Open Journal of Applied Sciences
Vol.05 No.01(2015), Article ID:53434,7 pages
10.4236/ojapps.2015.51002
Photodegradation of Atrazine on TiO2?Products Toxicity Assessment
Sarka Klementova1, Zuzana Rabova-Tousova2,3, Ludek Blaha3, David Kahoun1, Petr Simek4, Lucie Keltnerova1, Martin Zlamal5
1Faculty of Science, University of South Bohemia, Ceske Budejovice, Czech Republic
2Environmental Institute, Kos, Slovak Republic
3Faculty of Science, Masaryk University, Brno, Czech Republic
4Laboratory of Analytical Biochemistry, Institute of Entomology BC ASCR, Ceske Budejovice, Czech Republic
5Institute of Chemical Technology, Prague, Czech Republic
Email: sklement@jcu.cz
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 22 December 2014; accepted 15 January 2015; published 22 January 2015
ABSTRACT
Toxicity in reaction mixtures of atrazine irradiated on immobilized TiO2 at varying irradiation times was studied using an algal growth test on unicellular green alga Raphidocelis subcapitata and a cytotoxicity test with a RTgill-W1 cell line. The toxicity of atrazine samples to algae decreased exponentially with the time of irradiation on TiO2 up to 3 h for both IC50 and IC20 values calculated for growth rate inhibition and yield. A trend to increasing variability between replicates in atrazine samples irradiated on TiO2 for longer time periods was observed; the trend was particularly pronounced in samples irradiated for 3 and 5 hours, where the atrazine samples caused moderate stimulation in lower concentration treatments (up to 550 μg/l of the initial atrazine concentration). None of the atrazine samples showed significant cytotoxicity to the rainbow trout gill cell line (RTgill-W1).
Keywords:
Atrazine, Photodegradation, Toxicity Tests, Algae, Rainbow Trout Gill Cells

1. Introduction
Atrazine (2-chloro-4-ehtylamino-6-isopropylamino-1,3,5-triazine), one of the most common herbicides, is a persistent organic pollutant, and its residues are often found in groundwaters and surface waters [1] -[3] .
Some microorganisms are able to degrade the herbicide to metabolites such as desethyl atrazine, DEA, desisopropyl atrazine, DIPA, and desethyl desisopropyl atrazine, DEDIPA [4] . These compounds have also been found among the products of photochemical degradation of atrazine on semiconductors [5] [6] together with hydroxyderivatives of the parent compound as well as of its metabolites [7] and cyanuric acid [5] [8] . Herbicides, toxic by definition to some plant species, may display toxic effects to other species after short- or long-term exposure. The transformation products of herbicides represent an issue of emerging importance since in some cases they represent a greater risk to the environment than the parent molecules [9] .
The toxicity of atrazine and its metabolites has been evaluated in several studies. Tchounwouli et al. [10] compared the toxicities by Microtox Assay and concluded that DEA and DIPA are the least toxic, with EC50 81.86 and 82.68 mg/l, resp., followed by atrazine (EC50 = 39.87 mg/l) and DEDIPA (EC50 = 11.80 mg/l). Ralston-Hooper et al. [11] evaluted the acute and chronic toxicity in the amphipods Hyalella azteca and Diporeia spp., and in the unicellular algae Pseudokirchneriella subcapitata. They found that acute and chronic toxicities were ranked ATRAZINE > DEA > DIPA. All 96-h median inhibition concentrations, IC(50), were above concentrations found in the environment (>1.500 mg/l), with the highest sensitivity seen in the algae. An atrazine toxicity reduction to the yeast cells due to its photocatalytic decomposition on TiO2 has previously been reported by Campanella and Vitaliano [12] . The photochemical degradation using a semiconductor as catalyst such as TiO2 is an area of environmental interest for the treatment of polluted waters, because the technique accelerates the formation of very reactive species such as hydroxyl radicals that cause an oxidative degradation of organic contaminants.
The aim of this study was to investigate toxicity in reaction mixtures of atrazine after photocatalytic degradation on immobilized TiO2 at varying irradiation times and to evaluate the toxicity of gradually formed products making use of ecotoxicological models relevant to natural surface waters (algae and rainbow trout gill cells).
