American Journal of Analytical Chemistry
Vol. 4 No. 2 (2013) , Article ID: 27798 , 6 pages DOI:10.4236/ajac.2013.42010
Intraspecific Chemical Variability of Essential Oil of Hoslundia opposita
1Laboratoire de Chimie Organique Biologique, UFR-SSMT, Université de Cocody, Abidjan, Côte d’Ivoire
2Laboratoire de Chimie des Hétérocycles et Glucides, Université Blaise Pascale de Clermont Ferrand, Campus des Cézeaux, Aubière, France
Email: *tonzibz@yahoo.fr
Received October 26, 2012; revised November 27, 2012; accepted December 4, 2012
Keywords: Hoslundia Opposita; Lamiaceae; Essential Oil Composition; Benzyl Benzoate; Phytol
ABSTRACT
Chemical investigation on leaves oil of Hoslundia opposita, aromatic plant used in traditional medicine, harvested from 20 localities of Côte d’Ivoire was realized by GC and GC/MS. In total, 101 compounds were identified accounting to 92% - 99%, aromatic compounds such benzyl benzoate and sesquiterpenes namely germacrene D, β-caryophyllene and α-humulene hydroxyl were found as the major constituents. As regard, the chemical profiles of our samples, it was found a great chemical variability suggesting a high polymorphism degree. In order to study this chemical variability, the results were submitted to the principal components and cluster analysis which allowed distinguishing two main groups of essential oils with respect to sampling site. The cluster I was dominated by benzyl benzoate (15%) and thymol (7.4%) comprising 6 localities, cluster II was characterized by phytol (8.5%) and α-humulene (7%) and constituted by 14 localities.
1. Introduction
Hoslundia opposita Vahl is a herbaceous shrub which is widely distributed in different parts of Africa [1]. The whole plant or various parts of it are used in traditional medicine as a purgative, diuretic, febrifuge, antiepileptic and antiseptic [2-5]. The traditional uses of this species led to several biological investigations, indicating numerous properties of extracts from different parts of Hoslundia opposita namely antimicrobial, analgesic, antiinflammatory activities, antimalarial, antidiabetic and acaricidal activity [6-10].
From a chemical point of view, several published papers reported the isolation of non-volatile compounds consisting of 5,7-dimethyl-6-methylflavone, hoslundiol and four abietal type esters [11] added to the pyrone and flavones were isolated more recently from the leaves by R. Salam [12]. While dealing with the volatile compounds, the literature revealed three types of essential oils from Hoslundia opposita. The first chemotypes consisted to sesquiterpene hydrocarbons and rarely diterpene namely germacrene D, β-caryophyllene, α-copaene and phytol [13-16]. When, the second chemotypes were known to be dominated by the aromatic constituents such as benzyl benzoate, thymol and eugenol [16,17]. The last oil types shown monoterpene oxygenated as major components comprising 1,8 cineole and camphor [18].
However, this study reports for the first time, the chemical investigations on the leaf oils from Hoslundia opposita growing in Côte d’Ivoire concerning twenty localities. In the front of the chemical variability noted in the samples, cluster analysis added to PCA was used to confirm clearly the relationship between the samples. However, it is well-known that the statistical methods applied to the chemical data have provided the results which allow the best interpretations. In occurrence, the cluster analysis which consists to group the samples in respect to their chemical similitudes in order to led to the formation of the chemical groups of oils.
2. Materials and Methods
2.1. Plant Material
2.1.1. Extraction of Essential Oil
Each sample (500 g by fresh leaves) of Hoslundia opposita was collected between May and July 2001 and was submitted to hydrodistillation for 3 h, using a Clevenger-type apparatus. The oils obtained by decantation were dried over anhydrous sodium sulfate, and then stored in sealed vials protected from the light at −20˚C before GLC analysis.
2.1.2. Analysis of the Essential Oils
GC: The essential oils were analysed on a AGILENT gas chromatograph Model 6890, equipped with a DB5 MS column (30 m × 0.25 mm; 0.25 µm), programming from 50˚C (5 min) to 300˚C at 5˚C/min, 5 min hold. Hydrogen as carrier gas (1.0 ml/min); injection in split mode (1:60); injector and detector temperature were 280˚C and 300˚C respectively. The essential oil was diluted in hexane: 1/3.
