Natural Resources
Vol.07 No.08(2016), Article ID:70130,13 pages
10.4236/nr.2016.78041
Mineralogy and Beneficiation of Lamujärvi Syenites
Xiaosheng Yang1, Jukka Laukkanen1, Akseli Torppa2, Neea Heino1
1Geological Survey of Finland, GTK Mintec, Outokumpu, Finland
2Geological Survey of Finland, Kuopio, Finland

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 25 July 2016; accepted 23 August 2016; published 26 August 2016
ABSTRACT
The Lamujärvi syenites intrusions occur within Svecofennian (ca. 1.92 Ga) gneisses, ca. 70 km west of the Otanmaki alkaline area in central Finland. Previous studies have revealed zones in the syenites that display strong enrichment in Zr (1587 ppm), Nb (up to 685 ppm), Ta (up to 82 ppm), and REE (up to 5350 ppm). The major REE-bearing minerals in the Lamujarvi syenites are allanite and monazite. A sample of the Lamujärvi syenites with the REE content of 4700 ppm was studied on the mineralogy and beneficiation at GTK Mintec. The mineralogical analysis was conducted by MLA. The REE-bearing minerals allanite, parisite, zircon, and apatite were identified at significant concentrations and high contents of titanite (15.4%), biotite (30.9%) and magnetite (8.2%) were displayed in the sample. The beneficiation experiments by flotation, wet low and high gradient magnetic separations and gravity separation with a shaking table were carried out. A composite REE-Ti concentrate with REE 23,000 ppm, Zr 5000 ppm, Nb 3400 ppm and TiO2 25.5% at REE and Ti recoveries of 57% and 54% was obtained. In addition, biotite and magnetite concentrates were gained as by-products.
Keywords:
The Lamujärvi Syenites, Mineralogy, Beneficiation, Allanite, Titanite

1. Introduction
No REE are currently mined in Europe, but potential resources are known to be widespread, and many are being explored [1] . Finland has high potential for exploration of REE and the known examples of REE mineralisation occur in carbonatitic and alkaline intrusives, granites, hydrothermal rocks and kaolinitic saprolites (Figure 1). There are currently no economic REE deposits in Finland, although REE were extracted between 1963 and 1972 from apatite concentrate mined as a by-product in the Korsnäs lead (Pb)-REE mine in western Finland [2] . REE could potentially be extracted in the future as a by-product of mining for commodities such as phosphorus (P) and gold (Au).
As shown in Figure 1, Finland is situated in the central part of the Fennoscandian shield, which is the largest outcropping Precambrian domain in Europe. The shield hosts a few sub-economic to economic REE deposits mostly with the intrusions of the Devonian Kola alkaline province in Russia [3] [4] . The Sokli carbonatite complex (total area 20 km2) and the Iivaara ijolite complex in Finland are the westernmost intrusions of the Devonian Kola alkaline province. Other potentially economic REE deposits in Fennoscandia include the Norra Kärr in Sweden. Currently, the most promising REE deposit is associated with the Sokli carbonatite complex in north eastern Finland. The Sokli carbonatite (approximately 360 - 380 Ma) hosts an unexploited deeply weathered phosphate deposit enriched in niobium (Nb), tantalum (Ta), zirconium (Zr), REE and uranium (U). The carbonatite intrusion consists of a magmatic phoscorite-carbonatite core, surrounded by metacarbonatite and a wide fenite aureole, altogether about 9 km in diameter. Late-stage carbonatite veins in the magmatic core and in the fenite zone have high potential for REE mineralisation [5] [6] .
The Lamujärvi plutons are located ca. 70 km west of the Otanmäki area, which hosts Nb-REE mineralisations in alkaline gneisses, but despite the spatial proximity, in light of the 200 Ma age difference suggested by recent U-Pb (zircon) dating of the Lamujärvi syenite at ca. 1.85 Ga and the Otanmäki granite at ca. 2.05 Ma, a genetic relationship between the two occurrences is improbable. The Lamujärvi syenites in central Finland display strong enrichment in Zr (1587 ppm), Nb (up to 685 ppm), Ta (up to 82 ppm) and REE (up to 5350 ppm). The major REE-bearing minerals in the enriched rocks are allanite and monazite [7] . Meanwhile, other valuable minerals such as titanite, biotite and magnetite are at high concentrations which could be economically recovered. In this paper, the mineralogy and the beneficiation of the Lamujärvi syenites are studied.