2. Materials and Methods
Atrazine, atrazine desethyl (DEA), atrazine desisopropyl (DIPA), atrazine desethyl desisopropyl (DEDIPA), 2- hydroxyderivatives of the above compounds and cyanuric acid (all HPLC quality standards) were purchased from Dr. Ehrenstorfer GmbH, Germany.
A saturated solution of atrazine was prepared by dissolving the substance in double deionised water (Ultrapur 10, Watrex); the solution was filtered through 0.45 μm membrane filters (Millipore) before irradiation. Irradiation was carried out in reaction vessels containing 5 cm2 of glass coated with TiO2, in batch arrangement. For irradiation, samples containing 5 ml of stock solution of 3 × 10−5 M atrazine per batch were used. After irradiation, each sample was quantitatively transferred into 10 ml volumetric flask and filled. The light source Phillips TLD 15 W 08 lamp (light with the wavelength range of 350 - 410 nm) was used for irradiation. The light intensity measured by a Lutron UV light meter (UVA 365) was 1.8 mW∙cm−2.
TiO2 layers were prepared by sedimentation of Evonik/Degussa Aeroxide P 25 on soda lime glass following the procedure described earlier [13] .
Degradation kinetics were monitored by HPLC, using a high pressure pump ConstaMetric 3200, column Phenomenex Luna 5 μm (C18) 100 A (250 × 4.60 mm), UV detector Delta Chroma UVD 200 (Watrex) and ClarityLite software. The flow rate was 1 ml∙min−1, mobile phase methanol-water 65:35 (v/v). All samples were filtered through 0.2 μm membrane filters (Millipore) before the analyses. The products were determined by reversed phase liquid chromatography-mass spectrometry (RP-HPLC-MS) with two sets of equipment ((a) and (b)):
LTQ XL ion trap (a) or LCQ Fleet ion trap (b); Accelaautosampler, Acella 600 HPLC pump; HESI electronspray ion source or APCI ion source (both Thermo Fisher Scientific, San Jose, CA, USA).
The components were separated by:
(a) gradient elution with A = 0.1% formic acid in MeCN and B 0.1% formic acid in water with a 150 × 2 mm id Gemini C18 column (Phenomenex, Torrance, CA, USA). The mobile phase flow rate was 200 µl/min; column temperature, 30˚C. The gradient program was from A:B = 5:95 to 90:10 in 13 min, 2 min hold and then back to A:B = 5:95 in 1 min and an equilibration for 4 min;
(b) isocratic elution with the mobile phase consisted of 35% (v/v) 5 mM ammonium acetate in water and 65% (v/v) methanol on a Phenomenex Luna C18 (250 × 4.6 mm I.D., 5 μm particle size) chromatographic column (Phenomenex, Torrance, CA, USA). The mobile phase flow rate was 1000 µl/min, the injected volume was 25 μl, the column temperature was set at 25˚C.
Positive ion mass spectra were acquired by scanning the 100 - 600 Da mass range every 25 seconds (a) or 50 - 500 Da mass range every 27 seconds (b). The full scan mode was combined with targeted MS/MS scanning of the MH+ ion products generated by their collision-induced decomposition (CID) at 30% relative collision energy mass isolation width, 1 Da (a) or 3 Da (b). The HESI ion source was heated to 200˚C; ion source voltage was 3 kV; capillary voltage was 30 V.
Bioassays: an algal growth test was performed according to the OECD Guideline 2011 [14] using the unicellular green alga Raphidocelis subcapitata (syn. Pseudokirchneriella subcapitata). The assay was modified for a 96-well microtiter plate as described earlier [15] . Algae in the exponential growth phase were exposed (72 h, continuous illumination 1500 lux, 24˚C) to a concentration series of atrazine samples (initial concentrations prior to irradiation 8; 25; 69; 138; 270; 550; 1110; 3330; 10,000 μg/l). Absorbance at 680 nm was measured every 24 hours as a surrogate parameter for biomass to determine algal growth. The inhibitions of growth rate and yield were calculated and effective concentrations based on nominal values were estimated according to OECD 2011 [14] . Each concentration treatment was tested in 5 replicates. Growth inhibition caused by the reference toxicant, potassium dichromate (Lach-Ner, s.r.o., Czech Republic), in a 5-point-concentration series was tested as a positive control in parallel to each experiment.
