International Journal of Clinical Medicine
Vol.4 No.3(2013), Article ID:28691,4 pages DOI:10.4236/ijcm.2013.43024
Role of Placenta Parameters in Predicting Significant Feto-Maternal Haemorrhage*
![]()
1Department of Obstetrics & Gynaecology, Ladoke Akintola University of Technology, Ogbomoso, Nigeria; 2Department of Haematology, Ladoke Akintola University of Technology Teaching Hospital, Osogbo, Nigeria.
Email: #tunji1802@yahoo.com, #aoadeniji@lautech.edu.ng
Received January 29th, 2013; revised March 5th, 2013; accepted March 13th, 2013
Keywords: Feto-Maternal Haemorrhage; Transplacental Haemorrhage; Placental Parameters; Rh D Factor; Kleihauer-Betke Test
ABSTRACT
Purpose: Feto-maternal haemorrhage (FMH) is a complication of pregnancy and large FMH may lead to life-threatening anaemia in the fetus or newborn. In addition, exposure of Rhesus (Rh) D negative women to small amounts of fetal Rh D positive red cells during pregnancy or delivery may result in sensitization with its attendant problems of isoimmunisation. In most cases, the cause of FMH IS unknown. Through this study, we sought to determine if placental weight & diameter have any direct relationship with incidence and severity of FMH. Methods: This was a prospective study of parturients for presence of fetal red cells in the maternal blood circulation. The prepared slide was processed as in the acid elution test described by Kleihauer-Betke. The FMH was calculated using Mollison formula. Baseline data included maternal biodata, blood group, Rh D factor, placenta weight and diameter. Data generated were analysed with Frequency tables, cross-tabulations and Odd ratio and confidence intervals as appropriate. Results: Three hundred parturients were studied. However, only two hundred and ninety-five parturients were analysed, with five excluded due to lysed blood samples. A total of 52 parturients (17.63%) had demonstrable FMH, of which 8 (2.71%) were large FMH (>15 ml foetal cells). Both the placenta weight (P < 0.005) and diameter (P < 0.042) were significantly associated with incidence of FMH, more with placenta weight than diameter. Incidence of demonstrable FMH was 24.12% (48/199) in the group with placenta weight greater than 500 g, in contrast to 4.17% (4/96) in the group with weight of placenta below or equal to 500 g. All the 8 parturients with large FMH had placenta weights greater than 500 g. Placenta diameters were greater than 22 cm in 41/197 (20.81%) who had demonstrable FMH, compared with 11/98 (11.23%) whose diameter was less than 22 cm. Conclusion: Both the placenta weight and diameter are significant predictors of FMH in parturients. However, placenta diameter appears to be a minor predictor. These are factors that can be assessed antenatally by ultrasonography and in conjunction with other known obstetric factors, may possibly be considered in risk-based scoring system for predicting feto-maternal haemorrhage.
1. Introduction
Feto-maternal haemorrhage (FMH) remains Obstetricians concern, because it is a complication of pregnancy. Substantial FMH may lead to life-threatening anaemia in the fetus or newborn. In addition, exposure of Rhesus (Rh) D negative women to small amounts of fetal Rh D positive red cells during pregnancy or delivery may result in sensitization with its attendant problems of isoimmunisation. The fetal circulation is separated from the maternal circulation by the placental barrier allowing exchange of metabolic and gaseous products. Transplacental passage of fetal cells into maternal blood is a common phenomenon in pregnancy and delivery. Cells from fetal origin were first recognized in 1893 by Schmorl [1], but in most cases, the cause of FMH IS unknown [2]. Obstetric risk factors (caesarean section, multiple pregnancy, placenta praevia, vasa praevia, miscarriage, therapeutic termination of pregnancy and manual placental extraction), in-utero therapeutic interventions procedures (external cephalic version, transfusion, surgery, amniocentesis and cordocentesis) and trauma to abdomen/placenta (motor vehicle accident, and placenta abruption) [3,4] have majorly been implicated as possible associated factors. Some studies have suggested that placental morphology and parameters can provide valuable information on pregnancy outcome and as predictors of future health of the mother and neonate [5,6].
While large volumes of studies on obstetric factors in FMH abound in the literature. It is remarkable that there is great paucity of research on possible correlation of placental parameters with FMH, especially in low-resource countries like Nigeria. Through this study, we sought to determine if placental weight & diameter have any direct relationship with incidence and severity of FMH.
