Open Journal of Orthopedics
Vol.2 No.2(2012), Article ID:20108,5 pages DOI:10.4236/ojo.2012.22005
Role of Neo-Adjuvant Chemotherapy in the Treatment of Osteosarcoma
![]()
1Department of Orthopaedics, Bankura Sammilani Medical College, Bankura, India; 2Department of Radiotherapy, Bankura Sammilani Medical College, Bankura, India.
Email: ranadeb.b@rediffmail.com
Received March 14th, 2012; revised April 18th, 2012; accepted May 6th, 2012
Keywords: Osteosarcoma; Amputation; Neo-Adjuvant Chemotherapy; Adjuvant Chemotherapy
ABSTRACT
Osteosarcoma is a tumour characterized by the production of osteoid by malignant cells. The incidence is approximately 1 to 3 million/year. The incidence is slightly higher in males. Onset can occur at any age; however, primary high grade osteosarcoma usually occurs in the second decade of life. Historically patients with osteosarcoma were treated with immediate wide or radical amputation. Despite the treatment, 80% patients with apparently isolated disease died of distant metastases. In recent years the number of patients with osteosarcoma of the limb treated by amputation + chemotherapy has increased. In our study, we divided the patients into two groups. One group (A) was treated with amputation + adjuvant chemotherapy. The other group (B) was treated with neo-adjuvant chemotherapy + amputation followed by adjuvant-chemotherapy. In our study, the margin negativity in post surgical specimen was significantly higher (P-value 0.0007) for the group treated with neo-adjuvant chemotherapy. Local recurrence in the group treated without neoadjuvant chemotherapy was significantly more (P-value 0.0005). The systemic recurrence at the end of 6 months was higher the group treated without neoadjuvant chemotherapy (P-value 0.0169). However systemic recurrence between 6 months -1 year and 1 year - 2 years were not significant (P-values 0.1501 and 0.4902). From the above figures it may be concluded that treatment with neo-adjuvant chemotherapy + amputation + adjuvant chemotherapy had definite advantages over upfront amputation + adjuvant chemotherapy.
1. Introduction
Osteosarcoma is a tumour characterized by the production of osteoid by malignant cells. It is second most common primary malignancy of bone behind multiple myeloma. It accounts 20% of primary malignancies of bone. The incidence is approximately 1 to 3 per million per year. Onset can occur at any age. However, primary high grade osteosarcoma usually occurs in the second decade of life. Parosteal osteosarcoma has a peak incidence in the third and fourth decades while secondary osteosarcomas are more common in the older individuals [1].
The incidence is slightly higher in males (except parosteal osteosarcoma which is more common in females). There are no significant differences among races and genetic factors rarely play any role. It is more common in patients with hereditary form of retinoblastoma, Rothmund-Thomson syndrome and Li-Fraumeni syndrome. All skeletal locations can be affected; however, most primary osteosarcomas occur at the site of most rapid bone growth including the distal femur, the proximal tibia (i.e. around the knee joint) and the proximal humerus [1].
Historically patients with osteosarcoma were treated with immediate wide or radical amputation. Despite the treatment, 80% patients with apparently isolated disease died of distant metastases. From this it can be deduced that many patients with osteosarcomas might have had non detectable micro-metastases at presentation and this justifies the use of additional systemic treatment i.e. chemotherapy (neo-adjuvant or adjuvant). Currently at most musculoskeletal oncologic centres, the treatment of high grade osteosarcomas consists of neo-adjuvant chemotherapy, wide or radical surgery (resection or amputation) and adjuvant chemotherapy. Pulmonary metastases likewise are resected if possible after neo-adjuvant chemotherapy. The histological response of the primary tumour to neo-adjuvant chemotherapy has been known to be a good predictor of long term survival. Low grade osteosarcoma can be treated with wide resection or amputation without chemotherapy [1].