GC/MS: The essential oils were analysed on a AGILENT gas chromatograph Model 7890, coupled to a AGILENT MS model 5975, equipped with a DB5 MS column (20 m × 0.20 mm, 0.20 µm), programming from 50˚C (5 min) to 300˚C at 8˚C/min, 5 min hold. Helium as carrier gas (1.0 ml/min); injection in split mode (1:250); injector and detector temperature were 250˚C and 280˚C respectively. The MS was mode electron impact at 70 eV; electron multiplier, 1500 V; ion source temperature, 230˚C; mass spectra data were acquired in the scan mode in m/z range 33 - 450.
2.1.3. Identification of Compounds
Compounds were identified by the computer search using their mass spectra either with the known components published spectra [19] and by comparison of their retention indices with those of known compounds [20].
2.2. Statistical Analysis
Principal component analysis (PCA) was applied to examine the interrelationships between populations and its chemical constituents using XLSTAT 7.5.3. Cluster analysis was also applied to the study of similarity of samples on the basis of constituent distribution.
3. Results and Discussion
The chemical composition of the oils obtained from the leaves of Hoslundia opposita collected on twenty localities of Côte d’Ivoire shown the predominance of sesquiterpe hydrocarbons with the amounts varing between 25% to 90% within 101 constituents identified acconting for 91% - 98%. The characreristic components of the oils are represented in Table 1, arranged in order of elution. The main group of constituents in most of the samples were composed by sesquiterpe hydrocarbons namely β-caryophyllene (9.5% - 40.9%), germacrene D (8.6% - 31.1%) and α-humulene (1.2% - 18.3%) in combination
Table 1. Main components of chemical composition on leaves oils of Hoslundia opposita.
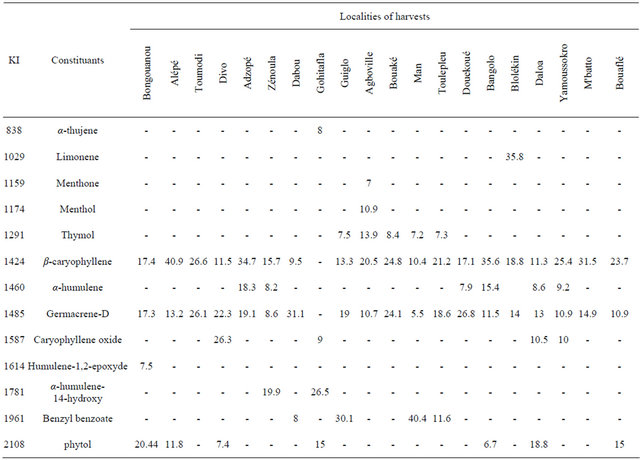
Figure 1. Main components of chemical composition oil of Hoslundia opposita.
according to the localities of harvests, with aromatic components as benzyl benzoate and thymol added to oxygenated sesquiterpenes namely caryophyl lene oxide, humulene-1,2-epoxy and α-humulene-14-hydroxy (Figure 1). The variability on chemical composition of theleaves oils from Hoslundia opposita shown clearly a chemical polymorphisme probably caused by exogenous factors like altitude, humidity, soils, etc. This fact could prevent a best description of the samples. Therefore, The results obtained from the principal component analysis (PCA) added to cluster analysis permitted to show interrelationships between these twenty samples. The PCA revealed the formation of two groups (Figure 2), in addition, the cluster analysis results in agreement with the PCA results (Figure 3) shown two clusters. Therefore, two types of volatile oils were found: cluster I (Man, Toulepleu, Guiglo, Bouaké, Dabou, Agboville) exhibiting the aromatic components predominance as shows the histogram resulting from chemical profiles of the samples which were grouped by the two analysis operations cited, benzyl benzoate (15%; sd = 15) (p < 0.001) and thymol (7.4%; sd = 4) (p < 0.001) (Figure 4) characterized this group. However, these findings are in agreement with our own studies [16], which revealed for the first time the predominance of aromatic components in the leaves oil of Hoslundia opposita. These results confirmed clearly the presence of a new chemotype in this species harvested in Côte d’Ivoire. The cluster II (Zé- noula, Toumodi, Douekoué, Adzopé, Bangolo, Alépé, M’batto, Blolékin, Yamoussokro, Gohitafla, Bongouanou, Dabou, Divo, Bouaflé) was characterized by phytol (8.5%; sd = 6.2%) (p < 0.001) and α-humulene (7%; sd = 4.7%) (p < 0.001) (Figure 5). It is noteworthy that the oil of Hoslundia opposita is known to be rich in sesquiterpene compounds, as regard to the earlier works. To date, the phytol type was described only by Onayade et al. [13] in the oil from Nigeria. To our knowledge, the combination of phytol and α-humulene, as principal components of volatile oils of this species, has never been published.
