including chemical burns and mechanical injuries to cornea. Autologous human limbal epithelial cells are cultured on human amniotic membrane. The extracellular carrier matrix is prepared by Reliance Life Sciences and they undertake cell culturing as well. The kit provides clinicians human amniotic membrane with cultured autologous limbal cells grown from limbal explants. The firm has obtained approval from the Drug Controller General of India and Food and Drug Administration to commercialize this product. It has also been patented with the World Intellectual Property Organization [17].
3.4. Government Agencies and Their Role in Translation
3.4.1. Fostering Translation through Support for Preclinical Neuro-RM Research
Financial support for basic research is usually obtained from The Indian Council of Medical Research (ICMR), the Department of Science and Technology (DST), Department of Biotechnology (DBT) and the Council of Scientific and Industrial Research (CSIR). The government funds preclinical research through grants to research groups and through investment in the creation of research centres focused on regenerative medicine innovation. Our interviewees seemed to appreciate these funding sources:
So like that, we have various funding agencies like ICMR, DST, Department of Science and Technology, CSIR… now it is easy for us to get grants, see if you submit a proposal and the proposal is convincing enough, we definitely get a lot of funding from Indian agencies.
The DBT allocated Indian rupees (INR) 53.4 million (CAN $1.15 million) for stem cell research in 2009-2010 [15]. This funding was directed towards several research institutions in order to develop isolation, expansion and storage protocols for adult stem cells. DBT allocates funds to create new labs focused on RM research, which house Neuro-RM projects. One example of this is the creation of a Stem Cell Facility within the All Indian Institute of Medical Sciences (AIIMS) in 2005 [18]. Currently, both the DBT and the Indian Council of Medical Research (ICMR) support projects therein. The Department of Science and Technology funds the generation of new facilities such as the stem cell facilities at the Christian Medical College in Vellore in India [19]. Emerging centres are equipped to connect scientists and clinicians in translating research into clinical trials. Furthermore, targeting funding for regenerative medicine supports new scientists entering into the field.
3.4.2. Established Guidelines and Their Role in Catylizing Translation
The ICMR Guidelines for Stem Cell Research and Therapy provide researchers and clinicians with recommendations for conducting research and clinical studies using stem cells [20]. These guidelines inform the development of research protocols and assist researchers in transitioning into trials. They indicate what types of research are permitted and which are prohibited. Stem cell research is grouped into three categories: permissive, restricted and prohibited. For example, any research related to reproductive cloning or human germ line genetic engineering is prohibited; creation of a human zygote by in vitro fertilization is restricted however, in vitro studies on established cell lines are permitted. Each category imposes different requirements on researchers. This can inform what types of research move forward and they facilitate government approval when a study is ready for clinical research.
The Guidelines recommend the formation of a National Apex Committee for Stem Cell Research and Therapy (NAC-SCRT) to review all stem cell protocols within the country. Stem cell research institutions must form an Institutional Committee for Stem Cell Review. However, during the period of this study a national committee for stem cell review had not been assembled to enforce these regulations. Currently, government research funding awards require recipients to follow established good practice ensuring adherence to the guideline until they are legislated.
So you know, on the one hand you can have a lot of regulations, on the other hand you can have, some isolated places… there may be some unscrupulous practices, say with the stem cells and since it is a huge country, very difficult to govern each and every person but at least the institutions that receives a government grant, can regulate them.
A key finding is that the challenges in enforcing the guidelines allow some facilities to offer therapies that have not been peer reviewed. Similarly an absence of a central registry and a reporting mechanism means that unsuccessful findings are not published or publicized.
Half of the people do it though it is unregulated, but it’s still not unlawful and until it’s made unlawful, they won’t stop.
Yeah, the whole thing, everyone you know is, I call it closet research, everybody’s doing it in secret. Doesn’t make sense, research is not meant for that, research has got to be shared, got to be open and nobody wants to talk about what they are doing…
Challenges in data collection and barriers to data sharing will prevent future studies from learning from current research. Addressing these and other barriers mentioned here barriers would require systemic changes that include implementation of extant guideline and regulations, appropriate legislative interventions, promotion of transparency and accountability, and promotion of data sharing amongst researchers in this field.
4. DISCUSSION
Based on our analysis, translation of Neuro-RM in India moves forward through the following four mechanisms.
