American Journal of Analytical Chemistry
Vol.5 No.3(2014), Article ID:42927,8 pages DOI:10.4236/ajac.2014.53019
HS-SPME/GC-MS Analysis of VOC and Multivariate Techniques Applied to the Discrimination of Brazilian Varieties of Mango
1Departamento de Ciências da Vida, Universidade do Estado da Bahia (UNEB), Salvador, Brazil
2Laboratório de Química Analítica, Universidade Estadual do Sudoeste da Bahia (UESC), Jequié, Brazil
3Instituto de Química, Universidade Federal da Bahia (UFBA), Salvador, Brazil
Email: jailsondeandrade@gmail.com
Copyright © 2014 Clícia Maria de Jesus Benevides et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Clícia Maria de Jesus Benevides et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received December 7, 2013; revised January 8, 2014; accepted January 16, 2014
KEYWORDS
Mango; Volatiles; Multivariate Techniques; HS-SPME/GC-MS; Chemometrics
ABSTRACT
The present study analyzed the volatile compounds of three mango varieties (Tommy Atkins, Rosa and Espada) using the static headspace technique with SPME coupled to CG-MS. Multivariate methodologies, such as factorial design and response surface methodology, were used to optimize the conditions of adsorption and desorption of these substances. The data were evaluated by using principal components analysis (PCA) and hierarchical grouping analysis, in order to visualize grouping tendencies of volatile compounds. Thirty-seven volatile compounds belonging to different chemical classes, such as esters, terpenes, alcohols and others, were tentatively identified in the three varieties of mango. Amongst them, twenty-three presented chromatographic peaks with relative areas larger than 2%. The multivariate analysis made it possible to visualize the grouping tendencies of the mango samples, according to the presence of their respective volatile substances, and enabled the identification of the groups of substances responsible for the discrimination among the three varieties.
1. Introduction
Globally, the mango (Mangifera indica L.) industry is the 5th largest tropical fruit industry with the production of over 34.3 million tons [1]. Although about 100 countries grow mangoes, about 80% of production comes from the top nine countries in order of production, India, China, Indonesia, Mexico, Thailand, Brazil, Philippines and Nigeria [2].
Chemical analysis of the flavor (volatiles) of several mango cultivars around the world has been reported [3-6] and has recently studied the spatial and temporal changes in the volatile profile of mango upon exogenous ethylene treatment, while [7] evaluating the attraction of west fruit flies to volatiles of three mango cultivars in field cage tests. In these works, a wide range of compounds has been identified, including esters, lactones, monoand sesquiterpenes. Monoterpenes such as cis-ocimene, α- and β-pinene, myrcene and limonene seem to be particularly important contributors to the flavor of the fresh fruit, depending upon the variety [8].
Aroma compounds are usually difficult to analyze, mainly due to their high volatility and low concentrations in samples. Therefore, many methods have been employed for the analysis of volatiles in foods. These methods are generally divided into two classes: classical methods (distillation, solvent extraction) and clean methods such as solid-phase extraction (SPE), supercritical fluid extraction (SFE), headspace extraction (HSE) and solidphase microextraction (SPME) [9]. The most commonly used clean methods are SPME, SFE, and static and dynamic headspace extraction. The multivariate statistical techniques applied to chromatographic methods have been basically employed for the optimization of the sample preparation and sample analysis steps [10].
The chemometrics associated with SPME-GC has been applied in several studies with different food matrices, such as coffee [11] and wines [12]. Chemometric methods (hierarchical cluster analysis (HCA), principal components analysis (PCA), and others) are especially indicated for the analysis of chromatograms of complex matrices, involving hundreds of compounds [11].
The purpose of this work was thus firstly to apply multivariate chemometric techniques for optimizing a SPME-GC-MS method, in order to extract and identify the most abundant VOC in three varieties of mangos, purchased in the city of Salvador, State of Bahia, Brazil and, secondly, to verify the clustering tendency of those VOC, in order to identify the most important one for the flavor associated with each variety studied.
2. Methods and Materials
2.1. Samples
Fresh samples of three varieties of mango (Espada, Rosa and Tommy Atkins) were purchased at different commercial establishments in the city of Salvador. The samples were selected carefully, washed, peeled, and their pulp homogenized in a blender. About 0.15 g of Tommy Atkins, 0.6 g of Espada and 1.6 g of Rosa mango were then placed in a 20 ml vial to which 10 mL of deionized water was added for headspace static extraction. The vial was then closed hermetically with a Teflon lid and an aluminum seal.
