Journal of Materials Science and Chemical Engineering
Vol.04 No.11(2016), Article ID:72064,8 pages
10.4236/msce.2016.411002
Biosorption of Zn2+ and Ni2+, Cu2+ by Four Kinds of Active Microalgae
Fang Chen1,2, Budan Chen1,2, Guojuan Gan1,3, Lin Zhang1,2, Yan Liang1,3, Sikai Wu1,2*
1Shenzhen Institutes of Advanced Technology, Chinese Academy of Science, Shenzhen, China
2Guangzhou Institute of Advanced Technology, Chinese Academy of Sciences, Guangzhou, China
3Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, China

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 19, 2016; Accepted: November 14, 2016; Published: November 17, 2016
ABSTRACT
To investigate the affinity of the terrestrial microalgae to metal ions (Zn2+, Cu2+ and Ni2+) in aqueous solutions, four kinds of living algae (Oscillatoria, Botryococcus, Scenedesmus and Spirulina platensis Geitl, abbreviated as CZ, PTZ, SZ and LXZ) were chosen to biosorption experiment, and the mechanism of algae biosorption to metal ions were explored by fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). Results show that four kinds of algae have a good affinity for Cu, Zn, the removal rate could reach more than 95%; while for Ni, compared with CZ, SZ and PTZ, the adsorption rate of LXZ is weak. The results of FTIR and SEM experiments revealed that the function groups such as carboxyl, hydroxyl, amino, sulfonic groups and so on are the main groups which played important role in the procedure of absorbing metal ions from water aqueous. Specific algae have different affinity to different metals which will be great helpful in the field of biosorption.
Keywords:
Microalgae, Biosorption, SEM, FTIR

1. Introduction
As we all know, heavy metal pollution has been a global problem, especially in the field of water pollution, which has caused great threaten to human [1] . Heavy metals discharged into the environment from various industries, such as textile, pigments, plastics, mining as well as electroplating, metallurgical processes can be ingested into the human through the chain of food, which may make humans more likely to get cancer, suffer some chronic diseases, incur disability and even cause genetic defect [2] [3] .
Biosorption is a useful and inexpensive process that utilizes inexpensive biomass to sequester poisonous metals and is particularly useful for the elimination of contaminants from industrial effluent [4] [5] . Compared with the conventional methods of removing toxic metals from the industrial wastewater, such as ion change, chemical precipitation, electrolysis and membrane filtration [6] [7] [8] [9] , the biosorption method offer many advantages, for instance, low operating cost, high efficient, selective adsorption, no secondary pollution and being available of large-scale applications, these advantages make it convenient and efficient to clean up heavy metal pollution [10] [11] [12] [13] . Thus, seeking for a new biological and environmental-friendly sorbents has become the focus in the field of wastewater treatment at the present.
Biosorption is a method which sequesters the heavy metal from water by the metablic process or physical-chemical route [14] . At present, most of studies are focused on sorbents like skin of fruits, bacteria, fungus and algaes, while studies on living biological materials are seldom heard [15] [16] [17] [18] . In this study, we have designate four kinds of living algaes (CZ, PTZ, SZ and LXZ) as the sorbents due to they are abundant in source and friendly-environment. What’s more, it will be helpful for us to find out the specificity about different algaes to separate heavy metals out from polluted water. Fourier transform infrared spectra (FTIR) and scanning electron microscope (SEM) were employed to study the mechanism of algaes biosorption to metal ions [19] [20] [21] .
2. Materials and Methods
2.1. Culturing the Algaes
The four puredalgaes were granted by institute of hydrobiology, Chinese academy of Sciences, and cultured in modified aquatic diatoms culture (HB-D1) at room temperature with continuing light intensity of 4000 ± 50 Lx, being standing incubation. The culture HB-D1 is consist of NaNO3, MgSO4∙7H2O, K2HPO4, KH2PO4, CaCl2, NaCl, Na2SiO3 and citrate which are needed during the growth of the four algaes. Enlarged culture has been used to increase the quantity of tested algas [22] .
2.2. Preparation of Metal Solution
A stock solution (1000 mg∙L−1) of metal solution were prepared by dissolving required quantity of corresponding metal chlorides with a small number of concentrated nitric acid, then diluted it to 1 L with distilled water. The initial pH of the uranium working solutions was adjusted by 0.1 M HCl or 0.1 M NaOH.