4.1. Capacity Building through Expansion of Existing Research Facilities
Basic research progresses when researchers and clinicians adapt existing infrastructure (facilities, human capital, funding opportunities) to emerging Neuro-RM studies. Previous work has shown that India is building capacity in regenerative medicine to address local health needs through investments in existing research institutions. Neuro-RM translation follows this trend as researchers branch into this field from related projects in regenerative medicine and neuroscience. Criteria such as detail of the anticipated study, adherence to ICMR guidelines and existing expertise in related fields (Neuroscience or Stem Cell research) may inform what institutions receive funds. Existing grants such as the DBT Centre’s of Excellence Program have criteria for identifying emerging centres of Neuro-RM translation. Nations can also allocate funds to adapt existing technology to what is required for Neuro-RM studies. Translation is also taking place in new facilities that are added to existing institutions. Stem Cell Facilities at the All India Institute of Medical Sciences and Christian Medical College exemplify this approach.
Other countries may benefit from some of the positive approaches India has taken to stimulate Neuro-RM and foster translation. The first task is to identify the current state of regenerative medicine as a whole within a country and to determine possible applications of Neuro-RM to treat neurological disorders. Our study suggests that countries aiming to begin clinical translation of NeuroRM technologies would benefit from reviewing any existing research infrastructure and funding opportunities that could be adapted for novel use as well at the onset of developing a Neuro-RM translation strategy.
4.2. Concensus Building Is Important in Setting Preclinical Research Goals
Setting research objectives at the national level may standardize good research practices and can set a national research agenda. In India, stakeholders working with stem cells reported varying techniques for isolating, culturing and transplanting stem cells in clinical studies. The reported lack of publication of unsuccessful studies hinders the discovery of effective strategies, which presents a barrier to clinical translation in the country. In other nations, consensus-building initiatives for stem cell research have helped scientists in setting research priorities. For instance, the European Society of Cardiology assembled a task force to [21] to investigate the current state of progenitor/stem cell therapy in the treatment of cardiovascular disease. In 2010, the International Mesenchymal Stem Cell Therapy (MSCT) Study Group published findings of a consensus meeting to share evidence concerning the use of stem cells to treat multiple sclerosis [22]. A harmonized national policy can unify researchers by recommending required research and allocating funds accordingly.
4.3. Standardization of Outcome Measures in Clinical Trials
Clinical trials that rely on qualitative outcome measures are met with skepticism according to clinicians interviewed in this study. However, neurodegenerative diseases cause symptoms that can be both quantitatively and qualitatively measured. Changes in patient perception of pain and movement are important in assessing the success of clinical interventions in the nervous system. Limited funding and equipment, as well as poor patient follow-up are the primary barriers to obtaining qualitative data. Qualitative trials with negative outcomes are published less than those with positive outcomes [23]. Neuro-RM clinicians facing these challenges either have their studies rejected or report that colleagues with unsuccessful trial results are not publishing findings. Nevertheless, qualitative measurements are being used increasingly in clinical trials, as physicians place more importance on reported experiences of patients. One example is the Patient-Reported Outcomes Measure Information System (PROMIS) initiative developed by the National Institutes of Health [24]. This scale combines patient reported outcomes with quantitative measures for trials. Clinical trials for neurodegenerative diseases are following this trend. Studies on qualitative outcome measures in dementia show the importance of understanding patient experiences and perceived changes during clinical trials [25-27]. However, quantitative data in conjunction with qualitative findings may facilitate knowledge dissemination and collaboration development nationally and internationally. This may ensure findings from one centre can be compared with studies from other institutions. Funding for equipment needed to perform quantitative measures and patient transportation should be incorporated into clinical study submissions.
Improving post-intervention observation is critical in determining the safety and efficacy of Neuro-RM. Loss of patients from studies is a recurring challenge in clinical studies [28,29]. In this study we found that research and clinical activities are located in large urban centres, so study participants are travelling great distances to receive initial treatments but fail to return for observation. It is recommended that measures be implemented (e.g. selection criteria) to select for patients who are able to complete studies, who have access to transportation for regular treatment, or can be provided with transportation, and who are informed of all responsibilities before enrollment. Access to treatment raises ethical questions. Those most in need of treatment are frequently the most difficult to reach for clinical trials and risk being further marginalized if they cannot enter studies. Exploration of this dilemma in future research may be needed as clinical translation moves forward.