2.2. SPME Conditions
The SPME extractions were performed using fiber coated with Carboxen/polydimethylsiloxane (75 µm) (Supelco, Bellefonte, PA, USA). This fiber was chosen due to the relative affinity between the compounds in the mango pulps and the fiber. The fibers were prepared according to the manufacturer’s instructions, which involved preconditioning them in the CG-MS system at 250˚C/20 min before each run. After this preconditioning, the SPME device was introduced manually into the vial and the fibers were exposed during the extraction time. The set was immersed on a double boiler with controlled temperature throughout the extraction and under constant magnetic shaking.
2.3. Optimization of the SPME Extraction
A fractional factorial design (24-1) was first carried out with 11 experiments, in which four variables were investigated (extraction (adsorption) and desorption temperature, ˚C; extraction (adsorption) and desorption time, min), applying three repetitions at the central point to evaluate the experimental error. The evaluated levels, as well as the experimental ranges, are shown in Table 1. Preliminary assays have demonstrated that extraction times and temperatures exceeding 5 min and 45˚C, respectively, led to a saturation of the detector signal, interrupting the run in the GC (data not shown). In the choice of the best condition, the response evaluated was the total sum of the peak areas, obtained in the GC-MS.
The factorial design made possible to select the significant variables (P < 0.05), from a Pareto chart, and point out their tendencies. However, in order to establish their optimum values, there is a need to perform a planning that could be capable to describe more adequately the data behavior. In this way, a Doelhert’s Experimental Matrix was applied (Table 2) and a response surface graph was obtained, in order to locate the optimal adsorption times and temperatures of the volatile compounds. This type of design is suitable for simultaneously evaluating the effect of several variables and allows for the introduction of quadratic terms in the model, which are fundamental for the description of response surface curves [13]. The not significant variables (desorption time and temperature) were kept constant.
The statistical analysis was performed using Statistica 6.0 software (StatSoft Inc., Tulsa, OK, USA).
2.4. GC-MS Analysis
The VOC were extracted from the headspace volume of

Table 1. Experimental levels applied to the factorial design (screening design).
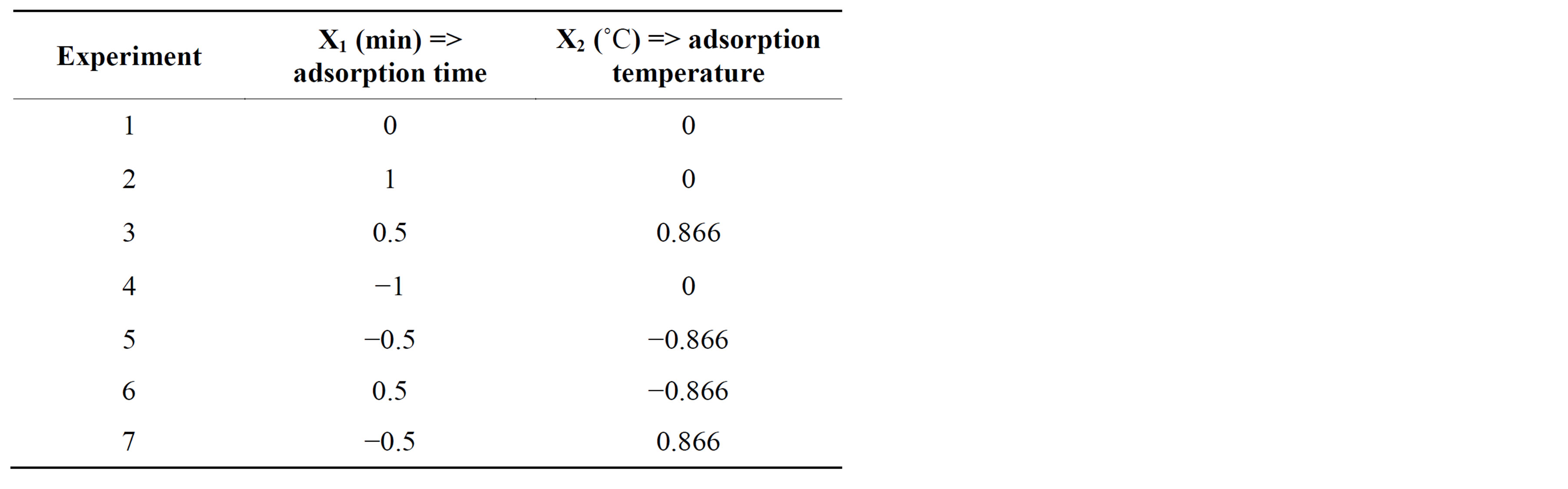
Table 2. Experimental levels employed for Doelhert matrix.
the samples and the trapped compounds were desorbed at 250˚C, for 5 min, in the GC injector and introduced directly into the GC column.