2.3. Adsorption Experiments by Four Microalgaes
Adsorption studies were carried out at room temperature by batch methods. Known amount of microalgae suspension was added into conical flask, mixed with 50 mL metal solution of required concentration, with the pH around 7.0. The mixture was stirred magnetically at approximately 150 r/min for the time necessary to reach the equilibrium, at the same time, keep continuing light intensity of 4000 ± 50 Lx [23] . After the desired contact time, the mixture was centrifuged for 10min at 3000rpm, and the liquid supernatant was used to analyze the metal concentration by atomic absorption spectrometr (AAS). At the same time, the lower algaes serum had been collected and washed throughly, then freeze dried, powder, for FTIR and SEM experiments.
All experiments were conducted in triplicate. The percentage of metal removal and the biomass sorption capacity were calculated in each case. The metal concentrations were calculated from the calibration curve. The biosorption efficiency (R) was calculated using the following equations [24] :
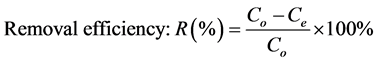
where Co and Ce are the initial and equilibrium metal concentration.
2.4. Fourier Transforms Infrared (FTIR) Spectra and SEM Experiments
Fourier transforms infrared (FTIR) spectra was used to probe the functional groups on the surface of the four algaes [25] [26] . The spectra were recorded with a spectrometer within the range 4000 - 400 cm−1 using a KBr window. The KBr background was automatically subtracted from the sample spectra.
Meanwhile, in order to obtain information about the surface morphologies of SZ, PTZ, CZ and LXZ, we have introduced SEM (Nova NanoSEM 430) at accelerating voltage of 20 kV. Before scanning process, all samples were dried and coated with gold to enhance the electron conductivity. SEM micrographs were taken at similar magnifications 10,000×.
3. Results and Discussion
3.1. Comparative Analysis of Adsorption
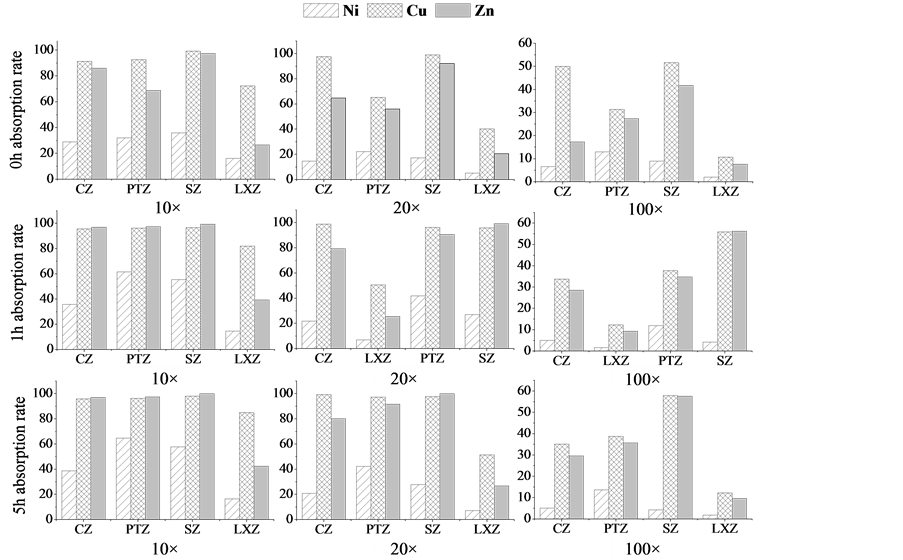
In this part, the initial density of algal cell was 2.45 × 107/ml for the four kinds of algae (CZ, PTZ, SZ and LXZ), then diluted them by 10 times, 20 times and 100 times for study. Here, the diluted algae suspension were abbreviated as 10CZ, 20CZ and 100CZ; 10XPTZ, 20PTZ and 100PTZ; 10SZ, 20SZ and 100SZ; 10LXZ, 20LXZ and 100LXZ. Results are shown in Figure 1.
From the picture, we can see that the four microalgae have a same biosorption behavior that the algae show a high affinity for the three metal ions when in high algae density, and then the removal rate tend to decrease with the decline of algae concentration. Within the first 1h, the removal rate has greatly increased which could be due to the interaction between the metal ions and algae such as coordination complexation, ions-exchange, micro-precipitation [27] . After 1 h, the removal rate tend to be slowly increasing, which could be explained by the gradually saturation of binding sites on the surface of the sorbents and the equilibrium between the metal ions and sorbents [28] .