An in vitro cytotoxicity test with a RTgill-W1 cell line was performed according to the method described by Tanneberger et al. [16] . RTgill-W1 cell line acquired from the American Type Culture Collection (ATCC) was cultured under standard conditions [17] in white L-15 medium (Sigma Aldrich, Czech Republic) supplemented with 10% fetal bovine serum (Sigma-Aldrich, Czech Republic). The cells were seeded to a 96-well plate (35,000 cells per well) and after 24 hours of incubation at 19.6˚C, exposed to atrazine samples for 24 hours. Cell viability was assessed using the combination of three fluorescent dyes that determine different cytotoxic mechanisms: Alamar blue (AB, Invitrogen-Life Technologies, Czech Republic), which indicates the metabolic activity, 5- carboxyfluorescein diacetateacetoxymethyl ester (CFDA-AM; Invitrogen-Life Technologies, Czech Republic), which is an indirect measure of the cell membrane integrity, and neutral red (NR; Invitrogen-Life Technologies, Czech Republic), which indicates the energetic state of a cell and is an indirect measure of the lysosomal membrane integrity. The results are reported as a fraction of control (FOC), which expresses the viability of cells exposed to atrazine compared to the negative control (i.e. cells in a clean medium with 50% v/v sterile distilled water, FOC = 1). Two concentrations (5 and 10 mg/l of initial atrazine concentrations prior to irradiation) were tested in triplicate and DMSO (Sigma Aldrich, Czech Republic) was used as the positive control (0.1%; 10%; 15%; 20% v/v).
Bioassays data were analyzed in GraphPad Prism 5 Software (GraphPad Software, Inc., San Diego, CA, USA), where Hill’s model was applied for sigmoidal dose-response curve fitting to estimate IC50 and IC20 values. Statistica 12 (StatSoft Inc., Tulsa, OK, USA) was used to perform ANOVA.
3. Results
The photocatalytic degradation of atrazine on TiO2 immobilised on a glass support proceeds as the 1st order reaction with a rate constant of 0.018 min−1 [13] . The irradiation procedure leads to the formation of a series of intermediates and final products. The results of LC-MS analyses revealed detectable amounts of the following products of atrazine degradation: atrazine desethyl, atrazine desisopropyl, atrazine desethyldesisopropyl and hydroxyderivatives of these products as well as of atrazine itself. Cyanuric acid was not found in the reaction mixtures.
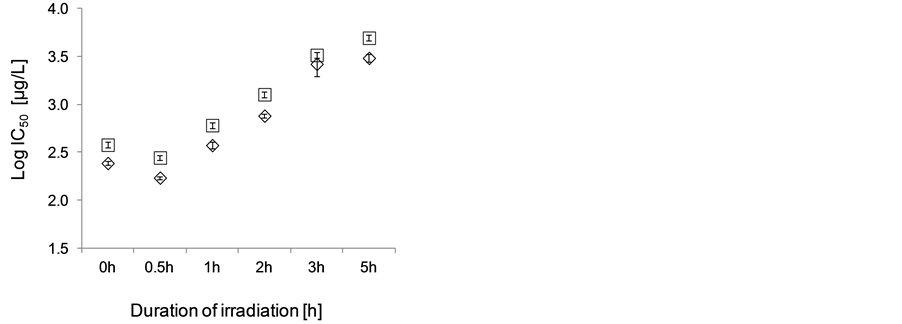
Algal growth inhibition tests were valid (specific growth rate of controls between 0.5 - 0.8 day−1, and IC50 of potassium dichromate for growth rate and yields of 4.04 mg/l and 3.18 mg/l respectively). All atrazine samples showed dose-response relationships and the highest tested concentration (10 mg/l) of the initial atrazine concentration treatments reached more than 80% inhibition (Figure 1). The toxicity of atrazine samples to algae decreased exponentially with the time of irradiation on TiO2 up to 3 h for both IC50 and IC20 values calculated for growth rate inhibition and yield (Table 1 and Figure 2). After 3 hours of irradiation the decrease in toxicity slowed down, this effect being more pronounced in IC20 values. There is a trend to increasing variability between replicates in atrazine samples irradiated for longer time periods on TiO2, which is particularly pronounced in samples irradiated for 3 and 5 hours, where the atrazine samples cause a moderate stimulation in lower concentration treatments (up to 550 μg/l of the initial atrazine concentration).