The volatile compounds were analyzed using a GCMS system (Shimadzu GC-2010/QP-2010, Japan) under the following operating conditions: DB5 column (30 m × 0.25 mm i.d. × 0.25 µm, J * W Scientific); helium gas flow: 109.2 mL∙min−1; temperature program: 50˚C - 100˚C (2˚C∙min−1) - 160˚C (6˚C∙min−1) and 280˚C (15˚C∙min−1); injector temperature and mode: 280˚C, splitless; split ratio: 1:00; ion source temperature: 230˚C; interface temperature: 280˚C and energy impact: 70 eV.
The compounds in each mango variety were identified by comparing their spectra with those available in the NIST digital library.
2.5. Principal Components Analysis
A set of 37 VOC were identified in the different mango varieties. Those with relative peak areas larger than 2% (23) were submitted to the PCA and HCA methods, in order to visualize possible clustering tendencies in the three mango varieties under study. The data were organized in X-type matrix containing 18 rows (6 replicates of each mango variety) and 23 columns (VOC). The data preprocessed by autoscaling was subjected to HCA, using the hierarchical agglomerative clustering procedure [14], while the PCA was calculated using the Statistica 7.0 and the Unscrambler® 8.0 software programs (Camo S.A.).
3. Results and Discussion
3.1. Optimization of the SMPE Conditions
The results obtained from the factorial design are summarized in the Pareto chart (Figure 1), which indicates that the extraction (adsorption) time and temperature were the most significant variables (p < 0.05).
Therefore, only these two variables were evaluated in the optimization process by means of the Response Surface Methodology (Doelhert matrix). In this design, three replicates were performed at the central point to estimate the experimental error and to evaluate the adjustment of the mathematical model employed. The response surface graph in Figure 2 shows that, as the adsorption time and temperature increase, so does the area of the chromatographic peaks. However, if the detector’s signal became very high, the chromatographic run was interrupted due to the detector saturation. Therefore, after this optimization the critical values of 4.0 min and 40˚C were employed, respectively, for the adsorption time and temperature, and were used in the analyses of all the mango samples.
The extraction temperature and time are interrelated and frequently investigated in optimization studies [12,

Figure 1. Pareto chart of the evaluated variables in the planned factorial design.

Figure 2. Response Surface graph obtained by the central composite design using encoded variables in which the response was the total chromatographic peak area.
15]. The extraction temperature has a dual impact on the analyses: although high temperatures allow for a rapid release of the analytes to the gas phase, they tend to reduce their partition coefficients between the fiber and the gas phase [16]. Relating to the extraction time, it needs to be long enough to enable phase equilibrium to be reached, without an excessive heating of the fiber [17].
3.2. Volatile Compounds Identified in the Mango Varieties
Amongst the thirty seven volatile compounds tentatively identified in the mango samples, Table 3 lists the twenty three whose chromatographic relative peak areas were larger than 2%, while Figure 3 shows the chromatographic profile of these compounds. The VOC identified

Figure 3. Chromatographic VOC profiles of Tommy-atkins (a), Rosa (b) and Espada (c) mangoes.
belong to different chemical classes of compounds, mainly esters and terpenes. Among them, α-pinene, β- myrcene and caryophyllene were common to the three mango varieties. The relative abundances of the VOC varied among samples of the three varieties. For example, ethyl butanoate was detected only in the Tommy Atkins mango, with an average relative abundance of 17.7%; α-pinene was detected with average relative abundances of 17.9%, 31.3% and 2.8% in the Tommy Atkins, Rosa and Espada varieties, respectively, and 3-carene had average relative abundances of 51% in the Tommy Atkins and 17% in the Espada mango varieties, but was not detected in the Rosa variety. These variations may be due, among other factors, not only to the differences amongst different varieties, but also to the different degrees of ripeness and peculiarities of different cultivation sites.
Some of the compounds listed in Table 3 had also been identified in the mango varieties Rosa (α-pinene, β- pinene and myrcene), Tommy Atkins (d-limonene) and Espada (α-pinene and myrcene) [18].
VOC present in Tommy Atkins mangoes, produced in the São Francisco Valley, were investigated at three different stages of ripeness, using SPME-GC-MS and GCFID [19]. The data obtained was in accordance with those of this work, since they identified 32 compounds, consisting mainly of monoterpenes, and found that 3- carene was the main VOC present in all the stages of ripeness, followed by α-pinene.