2. Experimental and Reagents
A sample of the Lamujarvi syenites over 100 kg collected from Lamujärvi area was received at GTK Mintec in 2012. The sample was crushed by a jaw and a roll crusher to −1.5 mm and split into 1.5 kg amount of subsamples which were stored for beneficiation experiments. One of the subsamples was further split into 100 g amount of samples for chemical and mineralogical analyses.
Chemical analyses of the sample were conducted by XRF, ICP-OES and ICP-MS at Labtium Oy in Finland. A Mineral Liberation Analyzer (MLA) was used for mineralogical analysis at GTK Mintec which consists of the standard modern SEM (FEI Quanta 600) with the energy dispersive X-ray analyzer (EDAX Genesis with two detectors) and the software package developed originally by JKTech (Australia) [8] [9] . The modal mineralogy of the samples was analyzed by the XMOD-STD and XBSE-STD methods, which were point counting methods, and the grain size was analyzed by the XBSE method.
The samples of 1.5 kg with the size of −1.5 mm were ground at a laboratory rod mill as the head samples for beneficiation experiments. Flotation experiments were carried out using an Outotec laboratory flotation machine. Flotation cells with the volumes of 4 and 1.5 liters were used in rougher and cleaner flotation, respectively. Tap water was used for wet grinding and flotation. The room temperature (20˚C - 23˚C) was applied for flotation conditioning. A laboratory shaking table was used for gravity concentration at the conditions of inclination 3˚ - 4˚, feed rate 0.3 kg/h, stroke 350/min, slurry density 25% and rate of rinse water 6 l/min. Wet low intensity magnetic separation (WLIMS) experiments were performed by using a Sala laboratory roller type magnetic separator with the magnetic field of 0.07 Tesla. A laboratory high gradient magnetic separator (Sala HGMS 10-15- 20 SCR) was used for the high gradient magnetic separation (HGMS) experiments. The matrix type of 3.5 XRO with the aperture size of 750 µm was used. The magnetic intensities of 1.5 and 2 Tesla were applied for roughing process and 0.2 Tesla for cleaning process.
To avoid negative effects of slimes on beneficiation desliming was conducted by a hydraulic sedimentation method to remove the fine fraction of −20 µm after material grinding. Acid leaching testwork by using hydrochloric acid (HCl) was performed for the treatment of slimes. The 37% HCl was used and diluted into required concentration for leaching. The volume of the leaching tank was 1000 ml, the ratio of solid to acid solution 75 g/500 ml and the agitation speed 180 rpm. The acid concentration was 6 mol/l and leaching temperature 60˚C which was controlled by circulation of heated water. The leaching times tested were 60 min, 120 min and 180 min. After leaching the residues were filtered, dried, weighted and assayed by XRF.
In biotite flotation, two cationic collectors tallow amine and Aero 3030C supplied by Cytec were used and in REE-Ti flotation hydroxamate type of reagent Aero6494 and sulfosuccinamate type of reagent Aero 845 from Cytec, FS-2 an anonic collector, and petroleum sulfonate were applied.
Figure 1. Simplified geological map of Finland showing the distribution of REE occurrences (the occurrences are classified by rock types) [7] .
3. Results and Discussion
3.1. Chemical Compositions
The REE contents determined by ICP-OES and ICP-MS are shown in Table 1. Total sixteen rare earth elements were found but seven of them including La, Ce, Pr, Nd, Sm, Gd and Y with significant contents over 100 ppm. The REE with the highest content is Ce that is 2060 ppm. The total REE (TREE) is 4729 ppm or 0.47% and the ratio of the light (LREE) and heavy REE (HREE) is 91%/9%. The contents of the major elements determined by XRF are shown in Table 2. The elements Nb and Zr were found to be with significant contents, i.e., ZrO 0.18% or 1800 ppm, Nb 0.066% or 660 ppm. The contents of Th and U are ignorable. The relatively high concentrations of TiO2, FeO and MgO in the sample are explained by abundant titanite, biotite and magnetite which could be recovered as co-product and by-products.