2. Methods
This was a prospective study of parturients at Ladoke Akintola University of Technology Teaching Hospital, Osogbo, Nigeria. The study was over a 3-year period (2008-2010) and was approved by the Institutional Ethical Review Committee. The exclusion criteria were haemoglobinopathy, diabetes mellitus, multiple pregnancy, intrauterine growth restriction and patient’s refusal of consent to participate in the study. Sample size was determined using Fischer’s formula, corrected for population less than 10,000 and FMH incidence was set at 10.43% [7]. Placentae of all consenting parturients were collected at delivery. Excess fluid, mucous and maternal blood was wiped off, attached membranes and blood coagula trimmed and the umbilical cord ligated at approximately 5 mm from the insertion point. The processed placental was weighed using an electronic balance and the diameter determined on a graduated board. Limits of average Placenta weight was set at 500 gram and diameter at 22 centimetres [8].
FMH was determined according to the method of Kleihauer-Betke’s [9]. Two ml of maternal blood was obtained into EDTA bottle within 2 hours of the postpartum period. The maternal whole blood sample was diluted 1:2 with normal saline. The diluted sample was mixed well and a standard blood smear was prepared. Each slide was spread evenly and examined under the microscope to ensure that the red cells were touching, but not overlapping each other. An acid bath was then used, which would remove all adult haemoglobin, but not the fetal haemoglobin. Subsequent staining made fetal cells (containing fetal haemoglobin) rose pink while adult’s cells were only seen as “ghosts”. A minimum of 25 fields was examined using × 10 objective. Slides giving positive screening results were examined further to estimate the number of fetal cells present. A large number of cells (≥5000) were counted under the microscope and a ratio offetal to maternal cells generated. The FMH was calculated using Mollison formula [10]. This assumed that the maternal red cell volume is 1800 ml, fetal cells are 22% larger than the maternal cells and only 92% of fetal cells stain darkly. The fetal bleed was calculated thus:
Uncorrected volume of bleed = 1800 × fetal cells counted (F)/Adults cells counted (A)
Corrected for fetal volume (1.220 = (1800 × F/A) × 1.22 = J Corrected for staining efficiency = J × 1.09 = Fetal bleed.
On each day of our testing, blood from a newborn and blood from an adult male (without any haemoglobinopathy) were used and inspected on the same slide and served as positive and negative controls respectively. Baseline data included maternal biodata, blood group, Rh D factor, placenta weight and diameter. Data generated were analysed with the Statistical Package for Social Scientists (SPSS) version 17 software. Frequency tables, cross-tabulations and Odd ratio, with confidence intervals were determined. Level of statistical significance was set at P < 0.05.
3. Results
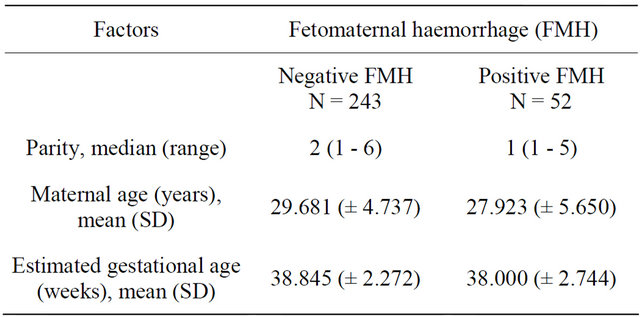
Three hundred parturients were studied. However, only two hundred and ninety-five parturients were analysed, with five excluded due to lysed blood samples. A total of 52 parturients (17.63%) had demonstrable FMH, of which 8 (2.71%) were large FMH (>15 ml foetal cells). The median parity of the group with FMH and without FMH was 2.000 (1 - 6) and 1.000 (1 - 5) respectively, while the mean of the maternal age of the two groups were 29.681 (±4.737) and 27.923 (±5.650); and estimated gestational age 38.845 (±2.272) and 38.000 (±2.744) respectively (Table 1). Sixteen of the parturients with positive FMH were Rhesus negative (Table 2).
Both the placenta weight (P < 0.005) and diameter (P < 0.042) were significantly associated with incidence of FMH. When placenta weight was categorised, 48/199 (24.12%) in the group with above average (>500 g) placenta weight had demonstrable FMH, in contrast to 4/96 (4.17%) in the group whose placenta weight was less or equal to 500 g. All the 8 parturients with large FMH had placenta weights greater than 500 g. Similar, but lesser pattern of association was observed among parturients
Table 1. Socio-demographic factors of study population.
whose placenta diameters were greater than 22 cm, among whom 41/197 (20.81%) had demonstrable FMH, compared with 11/98 (11.23%) whose diameter was less than 22 cm (Table 3).