4.4. Supports for Collaborations to Catalyze Neuro-RM Translation
This study has looked at collaborations at each stage of Neuro-RM translation and has determined the impact of each in moving research forward. National and international collaboration between scientists generates new projects, and nurtures the expansion of current studies into Neuro-RM research. Collaborations generate new knowledge and build capacity in an emerging field [30, 31]. Likewise, research on international collaborations concerning health biotechnologies has shown that collaborations benefit developing world partners by providing strategic regulatory, financial and scientific knowledge [32]. This study has identified areas where collaborations are promoting translation and presents instances where no collaboration is taking place, which slows clinical translation. The following are key points concerning collaboration in translation of Neuro-RM:
· Physician-researcher collaborations are critical when conducting clinical trials;
· Limited clinician-clinician collaborations hinder protocol development;
· Government intervention is important in establishing collaborations between stakeholders.
Clinician-researcher collaborations can be developed through the creation of research centres with embedded clinical departments. Alternatively, the expansion of clinical departments within research institutions will connect stakeholders and foster translation. Interaction between clinicians and regulators, including networking events, may promote standardization and harmonization of practices. Additionally, support and oversight from the government for multi-hospital clinical trials would connect principle investigators and facilitate collaboration. One example of this is the Canadian Networks of Centres of Excellence Program, which has three objectives according to a 2009 evaluation report by the Government of Canada [33]:
· Facilitate the creation of networks on a national and an international level;
· Support networking activities among well-established researchers or research teams to encourage them to develop new collaborations with receptor communities;
· Respond to the needs of both researchers and receptor communities for interaction, collaboration, and networking.
5. CONCLUSION
India is moving forward in this area by building on existing resources. This is reflected in the development of commercial products and reported clinical trials. Communication between clinicians, scientists and government agencies is integral to generating new ideas, translating discoveries into clinical trials and ensuring that good practices are followed. Enforcement of guidelines through grants ensures good practices are followed. However, the enactment of guidelines into law is needed to ensure all institutions will follow accepted practices. Neurodegenerative diseases are a distinct category of disorders that can impact mobility, cognition and vision. Accordingly, treatments for these conditions are currently in high demand as reflected by the emergence of stem cell tourism. Regarding the phenomenon of medical tourism for neuroregenerative trials, representatives of one firm offering treatment to international patients did not wish to be interviewed for our study; and none of the stakeholders in this study recruited patients from overseas for treatment. Over the course of this study, clinicians, scientists, policy makers and private firms all agreed that such practices are unethical and further study on the state of medical tourism in this field is recommended. It is hoped that this study fosters discussion of the current state of Neuro-RM translation in India and globally.
6. ACKNOWLEDGEMENTS
The authors want to thank all the experts interviewed for generously sharing their expertise and time. Grant support for this project was primarily provided by a Canadian Institutes of Health (CIHR) Net grant (RMEthnet). Further support was provided by the McLaughlin-Rotman Centre for Global Health.
REFERENCES
- Kim, J., Schafer, J. and Ming, G.L. (2006) New directions in neuroregeneration. Expert Opinion on Biological Therapy, 6, 735-738. doi:10.1517/14712598.6.8.735
- Shaji, K.S., Jotheeswaran, A.T., Girish, N., et al. (2009) The dementia India report: Prevalence, impact, costs and services for dementia. Report Prepared for the Alzheimer’s & Related Disorders Society of India.
- Daar, A.S. and Greenwood, H.L. (2007) A proposed definition of regenerative medicine. Journal of Tissue Engineering and Regenerative Medicine, 1, 179-184. doi:10.1002/term.20
- Littman, B.H., Di Mario, L., Plebani, M. and Marincola, F.M. (2007) What’s next in translational medicine? Clinical Science, 112, 217-227.
- Gourie-Devi, M. and Organization of Neurology Services in India (2008) Unmet needs and the way forward. Neurology India, 56, 4-12. doi:10.4103/0028-3886.39304
- World Health Organization (2006) Neurological disorders: Public health challenges. World Health Organization, Geneva.
- Greenwood, H.L., Singer, P.A., Downey, G.P., Martin, D.K., Thorsteinsdóttir, H. and Daar, A.S. (2006) Regenerative medicine and the developing world. PLOS Medicine, 3, 1496-1500. doi:10.1371/journal.pmed.0030381
- Owens, D.F. and Panchision, D.M. (2012) Institutional profile: National Institute of Neurological Disorders and Stroke and National Institute of Mental Health. Regenerative Medicine, 7, 33-36. doi:10.2217/rme.11.106
- Munoz-Sanjuan, I. and Bates, G.P. (2011) The importance of integrating basic and clinical research toward the development of new therapies for Huntington disease. Journal of Clinical Investigation, 121, 476. doi:10.1172/JCI45364
- Tandon, P.N. (2009) Transplantation and stem cell research in neurosciences: Where does India stand? Neurology India, 57, 706-714. doi:10.4103/0028-3886.59464
- International Society for Stem Cell Research (2009) ISSCR guidelines for the clinical translation of stem cells. Current Protocols in Stem Cell Biology, Appendix 1B.