Other mango varieties were studied by different authors and also have shown VOC which are similar to those of this work. The monoterpenes (α-pinene, camphene, β-pinene, ocimene and d-limonene) were identified in mango juice through HS-SPME-GC-MS⁄FID [20]. The influence of changes in the geographical origin had been studied on the VOC profile of “Alfonso mangoes” in three regions of India [4]. Amongst other VOC, they found α-pinene, β-myrcene, d-limonene, o-cimene and caryophyillene. A total of twenty nine VOC were determined in “Nam Dok Mai” mangoes and, amongst them, four were in this work (o-cymene, carene, limonene and

Table 3. VOC tentatively identified in samples of the three mango varieties.
caryophyllene) [5].
3.3. Data Analysis
A multivariate analysis of the results was performed in order to visualize grouping tendencies between objects (samples) and possible dispersed variables which could distinguish the mangoes varieties through their aroma constituents, for each specific variety, or through the variations in concentrations of these constituents. In this way, the Hierarchical Cluster Analysis was used.
The resulting data are presented in the dendrogram in Figure 4, in which three clusters correspond to the mango varieties (Tommy Atkins, Espada and Rosa) at a distance level of 50. From the left, the MR, ME and MT correspond to the clusters of Rosa, Espada and Tommy Atkins samples, respectively.
Figures 5(a) and (b) show the scores and loading graphs, for samples and variables respectively. The first two principal components were able to explain 76.20% of the total variance, indicating that the studied volatile compounds could explain most of the characteristics of the samples.
The scores graph clearly shows the distinction among the groups of the mango varieties, i.e., the clustering tendency of these samples. Additionally, the variables described in the loading graph, which are located in the same quadrant as the groups of samples in the scores graph, comprise some of the most important information in the sample description. When scores and loadings graphs are analyzed together, the association between groups and the respective discriminator VOC becomes clear.
Table 4 shows clearly the information contained on the loading graph of Figure 5(b), namely the VOC which allow for the discrimination between the Rosa, Tommy Atkins and Espada mangoes. Amongst the 23 identified VOC (Table 3), only α-pinene, β-myrcene and caryophyllene were found in the three mango varieties. It was also observed that six VOC (2-carene, tujene, 3- carene, 4-carene, 2-ethyl-1,3-dimethylbenzene and terpinolene) were present in both Tommy Atkis and Espada varieties, one (α-caryophyllene) in Espada and Rosa and one (camphene) in Tommy Atkis and Rosa varieties. The other VOC were identified in just one of each variety, and thus they can be considered as possible flavor markers. According [3], esters, terpenes and aldehydes are very impactant on the fruit’s flavor.
The results presented by the PCA also indicate that there was a greater dispersion among the samples of Tommy mango, which is consistent with the dendrogram and can be explained by the higher quantity of compounds detected in smaller concentrations. The samples of the other varieties (Espada and Rosa) presented a higher clustering tendency.
Principal components analysis shows that volatile compounds provide enough information to develop a classification method for the studied mangoes, including their geographical origin. In addition, the agreement between HCA and PCA is consistent with this idea. Was observed a geographical differentiation in the VOC profile of Alphonso mangoes and stated this variation was reflected in the cluster separation in the PCA [4].
Was also studied the spatial and temporal changes in the VOC profile of Alphonso mango on effect of preclimacteric ethylene treatment using PCA and the study revealed accelerated ripening in terms of early appearance of ripening-specific compounds upon ethylene treatment and the increase in the terpene level during ripening appears to be independent of ethylene [6].