However, though reasons supporting the use of neoadjuvant chemotherapy in the treatment of limb osteosarcoma are convincing, superiority of neo-adjuvant therapy over adjuvant therapy is yet to be proved. We conducted this study to compare neo-adjuvant chemotherapy + amputation + adjuvant chemotherapy and amputation + adjuvant chemotherapy in the treatment of osteosarcoma at our institute.
2. Materials and Methods
This prospective randomized and comparative study was conducted on the patients admitted in the Department of Orthopaedics of BSMC&H. Our study population mainly consisted of patients younger than 40 years age with primary osteosarcoma of the extremity. The study period was about 2 years from April 2009 to March 2011. Eligibility criteria for the patients included in the study were as follows:
1) Patients who were younger than 40years age;
2) Had typical radiographic and histological features of osteosarcoma (primary, central and high grade);
3) Tumour was located in extremity;
4) No evidence of metastasis.
The parameters studied were:
1) Margin negativity in post surgical specimen;
2) Local recurrence and;
3) Systemic recurrence: At 6 months, 1 year and 2 years following surgery.
After obtaining ethical clearance from the institutional Ethics committee, study was conducted among the study populations after taking written informed consent. The relevant information collected by using a pre-designed proforma including history, general and systemic examination findings. Initial CT scan of lungs and abdomen were conducted besides routine pre anesthetic investigations. MRI Scan of the affected limb, which would have been helpful to know the extent of the disease before surgery, could not be done, as, at the time of this study, this institution had no MRI machine, nor was the facility for MRI available in the locality.
The patients under Group A were posted for amputation of the involved limb, while group B were treated with neo-adjuvant chemotherapy initially before amputation. Both the groups were given adjuvant chemotherapy.
All patients in the study received six cycles of chemotherapy. Patients under Group A received all those six cycles as adjuvant therapy, whereas patients under group B received three cycles before surgery as neo-adjuvant therapy and three cycles as adjuvant.
In this study, for patients receiving neo-adjuvant chemotherapy (i.e. Group B), we followed the protocol used in the landmark study of the European Osteosarcoma Intergroup [2]:
Inj. Doxorubicin 25 mg/m2 i.v. bolus daily for three consecutive days (Day 1 - 3);
Inj. Cisplatin 100 mg/m2 i.v. infusion on Day 1.
The cycles were repeated every three weeks followed by surgery. After a two weeks gap following surgery, further three cycles chemotherapy were given in the same protocol.
Patients in the Group A received chemotherapy in the same regimen as mentioned above with an interval of three weeks between the cycles (all six cycles as adjuvant therapy).
5HT3 antagonists were given during chemotherapy and for continued for three days afterwards. Post-chemotherapy adverse effects were minimal. Patients usually developed anorexia (loss of appetite, CTC Grade 1), some degree of nausea (were able to eat, CTC Grade 1), which were managed with reassurance and supportive care.
Specimen was sent for HPE for marginal negativity in both cases. As percentage of necrosis in post-neoadjuvant chemotherapy specimens are not routinely reported in our institutional histopathology reports, we refrained from taking that as our study parameter.
Both the groups were followed with CT scan of lungs and abdomen at the end of 6 months, 1 year and 2 years. The surgery conducted was amputation of the involved limb. Since frozen section could not be obtained in our set up, so an approximate margin of 5 cms was kept.
The marginal negativity, local recurrence and systemic recurrence were evaluated, and Fisher’s exact test was used in the analysis of data to calculate a two tailed Pvalue.
3. Results
The patients under the study were divided into 2 groups.
Group A: Upfront amputation + adjuvant chemotherapy;
Group B: Neo-adjuvant chemotherapy + amputation + adjuvant chemotherapy.
The results were statistically analyzed using Fisher’s exact test and the two tailed P-value was evaluated.
The post surgical specimen assessed for margin positivity showed margin + vity. The percentage of specimens which had a negative margin was 13.33% (2 of 15) in Group A and 80% (12 of 15) for Group B which was statistically highly significant (P-value 0.0007) (Table 1, Figure 1).