3.2. Mineralogical Studies
Modal mineralogy of the sample with the chemical formulas and physical properties of the contained minerals are shown in Table 3. A total of 18 minerals were identified by MLA (over 52000 points were measured) in which 89.5% are silicates, and other remained are Fe-oxides 8.6%, phosphates 1.24% and carbonates 0.48%. The main silicate minerals are biotite 31.6%, plagioclase 30.9%, titanite 15.4%, garnet 2.4% and allanite 1.9%. The Fe-oxides are magnetite 8.2%, goethite 0.3% and ilmenite 0.1%. The phosphate is apatite 1.24% and the carbonate calcite 0.48%. The identified REE minerals are allanite and parisite. Most the REE are contained in allanite because of very low concentration of parisite. Meanwhile, REE may be also contained in apatite and zircon in trace contents.
The SEM images of REE minerals allanite, parisite and zircon are shown in Figure 2. The allanite particles are clearly found in liberated and coarse grain sizes. The particles of zircon also appear in smaller grain sizes and some are liberated. But the parisite particles are found in very fine grain size and tightly locked in other minerals like silicates, titanite, calcite and allanite etc. The grain sizes of REE minerals allanite, parisite and zircon are presented in Figure 3. Allanite has a coarse grain size, i.e., P80 = 400 µm, however, zircon and parisite are much finely grained, i.e., P80 = 70 µm for zircon and P80 = 50 µm for parisite.
3.3. Beneficiation Studies
3.3.1. Design of Flowsheet
As shown in Table 3, the sample contains high contents of magnetite and biotite. Magnetite is a ferromagnetic
Table 1. The REE contents in the sample.
aLight REE to heavy REE ratio.
Table 2. The major element contents in the sample (wt%).
Table 3. Modal mineralogy of the sample.
Figure 2. SEM images of REE minerals, allanite, parisite and zircon.
Figure 3.The grain sizes of REE minerals allanite, parisite and zircon.
mineral and easily separated by low intensity magnetic separation. With a good floatability by using amine collector biotite can be recovered by flotation effectively. As the most important REE mineral in the sample allanite is a paramagnetic mineral and also a heavier mineral comparing to other major gangue minerals in the sample (plagioclase, K_feldspar and quartz) and could be concentrated by gravity and high gradient or intensity magnetic separations, and flotation. Meanwhile, titanite is also a paramagnetic and heavier mineral and could be recovered with allanite as co-product.
Rough experiments of gravity separation, high gradient magnetic separation and flotation were performed and a combination flowsheet was determined as illustrated in Figure 4. After grinding the slimes of −20 µm is removed by desliming. Then magnetite is recovered by WLIMS followed by biotite flotation. Finally, allanite and titanite are concentrated by gravity concentration with shaking tabling, flotation and HGMS as bulk REE-Ti concentrates.
3.3.2. Parametric Experiments and Results
The amount of the feed sample for each experiment was 3 kg and the grinding size was P80 = 85 µm which was determined based on overall analysis of mineral associations and liberations. Through roughing and cleaning process of WLIMS the magnetite concentrate with the grade of FeO 87% was obtained with low losses of REE and TiO2 (<1.0%). The WLIMS tailings (rougher and cleaner tailings combination) was reported to the biotite flotation in which the slurry was conditioned for 5 min at a high density (˃60%) with addition of amine collector and kerosene at pH 3.0 by using H2SO4 and then the biotite flotation was performed at pH 3.0. Two kinds of amine collectors tallow amine and Aero3030C were tested with kerosene as the assistant collector. The biotite flotation results indicated by MgO grade and recovery using the two collectors are shown in Figure 5. It is demonstrated that higher recovery of MgO (biotite) was obtained by using Aero 3030C. The losses of REE (indicated by Ce) and TiO2 during the biotite flotation for both collectors are compared in Figure 6. Generally, the loss of REE in the biotite concentrate was quite low (<20%) for both collectors but slightly lower recovery of REE was obtained while using tallow amine. Comparing to Aero 3030C, the loss of TiO2 in the biotite concentrate was significantly lower while using tallow amine.
After the biotite flotation gravity concentration by shaking tabling, flotation and HGMS were performed for recovery of REE-bearing minerals (mainly allanite) and titanite. The operation conditions of shaking tabling were inclination 3˚ - 4˚, feed rate 0.3 kg/h, stroke 350/min, slurry density 25% and rate of rinse water 6 l/min
Figure 4. Beneficiation flowsheet of the Lamujärvi syenites.