The responses of the negative and positive control in the cytotoxicity test with a rainbow trout gill cell line
Figure 1. Inhibition of algal growth by atrazine samples with increasing duration of irradiation on TiO2. The graphs show the initial atrazine concentrations [μg/l] in the non-irradiated sample (0 h), which is the theoretical maximum concentration of atrazine itself in the irradiated samples. Closed symbols show growth rate inhibition, open symbols yield inhibition and error bars standard deviation (N = 5).
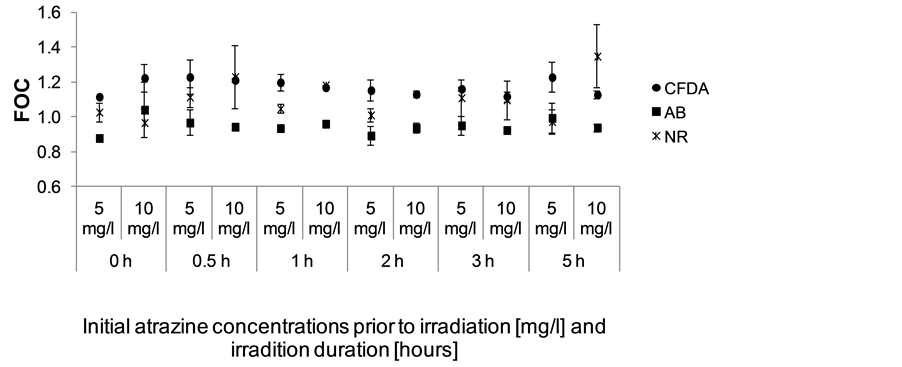
(RTgill-W1) were in line with the long term laboratory average (EC50 DMSO = 10% v/v). None of the atrazine samples showed significant cytotoxicity to the rainbow trout gill cell line (RTgill-W1) as the FOC of all samples exceeded 0.8 (80% of negative control). No differences were observed between non irradiated and irradiated atrazine samples (Figure 3). None of the three dyes used (AB, CFDA and NR) indicated a significant cytotoxicity and comparable response profiles for all samples were seen.
4. Discussion
The occurrence of atrazine and its degradation products in surface and ground waters have previously been addressed by several studies due to growing concerns about the adverse effects of atrazine on aquatic ecosystems [18] . A monitoring study in the Mississippi River Basin reported an atrazine occurrence ranging from 255 to 694 ng/l and its major degradation products DEA and DIPA at concentrations of 255 - 694 ng/l [19] . The hydroxylated degradation products of atrazine measured in the Missouri River Basin in 1992 were dominated by hydroxyatrazine; the ratio of hydroxyatrazine:atrazine:DEA being 20:5:1. Hydroxylated degradation products persist longer in the aquatic environment compared to atrazine and desethylatrazine [20] .
The photocatalytic degradation of atrazine is an efficient tool for its removal from drinking and waste waters [21] . However, the toxicity of the mixtures of photodegradation products arising under distinct irradiation condi-
Table 1. Effective concentrations [µg/l] of atrazine samples irradiated up to five hours on TiO2. Reported IC50 and IC20 estimates for growth rate and yield of green alga Raphidocelis subcapitata are based on the nominal initial atrazine concentration in the non-irradiated sample (0 h), which is the theoretical maximum concentration of atrazine in the irradiated samples. Values in brackets show the 95% confidence intervals.
Figure 2. Decrease of atrazine toxicity to green alga Raphidocelis subcapitata with the duration of irradiation on TiO2. Shown are the logarithms of estimated inhibitory concentrations [µg/l] based on the nominal initial atrazine concentration in the non-irradiated sample (0 h), which is the theoretical maximum concentration of atrazine in the irradiated samples. Diamonds show growth rate inhibition and squares yield inhibition. Error bars denote standard deviation of the Hill’s model fitting.