4. Discussion
The incidence of FMH recorded in this study was 17.63%, while large FMH was 2.71%. These two figures are at variance with earlier findings from our centre [7], but conform to observation of increasing prevalence in any centre focussing research on any clinical condition, because of more vigilance with diagnosis.
From our results, both the placental weight and diameter appeared to be predictors of FMH. Our literature search did not yield any result on placental parameters as determinants of FMH. However, it is known that large placentae are associated with some pregnancy conditions such as multifetal gestations, diabetes mellitus in pregnancy, erythroblastosis fetalis amongst others and earlier studies had shown multifetal gestation to be a determinant of FMH [7,11], though, reports in literatures are conflicting on the contributions of multifetal gestations as determinants of FMH [12]. Findings from this study, however, possibly further reinforce the confounding interplay of mutifetal gestation and placental parameters-weight and diameter, as determinants of FMH.
Placental weight greater than 500 gramme was significantly associated with incidence of FMH, with the odds of seven times more than weight less than or equal to 500 gramme (OR 7.311, C.I = 2.552 - 20.944). In the group with above average (>500 g) placenta weight 48/199 (24.12%) had demonstrable FMH, in contrast to 4/96 (4.17%) in the group whose placenta weight was less or equal to 500 g. Moreover, all the 8 parturients with large FMH had placenta weights greater than 500 g. It is a well-known fact that FMH occur transplacentally, though the precise mechanism remain speculative. It is thought to develop when damage occurs to the trophoblastic covering of the villi [13], but in most cases there is usually no history of trauma, but it is seen with increased frequency in the presence of placental choriocarcinomas and large placental hemangiomas (chorangiomas) [14,15].
Though less pronounced, placental diameter, showed twice an odd of FMH in patients with greater than 50 cm placenta. This finding is however difficult to explain, especially in the context that it is expected that increase placenta surface area, which is a factor derivable from radius and diameter, could be associated with FMH. It would seem from this study that placenta diameter did not correlate with placenta weight. In this instance, it could be that placenta thickness, which our study did not address, would be more related to placenta weight. The choice of placenta diameter in this study was because it is mathematically directly related to placenta surface area and volume. It was hypothesized at the study design that placenta surface area might be a major determinant of the incidence and degree of feto-maternal haemorrhage. However, our findings have proven this hypothesis to be much less so. Adjusted for co-variates, placenta weight remained significant with twice an odd than placenta diameter, as a determinant of feto-maternal haemorrhage.
The significance of findings from this study, when extrapolated to Rh D negative patients, is that, placental
Table 2. Maternal blood group and fetomaternal haemorrhage.
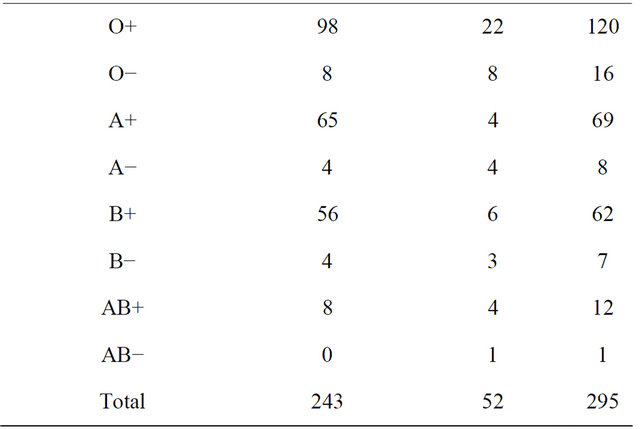
Table 3. Fetomaternal haemorrhage and placental parameters.

parameters being determinable by ultrasonography antenatally may be useful as predictor of possible FMH, alongside other known obstetric factors. Ultimately, a risk-based scoring system may be possible if these findings are consistent with findings from other centres. However, it is worth mentioning that the size of population studied and non-inclusion of placenta thickness in our study design parameters are limitations. We consequently recommend larger studies, preferably multi-centred and multi-racial, to further establish the placental parameters determinant of FMH.
5. Conclusion
Our findings suggest that placenta weight is a significant predictor of FMH in parturients. Possibly, due to sample size limitation, placenta diameter appears to be a minor predictor for FMH. These are factors assessable by ultrasonography and in conjunction with other known obstetric factors, may possibly be considered in risk-based scoring system for predicting feto-maternal haemorrhage.