The rate of local recurrence was 73.33% (11 of 15) in Group A while in only 6.67% (1 of 15) of patients in Group B tumour recurred locally, which was also highly significant (P-value 0.0005) (Table 2, Figure 2).
The rates of systemic recurrence at the end of 6 months were 40% (6 of 15) in Group A and 0% (0 of 15) in Group B which was significant (P-value 0.0169). Systemic recurrence between 6 months - 1 year was 44.44% (4 of 9) in Group A while 13.33% (2 of 15) in Group B which was not statistically significant (P-value 0.1501). Between 1 year - 2 years, systemic recurrence occurred in 20% (1 of 5) in Group A and 7.692% (1 of 13) in Group B which again was not significant (P-value 0.4902) (Table 3, Figure 3).
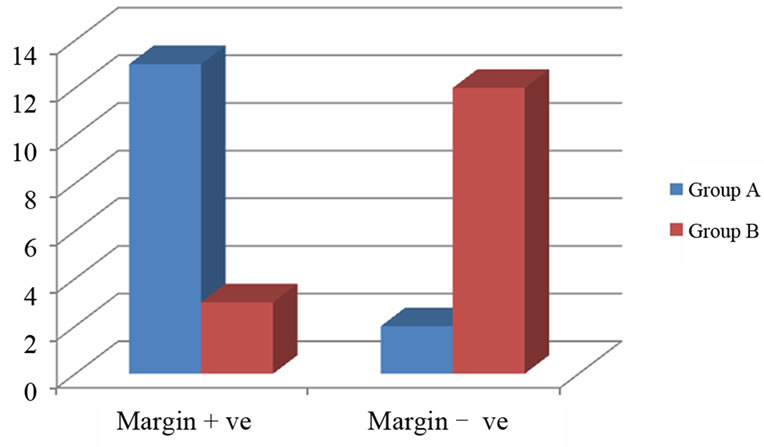
Figure 1. Margin negativity in post-surgical specimen.
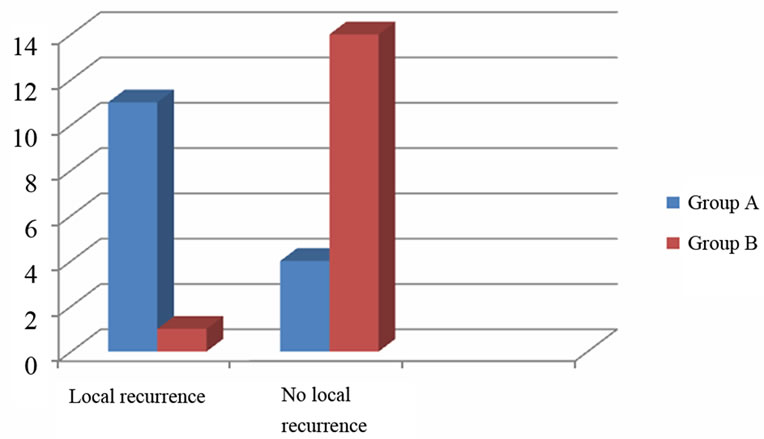
Figure 2. Local recurrence.

Figure 3. Systemic recurrence.

Table 1. Margin negativity in post-surgical specimen.
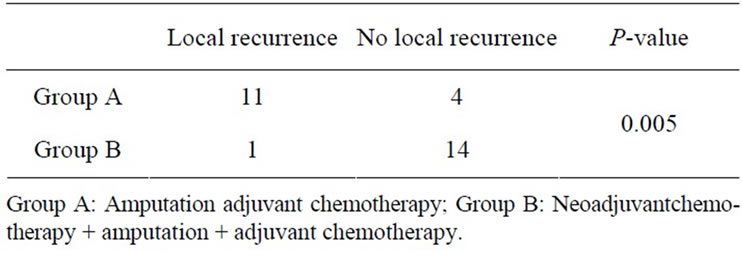
Table 2. Local recurrence.