4. Conclusions
The results of this work highlight the importance of chemometrics, both for optimizing important parameters in the analysis of VOC in biological samples and in the analysis of obtained data. The use of statistical tools,
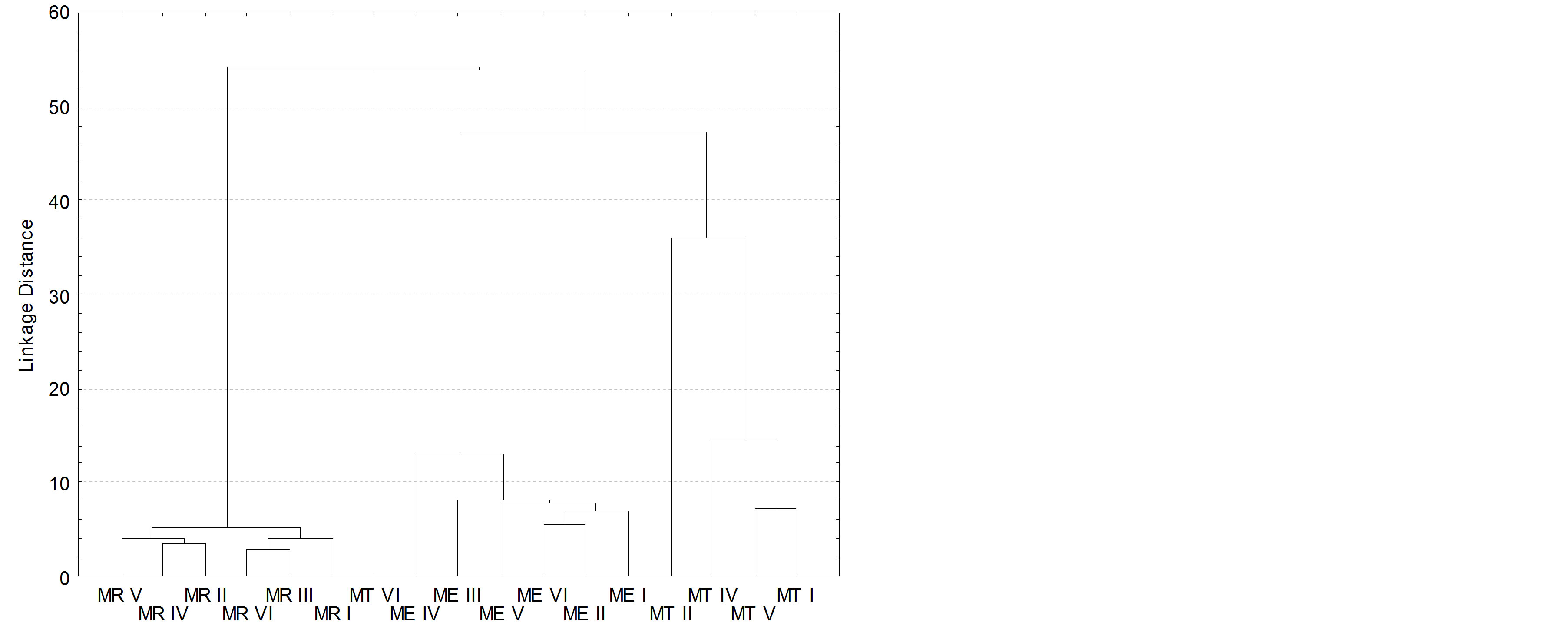