For CZ, it has been shown in the picture that the algae has a better adsorption capac-
Figure 1. Comparison of the biosorption efficiency of four algae with different concentrations for three metals at 0 h, 1 h, 5 h.
ity of Zn and Cu compared with Ni, the removal rate even could surpass 95%. For PTZ and SZ, same results could be found from the picture, while for LXZ, the algaes has a strong affinity of Cu, and a relative low of Zn, and the efficiency for Ni are pretty low.
Above all, for Zn and Cu, the CZ, SZ, PTZ and LXZ could be chose as sorbents with a removal efficiency more than 90% around the algal cell density of 2.45 × 107/ml, and for Ni, PTZ could be designated as sorbent with a removal efficiency around 50%.
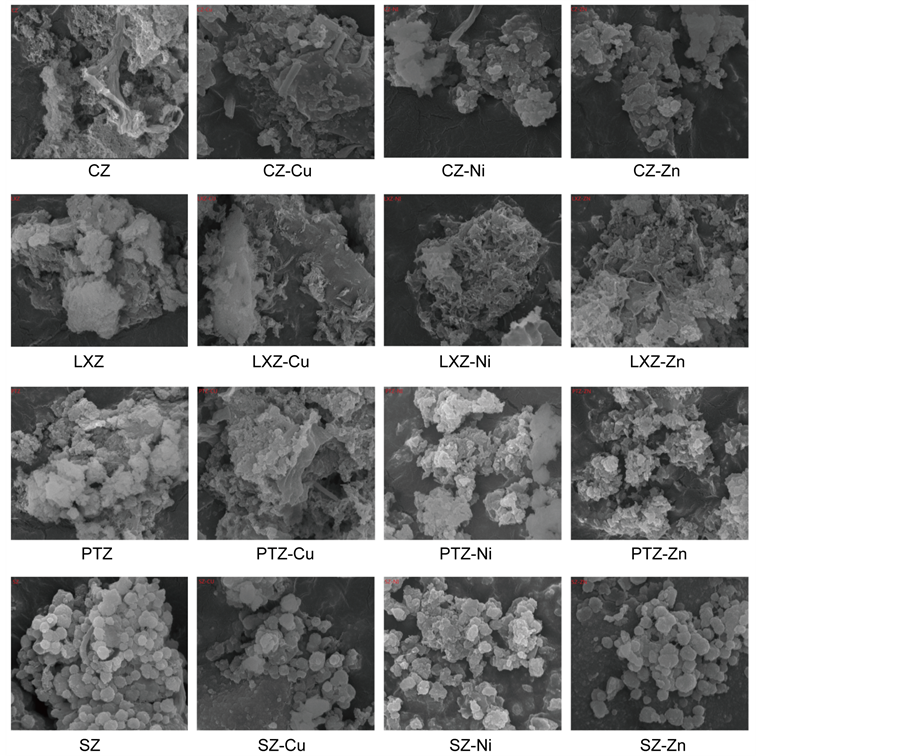
3.2. Specific Results on SEM
To further investigate the adsorption process, the native and metal-loaded algae were observed under the Scanning electron microscopy, and the SEM images of the tested algae were shown in Figure 2. The image of the virgin algae shows a regular symmetry structure in the material (Figure 2). And amounts of small pores can be also seen, which indicates that this material presents good characteristics to be employed as a natural adsorbent for metallic ions uptake, as previously reported, while the surface of metal-loaded biomass appears a rough asymmetry structure. These observations denote that the tested sample especially in the virgin biomass can provide ready access and rich surface area for metal ions onto the binding sites [29] . The same phenomenon could be found in the pictures for the four algaes.
From the above pictures, it is clearly that the cell morphology of the four kinds of algae became disorder after metal-loaded, and metals sedimentation can be found on the surface of the algae caused by the interaction between algae and metal ions. At the
Figure 2. Comparison of the images of four raw algae and metal-loaded algae.
same, the metal had brought toxic effects to the algae cells which led to the algae cell deformation. What’s more, the diameter of particles has expanded significantly.