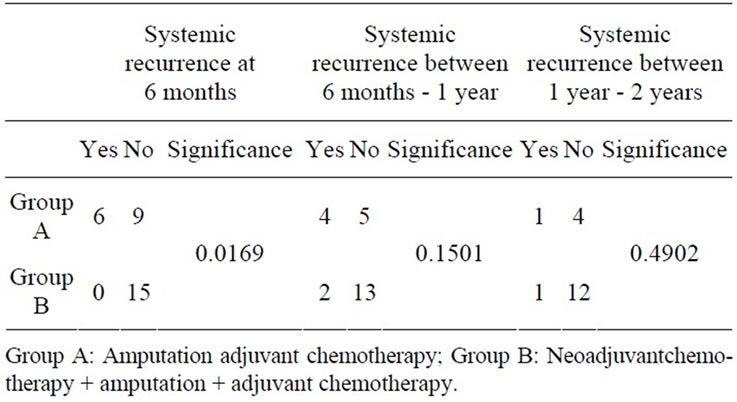
Table 3. Systemic recurrence.
4. Discussion
In recent years the number of patients with osteosarcoma of the limb treated by amputation has increased [3-7]. This is due to the improved efficacy of chemotherapy, which can reduce the extent of surgery required, the development of modern radiological tools such as CT and MRI and the availability of new reconstructive procedures. All these can allow good functional results and offer alternatives determined by the location of the tumour, the age of the patient and the desired lifestyle. Neo-adjuvant chemotherapy in the treatment of limb osteosarcoma evolved along with the concept of limb-salvage surgery. Even when limb salvage is not the goal, the fact that a larger number of patients receiving neo-adjuvant chemotherapy is inevitably associated with a reduction of surgical margins. This might increase the incidence of local recurrence. Previously, in patients who did not receive associated chemotherapy, local recurrence after surgery correlated strictly with surgical margins [8- 11]. Now, as most patients are getting neoadjuvant chemotherapy before surgery, it is not yet clear whether the reduction of surgical margins is associated with an increased risk of local recurrence. The reported results for the incidence of local recurrence in patients undergoing amputation + adjuvant chemotherapy and those treated by amputation and neoadjuvant chemotherapy are contradictory. In multicentre studies the incidence of local recurrence seems to be lower in patients treated with neoadjuvant chemotherapy [12,13] conversely, in two uniinstitutional studies with a smaller number of patients, the incidence of local recurrence was the same for both groups [14].
In our study, we divided the patients into two groups. One group (A) was treated with amputation + adjuvant chemotherapy. The other group was treated with neoadjuvantchemotherapy + amputation + adjuvant chemotherapy. Chemotherapy regime followed was cisplatin + doxorubicin, as followed in the study of the European Osteosarcoma Intergroup.
In our study, the margin negativity in post surgical specimen was significantly higher (P-value 0.0007) for the group treated with neo-adjuvant chemotherapy. Also, the local recurrence for the group treated with neoadjuvant chemotherapy was significantly lower (P-value 0.0005). Percentage of patients suffering systemic recurrence at the end of 6 months was significantly less for the group treated with neoadjuvant chemotherapy (P-value 0.0169). However, lower rates of systemic recurrence afterwards i.e. between 6 months - 1 year and 1 year - 2 years were not significant (P-values 0.1501 and 0.4902).