Figure 3. Results of cytotoxicity test with rainbow trout gill cell line (RTgill-W1) expressed as a fraction of control (FOC). Atrazine concentrations [mg/l] are based on the nominal initial atrazine concentration in the non-irradiated sample (0 h), which is the theoretical maximum concentration of atrazine in the irradiated samples. Error bars indicate standard deviation (N = 3).
tions is still subject to current research since there is evidence that some degradation products, e.g. DEA, may elicit higher toxicity than the parent compound [22] . A decrease in the toxicity of atrazine after photocatalytic degradation has been described using the Microtox bacterial bioluminescence assay and a yeast model [12] [23] . Our toxicity data from the algal growth test showed a similar trend with a significant decrease in toxicity to algae after 1 h (or longer periods) of irradiation. The test species of green alga, Raphidocelis subcapitata, is considered a sensitive and environmentally relevant model. The results observed in the present study for the non-ir- radiated atrazine samples are comparable with the results of Van der Heever et al. [24] who reported IC50 359 mg/l, even though lower IC50 values for the same species were reported by Weiner et al. [25] and Pérez et al. [26] (48.77 µg/l and 196 µg/l, resp.)
Experiments with fish RTgill-W1 cells did not show any toxic effects in both non-irradiated and irradiated atrazine samples. This seems to correspond to in vivo ecotoxicity data for atrazine reported in the US EPA Ecotox Database where EC/LC50 of atrazine for rainbow trout in acute tests exceeded 10 mg/l [27] . Wan et al. [28] reported acute LC50s of atrazine 15 and 13 mg/l for 1- and 2 - 4-day exposures, respectively. However, in another study atrazine caused a significant reduction in gill Na + K + ATPase activity at environmentally relevant concentrations (2.0, 5.0 and 10.0 µg/l) in Atlantic salmon (Salmosalar) smolts [29] . According to Prasad et al. [30] atrazine altered the hemocyanin metabolism, hydromineral balance, and gill function in crabs (Oziotelphusa senex senex). Exposure to 5 µg/l of atrazine led to osmotic disfunctions in mummichog fish larvae (Fundulus heteroclitus) and exposure to 40 - 80 µg/l to behavioral and growth changes in red drum larvae (Sciaenops ocellatus) [31] . This evidence indicates that atrazine may pose a risk to the early developmental stages of fish and interfere with invertebrate gill functions. Therefore, further experiments with fish embryos might provide additional insights into the toxicity of atrazine and its photodegradation-product mixtures.
5. Conclusions
Our experiments confirm that the photocatalytic degradation of atrazine on TiO2 under UV irradiation leads to a gradual decrease in toxicity to algae with increasing irradiation duration. The results indicate no formation of by-products with equal or higher toxicity to algae than the parent compound atrazine. Samples with photocatalytic degradation products of atrazine seem to be non-toxic to the fish cell line. There were no observable differences from the control in the 3-dyes-bioassay or microscopic inspection.
Though more experiments with early developmental stages of fish might provide an additional insight into the toxicity of atrazine and its photodegradation products, we can conclude from our environmentally relevant model that photocalytic degradation on TiO2 semiconductor is a useful method leading to a decrease of harmful effects of atrazine in polluted surface waters with respect to the water organisms.
Acknowledgements
Authors acknowledge Faculty of Science University of South Bohemia for funding the research.
References
- Konstantinou, I.K., Hela, D.G. and Albanis, T.A. (2006) The Status of Pesticide Pollution in Surface Waters (Rivers and Lakes) of Greece. Part I. Review on Occurrence and Levels. Environmental Pollution, 141, 555-570. http://dx.doi.org/10.1016/j.envpol.2005.07.024
- Guzzella, L., Pozzoni, F. and Giuliano, G. (2006) Herbicide Contamination of Surficial Groundwater in Northern Italy. Environmental Pollution, 142, 344-353. http://dx.doi.org/10.1016/j.envpol.2005.10.037
- Arias-Estévez, M., López-Periago, E., Mart?ez-Carballo, E., Simal-Gándara, J., Mejuto, J.-C. and García-Río, L. (2008) The Mobility and Degradation of Pesticides in Soils and the Pollution of Groundwater Resources. Agriculture, Ecosystems & Environment, 123, 247-160. http://dx.doi.org/10.1016/j.agee.2007.07.011
- Ralebitso, T.K., Senior, E. and van Verseveld, H.W. (2002) Microbial Aspects of Atrazine Degradation in Natural Environments. Biodegradation, 13, 11-19. http://dx.doi.org/10.1023/A:1016329628618
- Minero, C., Pramauro, E. and Pelizzetti, E. (1992) Photosensitized Transformations of Atrazine under Simulated Sunlight in Aqueous Humid Acid Solution. Chemosphere, 24, 1597-1606. http://dx.doi.org/10.1016/0045-6535(92)90403-E
- Konstantinou, I.K. and Albanis, T.A. (2003) Photocatalytic Transformation of Pesticides in Aqueous Titanium Dioxide Suspensions Using Artificial and Solar Light: Intermediates and Degradation Pathways. Applied Catalysis B: Environmental, 42, 319-335. http://dx.doi.org/10.1016/S0926-3373(02)00266-7
- Scribner, E.A., Thurman, E.M., Goolsby, D.A. and Battaglin, W.A. (2005) Summary of Significant Results from Studies of Triazine Herbicides and Their Degradation Products in Surface Water, Ground Water, and Precipitation in the Midwestern United States during the 1990s. Scientific Investigations Report 2005-5094, US Geological Survey, Reston, 1-27.