- Department of Biotechnology. Centers of Excellence and Programme Support in Areas of Biotechnology. http://dbtindia.nic.in/uniquepage.asp?id_pk=20
- Government of India. Stem Cell Biotechnology. http://dbtindia.nic.in/uniquepage.asp?ID_PK=20
- Totey, S.M. and Kayshyap, S.D. (2005) Tissue system with undifferentiated stem cells derived from corneal limbus. WIPO A01N 1/00.
- All Indian Institute of Medical Sciences. Stem Cell Facility Project. http://www.aiims.edu/aiims/stemcell/project.htm
- BS Reporter (2010) Cipla ties up with stempeutics for stem cell therapies. Business Standard.
- Reliance Life Sciences. http://www.rellife.com/products_relinethra.html
- Department of Biotechnology. Centers of Excellence and Programme Support in Areas of Biotechnology. http://dbtindia.nic.in/uniquepage.asp?id_pk=20
- Lenka, A. and Anand, A. (2009) Advancements in stem cell research—An Indian perspective. Annals of Neuroscience, 16.
- Indian Council of Medical Research. Stem Cell Guidelines. http://www.icmr.nic.in/stem_cell/stem_cell_guidelines.pdf
- Bartunek, J., Dimmeler, S., Drexler, H., et al. (2006) The consensus of the task force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for repair of the heart. European Heart Journal, 27, 1338-1340. doi:10.1093/eurheartj/ehi793
- Freedman, M.S., Bar-Or, A., Atkins, H., et al. (2010) The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: Consensus report of the International MSCT Study Group. Multiple Sclerosis Journal, 16, 503-510. doi:10.1177/1352458509359727
- Stern, J.M. and Simes, R.J. (1997). Publication bias: Evidence of delayed publication in a cohort study of clinical research projects. British Medical Journal, 13, 7109.
- Stern, J.M. and Simes, R.J. (1997) Publication bias: Evidence of delayed publication in a cohort study of clinical research projects. British Medical Journal, 13, 7109.
- Sabat, S. and Harre, R. (1992) The construction and deconstruction of self in Alzheimer’s disease. Ageing & Society, 12, 443-461. doi:10.1017/S0144686X00005262
- Small, J.A., Geldart, K., Gutman, G. and Clarke-Scott, M.A. (1998) The discourse of self in dementia. Ageing & Society, 18, 291-316.
- Gibson, G., Timlin, A., Curran, S. and Wattis, J. (2004) The scope for qualitative methods in research and clinical trials in dementia. Age and Ageing, 33, 422-426. doi:10.1093/ageing/afh136
- Steeves, J.D., Lammertse, D., Curt, A., Fawcett, J.W., et al. (2006) Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: Clinical trial outcome measures. Spinal Cord, 45, 206- 222. doi:10.1038/sj.sc.3102008
- Norquist, B.M., Goldberg, B.A. and Matsen III, F.A. (2000) Challenges in evaluating patients lost to follow-up in clinical studies of rotator cuff tears. The Journal of Bone & Joint Surgery, 82, 838.
- Katz, J.S. and Martin, B.R. (1997) What is research collaboration? Research Policy, 26, 1-18. doi:10.1016/S0048-7333(96)00917-1
- Bozeman, B. and Corley, E. (2004) Scientists’ collaboration strategies: Implications for scientific and technical human capital. Research Policy, 33, 599-616. doi:10.1016/j.respol.2004.01.008
- Thorsteinsdóttir, H., Melon, C.C., Ray, M., Chakkalackal, S., Li, M., Cooper, J.E., et al. (2010) South-south entrepreneurial collaboration in health biotech. Nature Biotechnology, 28, 407-416. doi:10.1038/nbt0510-407
- Bertrand, F., Picard-Aitken, M., Lecomte, N., et al. Summative evaluation of the networks of centres of excellence—New initiatives final evaluation report. http://www.sshrc-crsh.gc.ca/about-au_sujet/publications/NCE-NI_FinalE.pdf