Our study also confirmed the popular belief that local recurrence is a serious problem in osteosarcoma of the limb. This has been reported previously by others [4,8, 12]. The main strength of our study is that all the patients had been treated at the same institution by the same team of surgeons. However shortcomings too existed [13-16]. Some of the patients with bulky tumour were treated with neo-adjuvant chemotherapy which could be a bias on our part. Also, as we could not do a MRI Scan of the affected limb beforehand, we had to undertake surgery without knowing the proper local extent of the disease. This might explain the high rate of margin positivity in the group of patients without neo-adjuvant therapy. However, it is interesting to note that, when the facilities of MRI is not available or when the patient cannot afford the financial cost of MRI, neo-adjuvant chemotherapy may offer some advantage regarding margin-negativity, as evident from this study. For those patients who undergo operations with inadequate surgical margins the risk of local recurrence is very high, especially when associated with a poor histological response to chemotherapy [17-21]. Because of the poor outcome in patients who develop local recurrences, should a pathological examination of the resected specimen demonstrate inadequate surgical margins and an associated poor response to chemotherapy, a secondary amputation should be recommended. This may be difficult for the patient, but we believe that it is our duty to suggest this to them. It is for this reason that we consider that patients with osteosarcoma of the limb should be surgically treated in selected centres. These should be fully equipped to undertake a pathological examination of the surgical margins.
Therefore, it may be concluded that use of neo-adjuvant chemotherapy have some definite advantages over upfront surgery. When neo-adjuvant chemotherapy is used, margin negative resection is more probable, which would lead to lesser incidence of systemic recurrence and which might have some effect on the incidence of systemic recurrence too. Though not assessed in this study, histopathological response to neo-adjuvant chemotherapy is an individual predictor for the post-treatment survival of the patient, which should be another advantage in favour of the use of neo-adjuvant chemotherapy.
REFERENCES
- R. K. Heck, “Malignant Tumours of Bone: Campbell’s Operative Orthopaedics,” 11th Edition, Mosby Elsevier, Philadelphia, 2010, pp. 901-904.
- R. L. Souhami, A. W. Craft, J. W. Van der Eijken, M. Nooij, et al., “Randomised Trial of Two Regimens of Chemotherapy in Operable Osteosarcoma: A Study of the European Osteosarcoma Intergroup,” Lancet, Vol. 350, No. 9082, 1997, pp. 911-917. HUdoi:10.1016/S0140-6736(97)02307-6U
- M. Natarajan , M. Paraskumar, G. Rajkumar, A. Sivaseelam and S. Natarajan, “Limb Salvage in Aggressive and Malignant Tumours in the Fibula,” International Orthopaedics, Vol. 28, No. 5, 2004, pp. 307-310. HUdoi:10.1007/s00264-004-0566-xU
- M. Hegyi, A. F. Semsei, Z. Jakob, M. Szendrol, M. Csoka and G. Kovacs, “Good Prognosis of Localised Osteosarcoma in Young Patients Treated with Limb Salvage Surgery and Chemotherapy,” Pediatric Blood Cancer, Vol. 57, No. 3, 2011, pp. 415-422. HUdoi:10.1002/pbc.23172U
- W. S. Song, D. G. Jeon, C. B. Kong, W. H. Cho and S. Y. Lee, “Outcome of Re-Excision of Intralesionally Treated Parosteal Osteosarcoma,” Journal of Surgical Oncology, Vol. 103, No. 3, 2011, pp. 264-268. HUdoi:10.1002/jso.21822U
- W. Guo, X. Sin and T. Ji, “Evaluation of Surgical Treatment of Pelvic Osteosarcoma,” Zhonghua Wai Ke Za Zhi, Vol. 48, No. 13, 2010, pp. 994-998.
- M. A. Ayerza, G. L. Farfalli, L. Aponti-Tinao and D. L. Musculo, “Does Increased Rate of Limb Sparing Surgery Affect Survival in Osteosarcoma?” Clinical Orthopaedics and Related Research, Vol. 468, No. 11, 2010, pp. 2854- 2859. HUdoi:10.1007/s11999-010-1423-4U
- P. Ruggieri, T. Calabro, M. Montalti and M. Mercuri, “The Role of Surgery and Adjuvants to Survival in Pagetic Osteosarcoma,” Clinical Orthopaedics and Related Research, Vol. 468, No. 11, 2010, pp. 2962-2968. HUdoi:10.1007/s11999-010-1473-7U
- S. Ferrari, E. Palmerini, E. L. Staals, M. Mercuri, B. Franco, P. Picci and G. Bacci, “The Treatment of NonMetastatic High Grade Osteosarcoma of the Extremity: Review of the Italian Rizzoli Experience. Impact on Future,” Cancer Treatment and Research, Vol. 152, No. 3, 2009, pp. 275-287.