Figure 5. MaO grade and recovery of biotite flotation concentrate.
which were determined by observing the stratification and distribution status of the material on the table. During the tabling most coarse particles of allanite and titanite were concentrated as the Gra REE-Ti concentrate and the tailings from the shaking table mainly containing fine allanite and titanite particles was reported to the REE-Ti flotation circuit where the slurry was conditioned for 5 min with additions of chemicals at high density (>60%) prior to flotation. The pH values were adjusted to the optimal depending on collectors which were in the range of pH 6 to 9. In the roughing stage three collectors Aero 6494, FS-2 and P Sulfonate (petroleum sulfonate) combined with Aero 845 were tested. Experimental results of the rougher flotation with different collectors displayed by the relationships between Ce and TiO2 recoveries and the weight recoveries (yields) of concentrate are shown in Figure 7. Comparing to Aero 6494 and FS-2, the combination of P Sulfonate and Aero 845 as the collector shows significantly more selective for flotation of REE (Ce) with higher Ce recoveries at the same yields. For flotation of TiO2 both collector FS-2 and the combination of P Sulfonate and Aero 845 have a very good selectivity although FS-2 performs slightly more efficient for TiO2 flotation.
Being more selective for flotation of REE the combination of P Sulfonate and Aero 845 was selected as the collector for the cleaner flotation and the experimental results are shown in Figure 8. It is seen that the curves of Ce and TiO2 grade-recovery display high efficiencies of both REE and TiO2 flotation.
The suitable magnetic intensities of the high gradient separation were defined by roughly testing, that is, 1.5 and 2 Tesla were applied for roughing process and 0.2 Tesla for cleaning process.
3.3.3. Overall Beneficiation Results
The overall beneficiation results of the Lamujärvi syenites are presented in Table 4 and Table 5. The REE-Ti concentrate which is the composite of gravity and HGMS REE-Ti concentrates has the grade of REE (La, Ce, Y) 15,235 ppm (TREE around 23,000 ppm) at the recovery of 57.2% and the grade of TiO2 25.5% at the recovery of 54.0%. In addition, as the by-products, the magnetic concentrate with the grade of FeO 86.6% at the recovery of 45.6% and the biotite concentrate with the grade of MgO 6.6% at the recovery of 51.0% were obtained.


Figure 6. Recoveries and grades of Ce and TiO2 in biotite concentrate.


Figure 7. Experimental results of the rougher flotation with different collectors.
For analyzing the experimental errors the element contents of the feed by calculation from the experimental results (Calc’d Feed (X1)) and by the analysis (Analysis Feed (X2)) are listed in Table 4 and Table 5. The standard deviation (SD) between the calculated feed (X1) and the analysis feed (X2) was determined by following Equation.
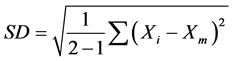
where Xi is the content of element i and Xm is the mean value of X1 and X2.
The values of SD for all the elements listed in Table 4 and Table 5 present that the experimental errors are very small.
3.3.4. Treatment of Slimes by HCl Leaching
It is noticed in Table 4 and Table 5 that 23.4% of REE and 20.8% of TiO2 were deposited in the slimes fraction which is hardly enriched by physical methods (flotation, gravity and magnetic separations) due to very fine particles. Acid leaching testwork using hydrochloric acid (HCl) was performed to assess the leachabilty of REE for the slimes. The extraction rates of elements in the solution are shown in Table 6. It is revealed that the recoveries of La and Ce in the solution are high. The 89.3% La and 79.8% Ce were obtained at the leaching time 180 min. The extraction of P2O5 was over 91%. That is, allanite as the major REE-containing mineral in the slimes and also apatite were mostly decomposed. Meanwhile, the extraction of TiO2 was low (<18.0%) and SiO2 as am impurity was remained in the solid in almost 100%.


Figure 8. Experimental results of the cleaner flotation (P sulfonate 25 g/t and Aero 845 50 g/t; conditioning for 5 min; pH 6).
Table 4. Beneficiation results of the Lamujärvi syenites (assayed by XRF).
Table 5. Beneficiation results of the Lamujärvi syenites (assayed by XRF).