3.3. Specific Results on FTIR Analysis
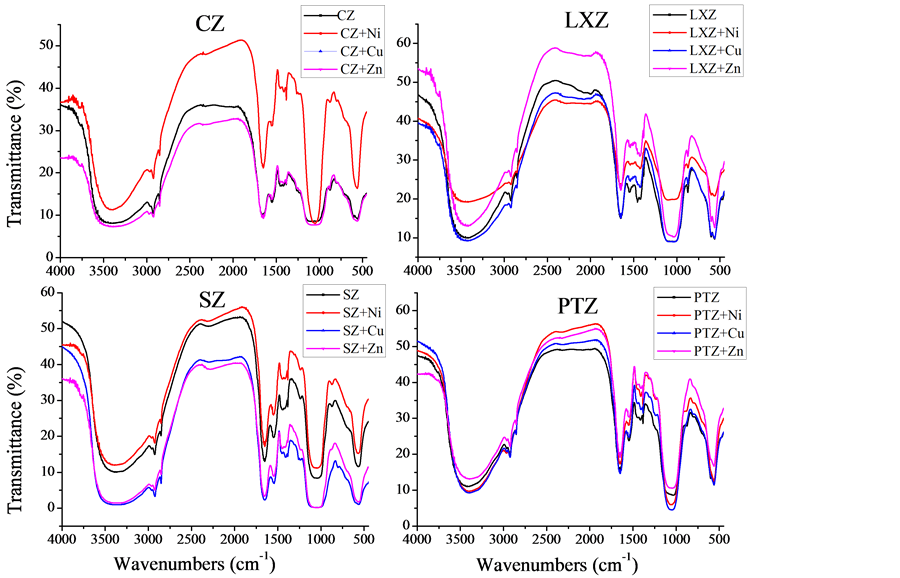
Objectively to investigate the mechanism of the algae absorbing the metals from water aqueous, we have introduced the Fourier transform infrared spectroscopy. Fourier transform spectroscopy was employed to monitor the changes of functional groups on the surface of algae.
The spectrum of the raw algae and metal-loaded algae were in shown in Figure 3. From the picture, we can see that the changes of vibration mode, vibration intensity and vibration position for the four algae could be found easily at 3600 cm−1 to 3400 cm−1, 2800 cm−1, 1700 - 1500 cm−1 and 1400 - 1300 cm−1, which was attributed to the changes of groups on the surface of the algae as their role in the process of absorbing metals. The metal ions were absorbed in the surface of algae by the role of the functions on the surface of the cell such as hydroxyl, amino, carboxyl, phosphate, sulfonic groups and so on.
Figure 3. Comparison of the FTIR spectroscopy of four raw algae and metal-loaded algae.
4. Conclusions
In this study, four kinds of algae have a good affinity for Cu, Zn, the removal rate could reach more than 95%; while for Ni, the adsorption rate is weak for LXZ compared with that of CZ, SZ and PTZ. The selectivity of specific algae having different affinity to different metals will be great helpful in the field of biosorption.
Scanning electron microscopy analysis showed that the surface of the four kinds of algae was distributed by metal sedimentation, indicating the metal being seized by the algae. After metal-loaded, the cell of the algae tends to distort. Combining with result of FIIR spectroscopy, we got the conclusion that the function groups such as carboxyl, hydroxyl, amino, sulfonic groups and so on are the main groups which played important role in the procedure of absorbing metal ions from water aqueous.
Acknowledgements
The work was financially supported by the Shenzhen municipal commission of Development and Reform (Grant No. 20140424111431649) and Guangdong Provincial Department of Science and Technology (Grant No. 2016A020222021, 2013B091300015 and 2013B010404035).
Cite this paper
Chen, F., Chen, B.D., Gan, G.J., Zhang, L., Liang, Y. and Wu, S.K. (2016) Biosorption of Zn2+ and Ni2+, Cu2+ by Four Kinds of Active Microalgae. Journal of Materials Science and Chemical Engineering, 4, 12-19. http://dx.doi.org/10.4236/msce.2016.411002
References
- 1. Ma, X.M., Cui, W.G., Lin, Y.G., Yang, Y.Y., Chen, H.F. and Wang, K.W. (2015) Efficient Biosorption of Lead (II) and Cadmium (II) Ions from Aqueous Solutions by Functionalized Cell with Intracellular CaCO3 Mineral Scaffolds. Bioresource Technology, 185, 70-78.
http://dx.doi.org/10.1016/j.biortech.2015.02.074 - 2. Volesky, B. and Holan, Z.R. (1995) Biosorption of Heavy-Metals. Biotechnology Progress, 11, 235-250.
http://dx.doi.org/10.1021/bp00033a001 - 3. Leusch, A., Holan, Z.R. and Volesky, B. (1995) Biosorption of Heavy-Metals (Cd, Cu, Ni, Pb, Zn) by Chemically-Reinforce Biomass of Marine-Algae. Journal of Chemical Technology and Biotechnology, 62, 279-288.