- H. J. Kim, P. M. Chalmers and C. D. Morris, “Pediatric Osteogenic Sarcoma,” Current Opinion in Pediatrics, Vol. 22, No. 1, 2010, pp. 61-66. HUdoi:10.1097/MOP.0b013e328334581fU
- A. Daigeler, M. Lehnhardt, A. Khadra, J. Hauser, L. Steinstrasses, S. Langer, O. Goertz and H. U. Steinau, “Proximal Major Limb Amputations: A Retrospective Analysis of 45 Oncological Cases,” World Journal of Surgical Oncology, Vol. 7, No. 1, 2009, pp. 7-15.
- A. Longhi, C. Errani, D. Gonzales-Arabio, C. Ferrari and M. Mercuri, “Osteosarcoma in Patients Older than 65 Years,” Journal of Clinical Oncology, Vol. 26, No. 33, 2008, pp. 5368-5373. HUdoi:10.1200/JCO.2007.14.9104U
- R. Lumbreras, A. Castro, S. Val, F. J. Modrego and M. L. Bello, “Salvage Surgery Treatment in Osteosarcoma of the Fibula with Seventeen Years Survival,” Revista de la Facultad de Ciencias Médicas de la Universidad Nacional de Córdoba, Vol. 64, No. 1, 2007, pp. 38-41.
- G. Bacci, S. Ferrari, M. Mercuri, et al., “Neoadjuvant Chemotherapy for Extremity Osteosarcoma: Preliminary Results of the Rizzoli’s 4th Study,” Acta Oncology, Vol. 37, No. 1, 1998, pp. 41-48.
- N. Delepine, G. Delepine, S. Alkallaf, et al., “Local Relapses Following Limb Sparing Salvage Surgery for Osteosarcoma: Prognostic Factors and Influence of Chemotherapy,” American Society of Clinical Oncology Proceedings, Vol. 15, 1996, p. 526.
- G. Saeter, T. Wiebe, T. Wiklund, et al., “Chemotherapy in Osteosarcoma: The Scandinavian Sarcoma Group Experience,” Acta Orthopaedica Scandinavica Supplementum, Vol. 285, No. 70, 1999, pp. 74-82.
- U. Nilsonne, L. A. Brostrom and T. Aparisi, “Local Tumor Resection in Interferon Treated Osteosarcoma Patients,” Annales Chirurgiae et Gynaecologiae, Vol. 84, No. 1, 1995, pp. 63-70.
- S. S. Bielack, B. Kempf-Bielack and K. Winkler, “Osteosarcoma: Relationship of Response to Preoperative Chemotherapy and Type of Surgery to Local Recurrence,” Journal of Clinical Oncology, Vol. 14, No. 10, 1996, pp. 683-684.
- O. Brosjo, “Surgical Procedure and Local Recurrence in 223 Patients Treated 1982-1997 According to Two Osteosarcoma Chemotherapy Protocols: The Scandinavian Sarcoma Group Experience,” Acta Orthopaedica Scandinavica Supplementum, Vol. 285, No. 70, 1999, pp. 58- 61.
- N. J. Lindner, O. Ramm, A. Hillmann, et al., “Limb Salvage and Outcome of Osteosarcoma: The University of Muenster Experience,” Clinical Orthopaedics, Vol. 358, 1999, pp. 83-89.
- http://www.graphpad.com/quickcalcs