Table 6. Testwork results of acid leaching using HCl for the slimes.
3.3.5. Process Mineralogy
Modal mineralogy of the flotation REE-Ti concentrate (Flo REE-Ti Conc) was determined by MLA analysis. The modal mineralogies of Flo REE-Ti Conc and the Feed are compared in Table 7. It is seen that target minerals allanite, apatite and titanite were significantly enriched, that is, allanite was enriched from 1.87% to 3.85%, apatite from 1.24% to 5.62%, and titanite from to 15.43% to 78.57%. Major silicate minerals biotite, plagioclase and K_felspar but garnet was removed in the flotation concentrate. Meanwhile, the contents of Fe-oxides are low in the Flo REE-Ti Conc. Garnet was concentrated into Flo REE-Ti Conc which could be due to its similarity to allanite, apatite and titanite on crystal properties and floatability [10] .
The contents of biotite, allanite and titanite in the gravity (Gra), HGMS and composite (COMP) REE-Ti concentrates and the biotite concentrate were calculated and the results are shown in Table 8.
Biotite was concentrated from 31.6% to 66.3% in the biotite concentrate. The contents of biotite in the REE-Ti concentrates were reasonably low, that is, 1.52%, 0.91% and 2.0% in the COMP, Gra and HGMS REE-Ti Concs, respectively.
Allanite was concentrated in the REE-Ti Concs, that is, from 1.9% in the feed to 5.48% in COMP REE-Ti Conc, 5.9% in Gra REE-Ti Conc and 5.2% in HGMS REE-Ti Conc. Meanwhile, titanite was significantly enriched in the REE-Ti Concs, that is, from 15.4% in the feed to 71.19% in COMP REE-Ti Conc, 66.1% in Gra REE-Ti Conc and 75.2% in HGMS REE-Ti Conc. The contents of allanite and titanite in the biotite concentrate are comparably low at 1.2% and 10.9%.
Modal mineralogy of the acid leaching residue of the slimes by MLA is shown in Table 9. The content of REE mineral allanite is dropped from 1.87% in feed to 0.06% in leach residue or it was almost dissolved into the solution, but titanite is still remained in the solid at a high content of 17.8%. Most remained minerals (>99%) in the residue are silicates including plagioclase 49.5%, titanite 17.8%, quartz 11.8%, K_felspar 10.9%, biotite 7.1% and garnet 1.9%.
3.3.6. REE-Ti Separation
The analyses of process mineralogy (Table 8) present that in bulk REE-Ti concentrates allanite and titanite consist of 70% to 80%. The further investigations in the continuation of this project will focus on the separation of allanite and titanite in the bulk REE-Ti concentrates.
According to the mineralogical studies of leaching residue of the slimes (Table 9) acid leaching with HCl is proved an effective approach to separate allanite and titanite by dissolving allanite into the solution and remaining titanite in the residue. Due to their similar floatability flotation would be challengeable to separate allanite and titanite. But testwork will be carried on to study the possibility such as by using selective collectors (cationic or anionic) and adjusting pH values etc.
4. Conclusions
Mineralogy and beneficiation of the Lamujärvi syenites were studied. In the sample, seven rare earth elements including La, Ce, Pr, Nd, Sm, Gd and Y were found with significant contents over 100 ppm and the total REE (TREE) is 4729 ppm. Meanwhile, the sample contains a high content of TiO2.
A total of 18 minerals were identified by MLA analysis in which 89.5% silicates, 8.6% Fe-oxides, 1.24% phosphates and 0.48% carbonates are contained. Allanite with content of 1.9% is the major REE-containing mineral and has a coarse grain size. Parisite was identified as another REE-containing mineral but at very low concentration and fine grain size. In addition, the sample has high contents of titanite (15.4%), biotite (31.6%) and magnetite (8.2%). Because allanite and titanite have similar physical (gravitational and magnetic) and surface properties, they could be recovered as co-products by gravity and magnetic separations and flotation. Biotite and magnetite could be recovered as by-products by cationic flotation and low intensity magnetic separation methods, respectively.