- Bianchi, C.L., Pirola, C., Ragaini, V. and Selli, E. (2006) Mechanism and Efficiency of Atrazine Degradation under Combined Oxidation Processes. Applied Catalysis B: Environmental, 64, 131-138. http://dx.doi.org/10.1016/j.apcatb.2005.11.009
- Vicente, A. (2004) Determination of Pesticides and Their Degradation Products in Soil: Critical Review and Comparison of Methods. Trends in Analytical Chemistry, 23, 772-789. http://dx.doi.org/10.1016/j.trac.2004.07.008
- Tchounwouli, P.B., Wilson, B., Ishaque, A., Ransome, R., Huang, M.-J. and Leszczynski, J. (2004) Toxicity Assessment of Atrazine and Related Triazine Compounds in the Microtox Assay, and Computational Modeling for Their Structure-Activity Relationship. International Journal of Molecular Sciences, 1, 63-74. http://dx.doi.org/10.3390/ijms1040063
- Ralston-Hooper, K., Hardy, J., Hahn, L., Ochoa-Acuna, H., Lee, L.S., Mollenhauer, R. and Sepúlveda, M.S. (2009) Acute and Chronic Toxicity of Atrazine and Its Metabolites Deethylatrazine and Deisopropylatrazine on Aquatic Organisms. Ecotoxicology, 18, 899-905. http://dx.doi.org/10.1007/s10646-009-0351-0
- Campanella, L. and Vitaliano, R. (2007) Atrazine Toxicity Reduction Following H2O2/TiO2-Photocatalyzed Reaction and Comparison with H2O2-Photolytic Reaction. Annali di Chimica, 97, 123-134.
- Klementová, S. and Zlámal, M. (2013) Photochemical Degradation of Triazine Herbicides-Comparison of Homogeneous and Heterogeneous Photocatalysis. Photochemical & Photobiological Sciences, 12, 660-663. http://dx.doi.org/10.1039/c2pp25223f
- OECD (2011) OECD Guidelines for the Testing of Chemicals, Guideline 201. Freshwater Alga and Cyanobacteria, Growth Inhibition Test. http://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-2-effects-on-biotic-systems_20745761
- Rojıckova, R., Dvorakova, D. and Maršálek, B. (1998) The Use of Miniaturized Algal Bioassays in Comparison to the Standard Flask Assay. Environmental Toxicology and Water Quality, 13, 235-241. http://dx.doi.org/10.1002/(SICI)1098-2256(1998)13:3<235::AID-TOX5>3.0.CO;2-8
- Tanneberger, K., Knöbel, M., Busser, F.J.M., Sinnige, T.L., Hermens, J.L.M. and Schirmer, K. (2013) Predicting Fish Acute Toxicity Using a Fish Gill Cell Line-Based Toxicity Assay. Environmental Science & Technology, 47, 1110- 1119. http://dx.doi.org/10.1021/es303505z
- ATCC (2014) Culture Method. RTgill-W1 (ATCC® CRL-2523TM). http://www.lgcstandards-atcc.org/products/all/CRL-2523.aspx?geo_country=cz#culturemethod
- Baxter, L.R., Sibley, P.K., Solomon, K.R. and Hanson, M.L. (2013) Interactions between Atrazine and Phosphorus in Aquatic Systems: Effects on Phytoplankton and Periphyton. Chemosphere, 90, 1069-1076. http://dx.doi.org/10.1016/j.chemosphere.2012.09.011
- Pereira, W.E. and Rostad, C.E. (1990) Occurrence, Distributions, and Transport of Herbicides and Their Degradation Products in the Lower Mississippi River and Its Tributaries. Environmental Science & Technology, 24, 1400-1406. http://dx.doi.org/10.1021/es00079a015