http://dx.doi.org/10.1002/jctb.280620311 - 4. Li, X.N., Li, A.R., Long, M.Z. and Tian, X.J. (2015) Equilibrium and Kinetic Studies of Copper Biosorption by Dead Ceriporia lacerata Biomass Isolated from the Litter of an Invasive Plant in China. Journal of Environmental Health Science and Engineering, 13, 1-8.
http://dx.doi.org/10.1186/s40201-015-0191-1 - 5. Mishra, A., Dubey, A. and Shinghal, S. (2015) Biosorption of Chromium (VI) from Aqueous Solutions Using Waste Plant Biomass. International Journal of Environmental Science and Technology, 12, 1415-1426.
http://dx.doi.org/10.1007/s13762-014-0516-0 - 6. Huang, S.M. and Lin, G. (2015) Biosorption of Hg (II) and Cu (II) by Biomass of Dried Sargassum fusiforme in Aquatic Solution. Journal of Environmental Health Science and Engineering.
- 7. Kapoor, A. and Viraraghavan, T. (1995) Fungal Biosorption—An Alternative Treatment Option for Heavy Metal Bearing Wastewaters: A Review. Bioresource Technology, 53, 195-206.
- 8. Pan, X.L., Wang, J.L. and Zhang, D.Y. (2005) Biosorption of Pb (II) by Pleurotus ostreatus Immobilized in Calcium Alginate Gel. Process Biochemistry, 40, 2799-2803.
http://dx.doi.org/10.1016/j.procbio.2004.12.007 - 9. Bagherifam, S., Lakzian, A., Ahmadi, S.J., Rahimi, M.F. and Halajnia, A. (2010) Uranium Removal from Aqueous by Wood Powder and Wheat Straw. Journal of Radioanalytical and Nuclear Chemistry, 283, 289-296.
http://dx.doi.org/10.1007/s10967-009-0348-4 - 10. Doke, K.M., Yusufi, M. and Joseph, R.D. (2012) Biosorption of Hexavalent Chromium onto Wood Apple Shell: Equilibrium, Kinetic and Thermodynamic Studies. Desalination and Water Treatment, 50, 170-179.
http://dx.doi.org/10.1080/19443994.2012.708565 - 11. Lezcano, J.M., Gonzalez, F. and Ballester, A. (2010) Biosorption of Cd(II), Cu(II), Ni(II), Pb(II) and Zn(II) Using Different Residual Biomass. Chemistry and Ecology, 26, 1-17.
http://dx.doi.org/10.1080/02757540903468102 - 12. Sari, A., Mendil, D., Tuzen, M. and Mustafa, S. (2008) Biosorption of Cd (II) and Cr (III) from Aqueous Solution by Moss (Hylocomium splendens) Biomass: Equilibrium, Kinetic and Thermodynamic Studies. Chemical Engineering Journal, 144, 1-9.
http://dx.doi.org/10.1016/j.cej.2007.12.020 - 13. Ioannis, A. and George, Z.K. (2015) Progress in Batch Biosorption of Heavy Metals onto Algae. Journal of Molecular Liquids, 209, 77-86.
http://dx.doi.org/10.1016/j.molliq.2015.05.023 - 14. Gadd, G.M. (2008) Biosorption: Critical Review of Scientific Rationale, Environmental Importance and Significance for Pollution Treatment. Journal of Chemical Technology and Biotechnology, 84, 13-28.
- 15. Torab-Mostaedi, M., Asadollahzadeh, M., Hemmati, A. and Khosravi, A. (2013) Equilibrium, Kinetic, and Thermodynamic Studies for Biosorption of Cadmium and Nickel on Grapefruit Peel. Journal of the Taiwan Institute of Chemical Engineers, 44, 295-302.
http://dx.doi.org/10.1016/j.jtice.2012.11.001 - 16. Cerino-Cordova, F.J., Diaz-Flores, P.E. and Garcia-Reyes, R.B. (2013) Biosorption of Cu (II) and Pb (II) from Aqueous Solutions by Chemically Modified Spent Coffee Grains. International Journal of Environmental Science and Technology, 10, 611-622.