Beneficiation flowsheet was developed containing sequent grinding, desliming, WLIMS, shaking tabling, flotation and HGMS. The composite REE-Ti concentrate with the grade of REE (La, Ce, Y) 15235 ppm (TREE 23000 ppm) at the recovery of 57.2% and the grade of TiO2 25.5% at the recovery of 54.0% was obtained. In addition, as the by-products, the magnetic concentrate with the grade of FeO 86.6% at the recovery of 45.6% and the biotite concentrate with the grade of MgO 6.6% at the recovery of 51.0% were achieved. The REE in the slimes fraction were efficiently leached (recoveries of 89.3% La and 79.8% Ce) with hydrochloric acid (HCl) at concentration 6 mol/l and temperature 60˚C.
Table 7. Modal mineralogy comparison of feed and REE-Ti flotation concentrate.
Table 8. Contents of biotite allanite and titanite in REE-Ti concentrates and biotite concentrate.
Table 9. Modal mineralogy of the acid leaching residue of the slimes.
Studies of process mineralogy present that allanite and titanite are successfully concentrated and that major silicate minerals biotite, plagioclase and K_felspar are significantly removed by gravity separation, high gradient magnetic separation and flotation processes for REE-Ti. Meanwhile, acid leaching with HCl could be an effective approach to separate allanite and titanite for further treatment of the REE-Ti concentrate.
Acknowledgements
The authors are grateful to the Ministry for Foreign Affairs of Finland for the financial support.
Cite this paper
Xiaosheng Yang,Jukka Laukkanen,Akseli Torppa,Neea Heino, (2016) Mineralogy and Beneficiation of Lamujärvi Syenites. Natural Resources,07,481-493. doi: 10.4236/nr.2016.78041
References
- 1. Al Ani, T. and Sarapää, O. (2013) Geochemistry and Mineral Phases of REE in Jammi Carbonatite Veins and Fenites, Southern End of the Sokli Complex, NE Finland. Geochemistry: Exploration, Environment, Analysis, 13, 217-224.
http://dx.doi.org/10.1144/geochem2011-088 - 2. Vartiainen, H. (1980) The Petrography, Mineralogy and Petrochemistry of the Sokli Carbonatite Massif, Northern Finland. Geological Survey of Finland, Bulletin 313, 126.
- 3. Sarapää, O., et al. (2013) Rare Earth Exploration Potential in Finland. Journal of Geochemical Exploration, 133, 25-41.
http://dx.doi.org/10.1016/j.gexplo.2013.05.003 - 4. Fandrich, R., et al. (2007) Modern SEM-Based Mineral Liberation Analysis. International Journal of Mineral Processing, 84, 310-320.
http://dx.doi.org/10.1016/j.minpro.2006.07.018 - 5. Gu, Y. (2003) Automated Scanning Electron Microscope Based Mineral Liberation Analysis An Introduction to JKMRC/FEI Mineral Liberation Analyser. Journal of Minerals & Materials Characterization & Engineering, 2, 33-41.
http://dx.doi.org/10.4236/jmmce.2003.21003 - 6. Zeh, A. (2004) Crystal Size Distribution (CSD) and Textural Evolution of Accessory Apatite, Titanite and Allanite during Four Stages of Metamorphism: An Example from the Moine Supergroup. Scotland. Journal of Petrology, 45, 2101-2132.
http://dx.doi.org/10.1093/petrology/egh049 - 7. Zaitsev, A.N., et al. (2014) Rare-Earth Elements Minerals in Carbonatites of the Kola Alkaline Province (Northern Fennoscandia). 1st European Rare Earth Resources Conference, Milos Island, 4-7 September 2014, 343-347.
- 8. Arzamastsev, A., et al. (2008) The Khibina and Lovozero Alkaline Massifs: Geology and Unique Mineralization. 33 IGC Excursion No. 47, Apatity, 22 July-2 August 2008, 1-58.
- 9. Sarapää, O. and Sarala, P. (2013) Rare Earth Element and Gold Exploration in Glaciated Terrain—Example from the Mäkärä Area, Northern Finland. Geochemistry: Exploration, Environment, Analysis, 13, 131-143.
http://dx.doi.org/10.1144/geochem2012-136 - 10. Goodenough, K.M., et al. (2016) Europe’s Rare Earth Element Resource Potential: An Overview of REE Metallogenetic Provinces and Their Geodynamic Setting. Ore Geology Reviews, 72, 838-856.
http://dx.doi.org/10.1016/j.oregeorev.2015.09.019