REFERENCES
- G. Schmorl, “Pathologisch—Anatomische Untersuchungenueber Puerperal Eklampsie,” Vogel, Leipzig, 1893.
- M. S. K. Lau, J. V. K. Tan, T. Y. T. Tan, J. M. Gomez and G. S. H. Yeo, “Case of FMH Resulting in Hydrops,” Annals Academy of Medicine, Singapore, 2003, pp. 642- 644.
- K. J. Moise, “Red Blood Cell Alloimmunization in Pregnancy,” Seminars in Hematology, Vol. 42, No. 3, 2005, pp. 169-178. doi:10.1053/j.seminhematol.2005.04.007
- American College of Obstetrics and Gynecology, “ACOG Practice Bulletin No. 75: Management of Alloimmunization,” Obstetrics & Gynecology, Vol. 108, No. 2, 2006, pp. 457-464.
- S. Sivarao, M. K. Vidyadaran, A. B. E. Jammal, S. Zainab, Y. M. Goh and K. N. Ramesh, “Weight, Volume and Surface Area of Placenta of Normal Pregnant Women and their Relation to Maternal and Neonatal parameters in Malay, Chinese and Indian Ethnic Groups,” Placenta, Vol. 23, No. 8, 2002, pp. 691-696. doi:10.1053/plac.2002.0817
- T. M. Mayhew, “The Human Placenta and the Search for Structural Correlates of Fetal Well-Being,” Proceedings of a Workshop on Comparative Placentology, Havemeyer Foundation Monograph Series No. 17, 2005.
- A. O. Adeniji, V. O. Mabayoje, A. A. Raji, M. A. Muhibi, A. A. Tijani and A. S. Adeyemi, “Fetomaternal Haemorrhage in Parturients: Incidence and Its Determinants,” Journal of Obstetrics & Gynaecology, Vol. 28, No. 1, 2008, pp. 60-63. doi:10.1080/01443610701812181
- Mandolin Ziadie, “Placenta,” Wikipedia, Wikimedia Foundation, Modified on 14 September 2012.
- E. Kleihauer, H. Braun and K. Betke, “Demonstration von Fetalemhemoglobin in der Erythrocyteneinesblutausstrichs,” Klinische Wochenschrift, Vol. 35, No. 12, 1957, pp. 637-638. doi:10.1007/BF01481043
- P. L. Mollison, “Quantification of Transplacental Haemorrhage,” British Medical Journal, Vol. 3, 1972, pp. 31- 34. doi:10.1136/bmj.3.5817.31
- L. Leyenaar, V. M. Allen, H. E. Robinson, M. Parsons and M. C. Van den Hof, “Peripartum Factors Predicting the Need for Increased Doses of Postpartum Rhesus Immune Globulin,” Journal of Obstetrics & Gynaecology Canada, Vol. 32, No. 8, 2010, pp. 739-744.
- R. Salim, I. Ben-Shlomo, Z. Nachum, R. Mader and E. Shalev, “The Incidence of Large Fetomaternalhemorrhage and the Kleihauer-Betke Test,” Obstetrics & Gynecology, Vol. 105, No. 5, 2005, pp. 1039-1044. doi:10.1097/01.AOG.0000157115.05754.3c
- K. Benirschke, P. Kaufmann and R. N. Baergen, “Pathology of the Human Placenta,” 5th Edition, Springer, New York, 2006.
- M. Santamaria, K. Benirschke, P. M. Carpenter, et al., “Transplacentalhemorrhage Associated with Placental Neoplasms,” Pediatric Pathology, Vol. 7, No. 5-6, 1987, pp. 601-615. doi:10.3109/15513818709161424
- K. Aso, K. Tsukimori, Y. Yumoto, S. Hojo, K. Fukushima, T. Koga, K. Sueishi, Y. Takahata, T. Hara and N. Wake, “Prenatal Findings in a Case of Massive Fetomaternalhemorrhage Associated with Intraplacentalchoriocarcinoma,” Fetal Diagnosis & Therapy, Vol. 25, No. 1, 2009, pp. 158-162. doi:10.1159/000209201
NOTES
*Financial disclosure: We declare that no funding/grant was received for this study and no conflict of interest or commercial relationship.
Conflict of interest notification: We declare that we have no conflict of interest; no funding/grant was received for this study and no commercial relationship.
#Corresponding author.