4. Conclusion
This first study, dealing with the chemical investigation expanded to twenty localities, on essential oil of Hoslundia opposita growing in Côte d’Ivoire revealed a high chemical polymorphism. However, the PCA and Cluster analysis have shown two different chemotypes. Furthermore, the richness of sesquiterpenes in the oil of this species has been noted. On the other hand, the aromatic components were found as chemical characteristic of the leaves oil of Hoslundia opposita growing in some localities of Côte d’Ivoire. The chemical variability found in our samples seemed to be linked to exogenous factors.
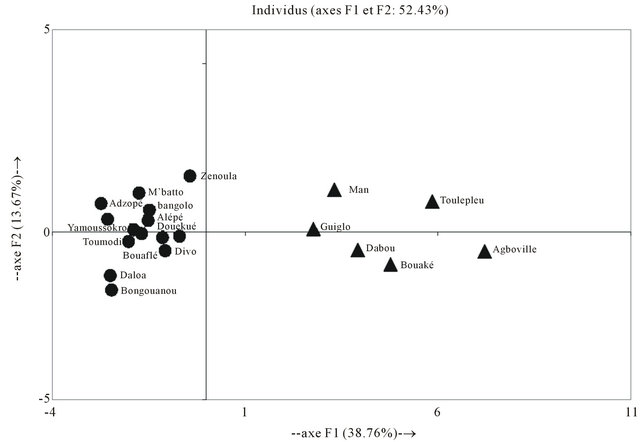
Figure 2. Principal component analysis of leaves oils of Hoslundia opposita.

Figure 3. Two-dimensional dendrogram representing chemical composition similarity relationships among twenty samples of H. opposita from Côte d’Ivoire.
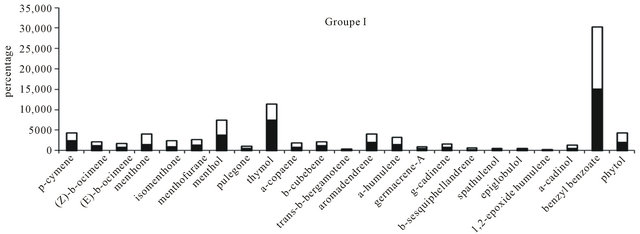
Figure 4. The mean chemical composition of leaves oil of cluster I of H. opposita.

Figure 5. The mean chemical composition of leaves oil of cluster II of H. opposita.
However, complementary investigations taking into account the geographic factors in the selected localities would confirm clearly the relationship between the samples and their correlation with the main constituents.
REFERENCES
- J. K. Morton, “Flora of West Tropical Africa,” J. Hutchinson and J. M. Daziel, Eds., Floras & Botanical Field Guides, Crown Agents, London, 1963, p. 450.
- M. M. Iwu, “Handbook of African Medicinal, Plants,” CRP Press, Boca Raton, 1993.
- J. M. Watt and M. G. Breyer-Brandwijk, “Medicinal and Poisonous Plants of Southern and Eastern Africa,” E & S Livingstone, Edinburg, 1962.
- E. S. Ayensu, “Medicinal Plant of West Africa,” Reference Publications, Algonac, 1978.
- A. Bouquet, “Féticheurs et de Médecine Traditionnelle du Congo Brazzaville,” Mémoire O.R.S.T.O.M. No.36, Paris, 1969, p. 142.
- K. Annan, N. Jackson, R. A. Dickson, G. H. Sam and G. Komlaga, “Acaricidal Effect of an Isolate from Hoslundia opposite vahl against Amblyomma variegatum (Acari: Ixodidae),” Pharmacognosy Research, Vol. 3, No. 3, 2011, pp 185-188. doi:10.4103/0974-8490.85004
- G. M. Gundidza, S. G. Deans, K. P. Svoboda and S. Mavi, “Antimicrobial Activity of Essential Oil from Hoslundia opposite,” Central African Journal of Medecine, Vol. 38, No. 7, 1992, pp. 290-300.