http://dx.doi.org/10.1007/s13762-013-0198-z - 17. Gorgievski, M., Bozic, D. and Stankovic, V. (2013) Kinetics, Equilibrium and Mechanism of Cu2+, Ni2+ and Zn2+ Ions Biosorption Using Wheat Straw. Ecological Engineering, 58, 113-22.
http://dx.doi.org/10.1016/j.ecoleng.2013.06.025 - 18. Sari, A. and Tuzen, M. (2008) Biosorption of Cadmium (II) from Aqueous Solution by Red Algae (Ceramium virgatum): Equilibrium, Kinetic and Thermodynamic Studies. Journal of Hazardous Materials, 57, 448-454.
http://dx.doi.org/10.1016/j.jhazmat.2008.01.008 - 19. Zhao, Y.C., Wang, B.L. and Liu, C.Q. (2013) Biosorption of Trace Metals from Aqueous Multimetal Solutions by Green Microalgae. Chinese Journal of Geochemistry, 32, 385-391.
http://dx.doi.org/10.1007/s11631-013-0646-y - 20. Mirghaffari, N., Moeini, E. and Farhadian, O. (2015) Biosorption of Cd and Pb Ions from Aqueous Solutions by Biomass of the Green Microalga, Scenedesmus quadricauda. Journal of Applied Phycology, 27, 311-320.
http://dx.doi.org/10.1007/s10811-014-0345-z - 21. Yasser, H., Amina, R., Abdelbasset, B.D. and Taoufik, B. (2015) Removal of Cadmium (II) from Aqueous Solutions by Biosorption onto the Brown Macroalga. Desalination and Water Treatment, 54, 1663-1673.
- 22. Delosme, R., Olive, J. and Wollman, F.A. (1996) Changes in Light Energy Distribution upon State Transitions: An in Vivo Photoacoustic Study of the Wild Type and Photosynthesis Mutants from Chlamydomonas reinhardtii Photoacoustic Study of the Wild Type and State Transitions. Biochimica et Biophysica Acta, 1273, 150-158.
http://dx.doi.org/10.1016/0005-2728(95)00143-3 - 23. Chen, F., Tan, N., Long, W., Yang, S.K., She, Z.G. and Lin, Y.C. (2014) Enhancement of Uranium (VI) Biosorption by Chemically Modified Marine-Derived Mangrove Endophytic Fungus Fusarium sp #ZZF51. Journal of Radioanalytical and Nuclear Chemistry, 299, 193-201.
http://dx.doi.org/10.1007/s10967-013-2758-6 - 24. Yuvaraja, G., Krishnaiah, N. and Subbaiah, M.V. (2014) Biosorption of Pb (II) from Aqueous Solution by Solanum melongena Leaf Powder as a Low-Cost Biosorbent Prepared from Agricultural Waste. Colloids and Surfaces B: Biointerfaces, 114, 75-81.
http://dx.doi.org/10.1016/j.colsurfb.2013.09.039 - 25. Ngah, W.S.W. and Fatinathan, S. (2009) Pb (II) Biosorption Using Chitosan and Chitosan Derivatives Beads: Equilibrium, Ion Exchange and Mechanism Studies. Journal of Environmental Sciences, 22, 338-346.
http://dx.doi.org/10.1016/S1001-0742(09)60113-3 - 26. Dang, V.B.H., Doan, H.D. and Dang-Vu, T. (2009) Equilibrium and Kinetics of Biosorption of Cadmium (II) and Copper (II) Ions by Wheat Straw. Bioresource Technology, 100, 211-219.
http://dx.doi.org/10.1016/j.biortech.2008.05.03 - 27. Tamilselvan, N., Saurav, K. and Kannabiran, K. (2012) Biosorption of Cr (VI), Cr (III), Pb (II) and Cd (II) from Aqueous Solutions by Sargassum wightii and Caulerpa racemosa Algal Biomass. Journal of Ocean University of China, 11, 52-58.
http://dx.doi.org/10.1007/s11802-012-1843-8 - 28. Chen, Y.W., Hu, J. and Wang, J.L. (2012) Kinetics and Thermodynamics of Cu (II) Biosorption on to a Novel Magnetic Chitosan Composite Bead. Environmental Technology, 33, 2345-2351.
http://dx.doi.org/10.1080/09593330.2012.668940 - 29. Sana, S., Roostaazad, R. and Yaghmaei, S. (2015) Biosorption of Uranium (VI) from Aqueous Solution by Pretreated Aspergillus niger Using Sodium Hydroxide. Iranian Journal of Chemistry and Chemical Engineering, 34, 65-74.