- Lerch, R.N., Blanchard, P.E. and Thurman, E.M. (1998) Contribution of Hydroxylated Atrazine Degradation Products to the Total Atrazine Load in Midwestern Streams. Environmental Science & Technology, 32, 40-48. http://dx.doi.org/10.1021/es970447g
- Lekkerkerker-Teunissen, K., Benotti, M.J., Snyder, S.A. and van Dijk, H.C. (2012) Transformation of Atrazine, Carbamazepine, Diclofenac and Sulfamethoxazole by Low and Medium Pressure UV and UV/H2O2 Treatment. Separation and Purification Technology, 96, 33-43. http://dx.doi.org/10.1016/j.seppur.2012.04.018
- Andreu, V. and Picó, Y. (2004) Determination of Pesticides and Their Degradation Products in Soil: Critical Review and Comparison of Methods. TrAC Trends in Analytical Chemistry, 23, 772-789.
- Ying, J.-C., Anderson, T.D. and Zhu, K.Y. (2008) Effect of Alachlor and Metolachlor on Toxicity of Chlorpyrifos and Major Detoxification Enzymes in the Aquatic Midge, Chironomus tentans (Diptera : Chironomidae). Archives of Environmental Contamination and Toxicology, 54, 645-652. http://dx.doi.org/10.1007/s00244-007-9067-4
- Van der Heever, J.A. and Grobbelaar, J.U. (1996) The Use of Selenastrum capricornutum Growth Potential as a Measure of Toxicity of a Few Selected Compounds. Water SA, 22, 183-191.
- Weiner, J.A., DeLorenzo, M.E. and Fulton, M.H. (2004) Relationship between Uptake Capacity and Differential Toxicity of the Herbicide Atrazine in Selected Microalgal Species. Aquatic Toxicology, 68, 121-128. http://dx.doi.org/10.1016/j.aquatox.2004.03.004
- Pérez, J., Domingues, I., Soares, A.M.V.M. and Loureiro, S. (2011) Growth Rate of Pseudokirchneriella subcapitata Exposed to Herbicides Found in Surface Waters in the Alqueva Reservoir (Portugal): A Bottom-Up Approach Using Binary Mixtures. Ecotoxicology, 20, 1167-1175. http://dx.doi.org/10.1007/s10646-011-0661-x
- US Environmental Protection Agency (2014) ECOTOX User Guide: ECOTOXicology Database System. Version 4.0. http://cfpub.epa.gov/ecotox
- Wan, M.T., Buday, C., Schroeder, G., Kuo, J. and Pasternak, J. (2006) Toxicity to Daphnia magna, Hyalella azteca, Oncorhynchus kisutch, Oncorhynchus mykiss, Oncorhynchus tshawytscha, and Rana catesbeiana of Atrazine, Metolachlor, Simazine, and Their Formulated Products. Bulletin of Environmental Contamination and Toxicology, 76, 52-58. http://dx.doi.org/10.1007/s00128-005-0888-4
- Waring, C.P. and Moore, A. (2004) The Effect of Atrazine on Atlantic salmon (Salmo salar) Smolts in Fresh Water and after Sea Water Transfer. Aquatic Toxicology, 66, 93-104. http://dx.doi.org/10.1016/j.aquatox.2003.09.001
- Prasad, T.A.V., Srinivas, T., Janardan, S. and Reddy, D.C. (1995) Atrazine Toxicity on Transport Properties of Hemocyanin in the Crab Oziotelphusa senex senex. Ecotoxicology and Environmental Safety, 30, 124-126. http://dx.doi.org/10.1006/eesa.1995.1015
- Del Carmen Alvarez, M. and Fuiman, L.A. (2005) Environmental Levels of Atrazine and Its Degradation Products Impair Survival Skills and Growth of Red Drum Larvae. Aquatic Toxicology, 74, 229-241. http://dx.doi.org/10.1016/j.aquatox.2005.05.014