- J. O. Akolade, N. O. Muhammad, L. Usman, T. A. Owolarafe and O. B. Oloyede, “Antidyslipidemic Effect of Leaf Essential Oil of Hoslundia Oppositavahl. In AlloxanInduced Diabetic Rats,” International Journal of Tropical Medicine and Public Health, Vol. 1, No. 1, 2011, pp. 6-13.
- H. Anchenbach, R. Waibel, H. H. M. Nkunya and H. Weenen,” Antimalarial Compounds from Hoslundia opposita,” Phytochemistry, Vol. 31, No. 11, 1992, pp. 3781- 3784. doi:10.1016/S0031-9422(00)97526-5
- O. A. Olajide, S. O. Awe and J. M. Makinde, “Central Nervous System Depressant Effect of Hoslundia opposita Vahl,” Phytotherapy Research, Vol. 13, No. 5, 1999, pp. 425-426.
- B. T. Ngadjui, F. J. Ayafor, B. L. Sondengam, J. D. Connolly and D. S. Rycroft, “Hoslundin, Hoslundal, and Hoslunddiol: Three New Flavonoids from the Twigs of Hoslundia opposite (Lamiaceae),” Tetrahedron, Vol. 47, No. 22, 1991, pp. 3555-3564. doi:10.1016/S0040-4020(01)80869-3
- R. Salam, Z. Cheikh-Ali, C. Bories, M. Adiko M, E. Poupon and P. Champy, “Pyrone and Unusually Furanone-Substituted Flavones from the Leaves of Hoslundia opposita,” Planta Medica, Vol. 78, No. 16, 2012, pp. 1777-1779.
- O. A. Onayade, L. Ntezurubanza, J. J. C. Scheffer and A. S. Borerheim, “Composition of the Essential Oil of Hoslundia opposita Collected in Nigeria and Rwanda,” Planta Medica, Vol. 55, No. 7, 1989, pp. 634-635. doi:10.1055/s-2006-962206
- M. A. Ayeodun, B. S. Adeoti, J., Setondji, F. Tchoumbougnang, J. R. Kuiate, P. H. A. Zollo, C. Menut, G. Lamaty and J. M. Bessiere, “Aromatic Plants of Tropical West Africa. XII, Essential Oils of Hoslundia opposita Vahl Growing in Benin and Cameroon,” Flavour and Fragrance Journal, Vol. 22, No. 12, 1999, pp. 1047- 1054.
- P. H. A. Zollo, L. Biyiti, F. Tchoumbougnang, C. Menut, G. Lamaty and Ph. Bouchet, “Aromatic Plants of Tropical Central Africa. Part XXXII. Chemical Composition and Antifungal Activity of Thirteen Essential Oils from Aromatic Plants of Cameroon,” Flavour and Fragrance Journal, Vol. 13, No. 2, 1998, pp. 107-114. doi:10.1002/(SICI)1099-1026(199803/04)13:2<107::AID-FFJ701>3.0.CO;2-G
- Z. F. Tonzibo, A. Ahibo Coffy, J. C. Chalachat and Y. T. N’guessan, “Chemical Composition of Essential Oils of Hoslundia opposita Vahl. from Ivory Coast,” Flavour and Fragrance Journal, Vol. 21, No. 5, 2006, pp. 789-791. doi:10.1002/ffj.1715
- L. S. Chagonda and J. C. Chalchat, “The Essential Oil of Wild and Cultivated Hoslundia opposita Vahl. from Zimbabwe,” Flavour and Fragrance Journal, Vol. 20, No. 2, 2005, pp. 193-195 doi:10.1002/ffj.1402
- L. A. Usman, M. F. Zubair, S. A. Adebayo, I. A. Oladosu, N. O. Muhammad and J. O. Akolade, “Chemical Composition of Leaf and Fruit Essential Oils of Hoslundia opposita Vahl Grown in Nigeria,” American-Eurasian Journal of Agricultural & Environmental Science, Vol. 8, No. 1, 2010, pp. 40-43.
- R. P. Adams, “Identification of Essential Oils by Ion Trap Mass Spectroscopy,” Academic Press, Inc., New York, 1989.
- A. A. Swigar and R. M. Silverstein, “Monoterpenes,” Aldrich Chemical Company, Inc., Milwaukee, 1981.
NOTES
*Corresponding author.