Figure 4. Dendrogram of the cluster analyses.
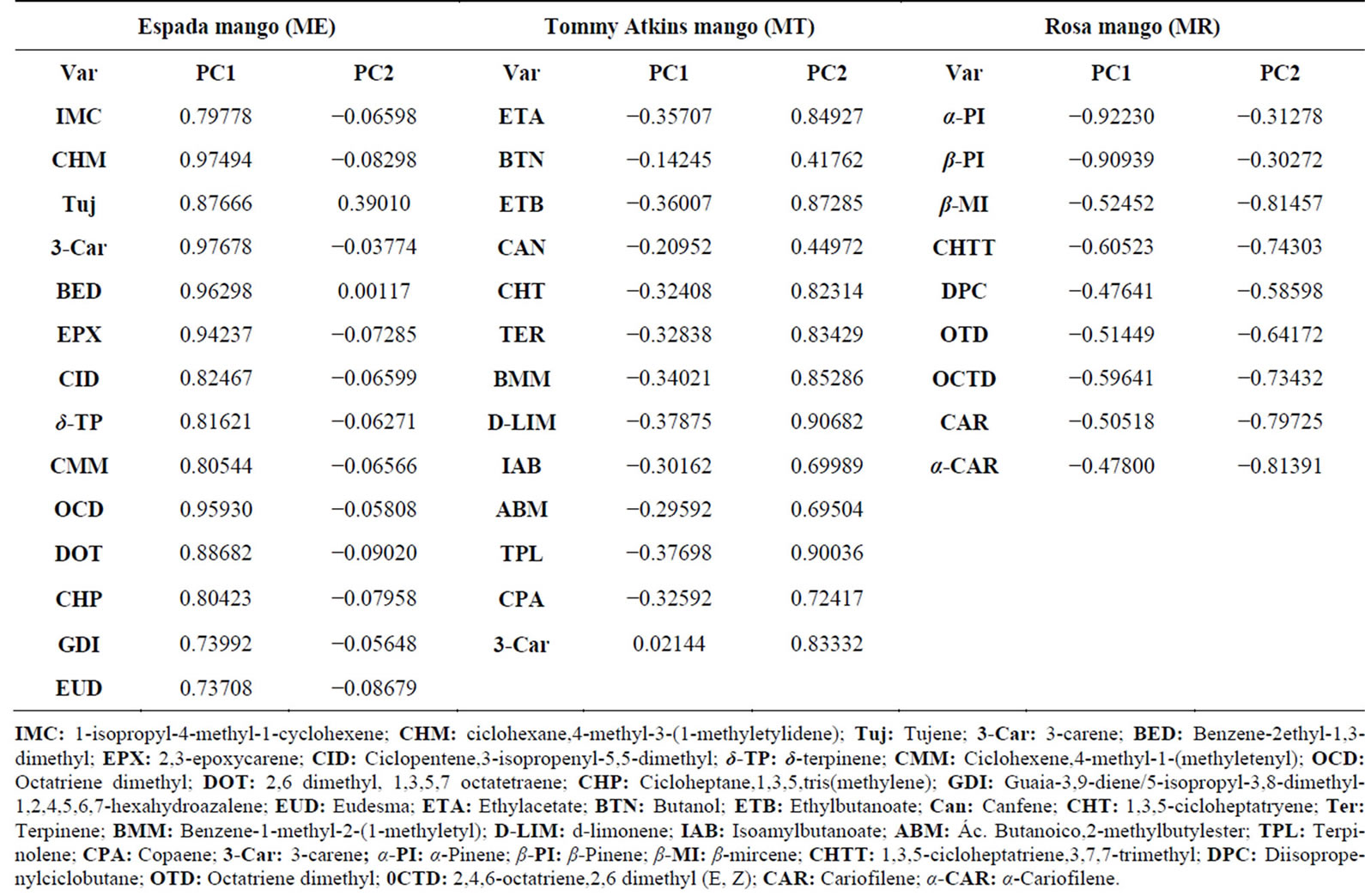
Table 4. Loading values of the variables for the two first PCs.
 (a)
(a)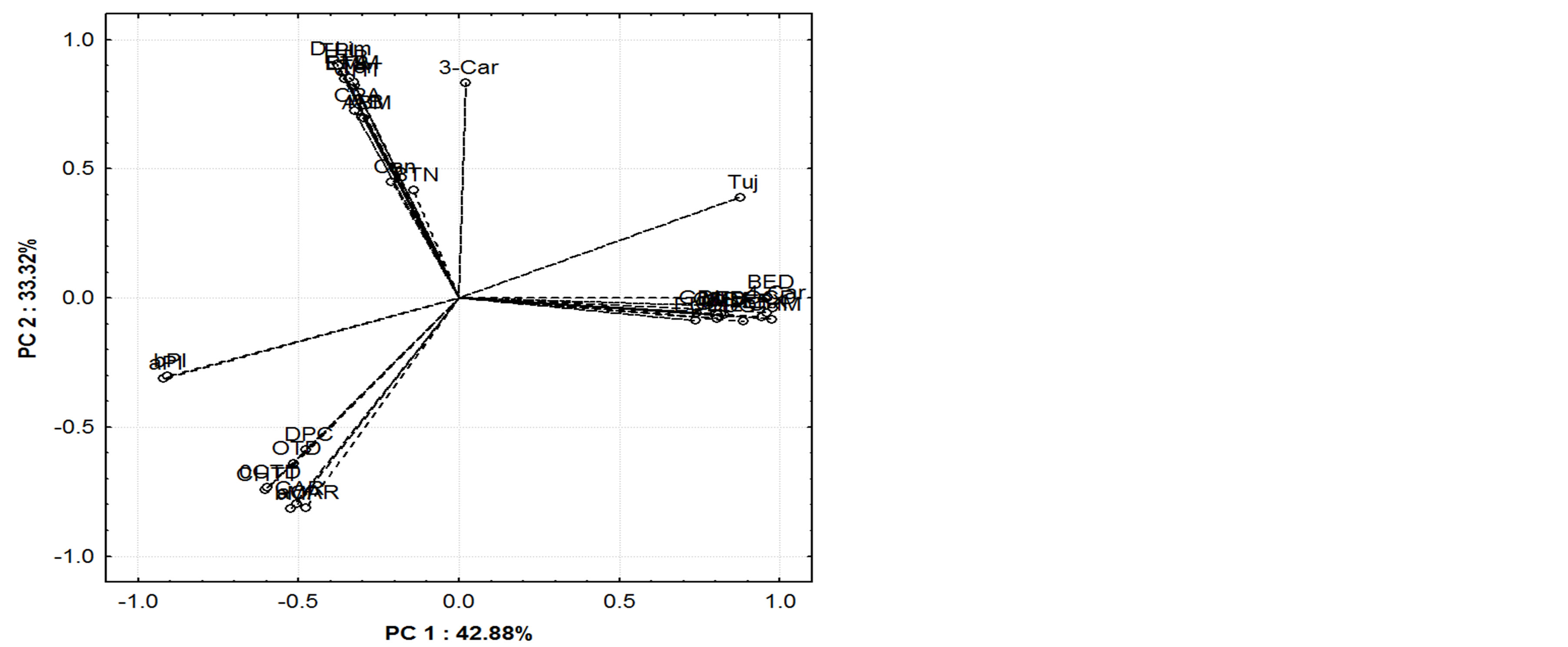 (b)
(b)
Figure 5. Dendrogram of the cluster analyses.
such as Factorial Plannig and Doehlet Matrix, has provided optimal conditions for using the SPME-CG/MS technique in the sampling and identification of 37 volatile substances in the three mango varieties.
The principal components analysis enabled the visualization of clustering tendencies of the mango samples as a function of the identified VOC, as well as the identification of groups of substances which were responsible for distinguishing among these three varieties.
REFERENCES
- FAOSTAT 2008, “FAO Statistics, Food and Agriculture Organization of the United Nations,” Rome, 2012. http://faostat.fao.org/
- S. V. Galán, “Worldwide Mango Production and Market. Current Situation and Future Prospects,” International Mango Symposiu on International Society for Horticultural Science, Sanya, 2010, pp. 9-12.
- C. Cheng, A. Seal, E. MacRae and M. Wang, “Identifying Volatile Compounds Associated with Sensory and Fruit Attributes in Diploid Actinidia chinensis (kiwifruit) Using Multivariate Analysis,” Euphytica, Vol. 181, No. 2, 2011, pp. 179-195. http://www.ingentaconnect.com/content/klu/euph/2011/00000181/00000002/00000392 http://dx.doi.org/10.1007/s10681-011-0392-3
- R. S. Kulkarni, H. G. Chidley, H. Keshav, K. H. Pujari, A. P. Giri and V. S. Gupta, “Geographic Variation in the Flavour Volatiles of Alphonso mango,” Food Chemistry, Vol. 130, No. 1, 2012, pp. 58-66. http://www.sciencedirect.com/science/article/pii/S030881461100940X http://dx.doi.org/10.1016/j.foodchem.2011.06.053
- N. Laohaprasit, D. S. Ambadipudi and G. Srzednicki, “Optimisation of Extraction Conditions of Volatile Compounds in ‘Nam Dok Mai’ Mangoes,” International Food Research Journal, Vol. 18, No. 3, 2011, pp. 1043-1049. http://www.ifrj.upm.edu.my/18%20(03)%202011/(27)IFRJ-2010-291.pdf
- H. G. Chidley, R. S. Kulkarni, K. H. Pujari, A. P. Giri and V. S. Gupta, “Spatial and Temporal Changes in the Volatile Profile of Alphonso mango upon Exogenous Ethylene Treatment,” Food Chemistry, Vol. 136, No. 2, 2013, pp. 585-594. http://www.sciencedirect.com/science/article/pii/S0308814612013131 http://dx.doi.org/10.1016/j.foodchem.2012.08.029
- E. A. Malo, I. Gallegos-Torres, J. Toledo, J. Valle-Mora and J. C. Rojas, “Attraction of the West Indian Fruit Fly to Mango Fruit Volatiles,” Entomologia Experimentalis et Applicata, Vol. 142, No. 1, 2011, pp. 45-52.
- J. A. Pino, J. Mesa, Y. Muñoz, M. P. Martí and R. Marbot, “Volatile Components from Mango (Mangifera indica L.) Cultivars,” Journal Agricultural Food Chemistry, Vol. 53, No. 6, 2005, pp. 2213-2223. http://pubs.acs.org/doi/abs/10.1021/jf0402633 http://dx.doi.org/10.1021/jf0402633
- E. T. Sousa, F. M. Rodrigues, C. C. Martins, F. S. Oliveira, P. A. P. Pereira and J. B. Andrade, “Multivariate Optimization and HS-SPME/GC-MS Analysis of VOCs in Red, Yellow and Purple Varieties of Capsicum chinense sp. Peppers,” Microchemical Journal, Vol. 82, No. 2, 2006, pp. 142-149. http://www.sciencedirect.com/science/article/pii/S0026265X06000117 http://dx.doi.org/10.1016/j.microc.2006.01.017
- S. L. C. Ferreira, R. E. Bruns, E. G. P. Silva, W. N. L. Santos, C. M. Quintella, J. M. David, J. B. Andrade, M. C. Breitkreitz, I. C. S. F. Jardim and B. B. Neto, “Statistical Designs and Response Surface Techniques for the Optimization of Chromatographic Systems,” Journal of Chromatography A, Vol. 1158, No. 1, 2007, pp. 2-14. http://www.sciencedirect.com/science/article/pii/S0021967307005298 http://dx.doi.org/10.1016/j.chroma.2007.03.051
- J. S. Ribeiro, F. Augusto, M. M. C. Ferreira and T. J. G. Salva, “Uso de Perfis Cromatográficos de Voláteis de Cafés Arábicas Torrados para a Diferenciação das Amostras Segundo o Sabor, o Aroma e a Qualidade Global da Bebida,” Química Nova, Vol. 33, No. 9, 2010, pp. 1897- 1904. http://quimicanova.sbq.org.br/qn/qnol/2010/vol33n9/14-AR10113.pdf http://dx.doi.org/10.1590/S0100-40422010000900015
- F. Pellati, S. Benvenuti, F. Yoshizaki, D. Bertelli and M. C. C. Rossi, “Headspace Solid-Phase MicroextractionGas Chromatography-Mass Spectrometry Analysis of the Volatile Compounds of Evodia species Fruits,” Journal of Chromatography A, Vol. 1087, No. 1-2, 2005, pp. 265- 273. http://www.sciencedirect.com/science/article/pii/S0021967305001305 http://dx.doi.org/10.1016/j.chroma.2005.01.060
- M. Zeaiter, J. M. Roger, V. Bellon-Maurel and D. N. Rutledge, “Robustness of Models Developed by Multivariate Calibration Part I: The Assessment of Robustness,” Trends in Analytical Chemistry, Vol. 23, No. 2, 2004, pp. 157-170. http://www.sciencedirect.com/science/article/pii/S0165993604003073 http://dx.doi.org/10.1016/S0165-9936(04)00307-3
- K. R. Beeb, R. J. Pell and M. B. Seasholtz, “Chemometrics: A Practical Guide,” John Wiley & Sons, New York, 1998.
- P. Díaz, F. J. Señoráns, G. Reglero and E. Ibañez, “Truffle Aroma Analysis by Headspace Solid Phase Microextraction,” Journal Agricultural Food Chemistry, Vol. 50, No. 22, 2002, pp. 6468-6472. http://pubs.acs.org/doi/abs/10.1021/jf025609t http://dx.doi.org/10.1021/jf025609t
- H. Prosen and L. Zupančič-Kralj, “Solid-Phase Microextraction,” Trends in Analytical Chemistry, Vol. 18, No. 4, 1999, pp. 272-282. http://www.sciencedirect.com/science/article/pii/S0165993698001095 http://dx.doi.org/10.1016/S0165-9936(98)00109-5
- S. Ulrich, “Solid-Phase Microextraction in Biomedical Analysis,” Journal of Chromatography A, Vol. 902, No. 1, 2000, pp. 167-194. http://www.sciencedirect.com/science/article/pii/S0021967300009341 http://dx.doi.org/10.1016/S0021-9673(00)00934-1
- E. H. A. Andrade, J. G. S. Maia and M. G. B. Zoghbi, “Aroma Volatile Constituents of Brazilian Varieties of Mango Fruit,” Journal of Food Composition and Analysis, Vol. 13, No. 1, 2000, pp. 27-33. http://www.sciencedirect.com/science/article/pii/S0889157599908414 http://dx.doi.org/10.1006/jfca.1999.0841
- K. M. Canuto, M. A. S. Neto and D. S. Garruti, “Composição Química Volátil, em Diferentes Estádios de Maturação, de Manga ‘Tommy Atkins’ Produzida no vale do São Francisco,” Quimica Nova, Vol. 32, No. 9, 2009, pp. 2377-2381. http://quimicanova.sbq.org.br/qn/qnol/2009/vol32n9/26-AR09028.pdf http://dx.doi.org/10.1590/S0100-40422009000900027
- X. Li, B. Yu, P. Curran and S. Q. Liu, “Chemical and Volatile Composition of Mango Wines Fermented with Different Saccharomyces cerevisiae Yeast Strains,” South African Journal for Enology and Viticulture, Vol. 32, No. 1, 2011, pp. 117-128. http://www.sawislibrary.co.za/dbtextimages/72569.pdf

