Advances in Materials Physics and Chemistry
Vol.4 No.7(2014), Article
ID:47685,7
pages
DOI:10.4236/ampc.2014.47016
Controllable Hydrothermal Synthesis of Cd2Ge2O6 Nanostructures
Qian Liu, Guanjie He, Kaibing Xu, Junqing Hu*
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai, China
Email: *hu.junqing@dhu.edu.cn
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 27 April 2014; revised 12 June 2014; accepted 30 June 2014
Abstract
Various Cd2Ge2O6 nanostructures, including nanorods, nanoparticles, nanowires and erythrocyte/ flower/disc-like superstructures have been successfully prepared by hydrothermal methods, which are simply tuned by changing the reaction temperature, surfactants, and the molar ratio of Cd and Ge precursors in aqueous solution. These morphologies can be simply controlled by only selecting the reactants and controlling experimental conditions with excellent reproducibility. These studies about the Cd2Ge2O6 nanostructures reveal that temperature is a crucial parameter to tune the morphologies from nanoparticles to nanorods. By adding various surfactants, different nanostructures such as flower/disc-like nanosticks could be obtained. Replacing Cd(CH3COO)2∙ 2H2O with CdO as the precusor results in the formation of ultralong nanowires with CTAB as surfactant. Molar ratio of GeO2 to CdO was demonstrated as an important factor to influence the surface smoothness of nanowires. It is believed that the simple hydrothermal route may be the useful route to synthesize variable germanate nanostructures for various applications.
Keywords
Hydrothermal, Cd2Ge2O6, Nanorods, Nanowires, Superstructures

1. Introduction
Metal germinates have attracted attention due to their applications in catalysis, adsorption, ion exchange, humidity sensors and high energy laser systems [1] -[3] . In recent years, considerable efforts have been devoted to synthesizing germinate nanomaterials, such as CuGeO3 [4] [5] , In2Ge2O7 [6] [7] , Bi2GeO5 [8] , ZnGeO3 [9] -[11] and PbGeO3 [12] . However, reports about the fabrication of the Cd2Ge2O6 nanostructures are still quite rare and the phase and morphology of the nanostructrues are still not well controlled. Thus, developing a simple route to synthesize various phases and shape for Cd2Ge2O6 nanostructures is of fundamental importance. In recent years, hydrothermal synthesis has attracted attention because it can control the shape of materials easily, which is simply processed and in large scale. For example, Liu, et al. [13] reported the synthesis of a family of highly uniform metal germinate nanowires in a hydrazine monohydrate/H2O binary solvent system, which facilitates CO2 photocatalytic reduction into renewable hydrocarbon fuel in the presence of water vapor at room temperature. Huang et al. [14] used hydrothermal route to obtain Cd2Ge2O6 nanorods photocatalyst for environmental purification of benzene in air with molecular oxygen under ambient conditions; Pei et al. [15] [16] synthesized Cd2Ge2O6 nanowires and flower-like Cd2Ge2O6 microstructures in the absence of any surfactants by hydrothermal treatment. Herein, we demonstrate a one-step hydrothermal route to synthesize Cd2Ge2O6 nanostructures with well controlling of their morphologies, including nanorods, nanowires, erythrocyte-like and flower-like microstructures, which are simply tuned by changing the hydrothermal reaction temperature, surfactants, and the molar ratio of Cd and Ge precursors in aqueous solution.
2. Experimental Section
2.1. Synthesis
All of the chemical reagents are analytically pure and used as received without further purification. GeO2, Ethylenediamine (EDA), Polyvinyl Pyrrolidone (PVP), Sodium Dodecyl Benzene Sulfonate (SDBS) and Cetyltrimethyl Ammonium Bromide (CTAB) were purchased from National Chemical Agent. Cd(CH3COO)2·2H2O and CdO were purchased from Aladdin Industry.
2.1.1. GeO2 and Cd(CH3COO)2∙2H2O as the Precursors
In a typical synthesis, 1.5 mmol GeO2 and 3 mmol Cd(CH3COO)2∙2H2O was dissolved completely in 20 mL and 20 mL deionized water, respectively. Then the GeO2 aqueous solution was transferred into a 60 mL Teflon-lined stainless steel autoclave, following dropwise adding of 20 mL Cd(CH3COO)2 aqueous solution. And more deionized water was added to reach 80% fill rate for the autoclave. Hydrothermal treatments were carried out at 180˚C, 160˚C, 140˚C or 120˚C for 24 h and then the autoclave was cooled down to room temperature naturally. White precipitates were collected by centrifugation, and washed with deionized water and ethanol several times to remove impurities. Finally, the precipitates were dried in air at 60˚C for 5 h.
2.1.2. GeO2 and CdO as the Precursors
2 mmol GeO2 was dissolved completely in 20 mL deionized water and then transferred into a 60 mL Teflonlined stainless steel autoclave, following dropwise adding of 20 mL CdO aqueous solution (The molar ratio of GeO2 to CdO is controlled at 1:1 and 2:1). Then 1 mmol CTAB was added into the uniform turbid solution under stirring and the hydrothermal treatment was carried out at 180˚C for 24 h, and then the autoclave was cooled down to room temperature naturally. White precipitates were collected by centrifugation, and washed with deionized water and ethanol several times to remove impurities. Finally, the precipitates were dried in air at 60˚C for 5 h.
2.2. Characterization
The products were characterized by X-ray diffractometer (XRD; Rigaku D/Max-2550 PC) equipped with Cu-Kα Radiation; scanning electron microscope (JEOL, JSM5600 LV) equipped with an X-ray energy dispersive spectrometer (EDS) (Oxford, IE 300 X).
3. Results and Discussion
To study the role of the temperature, we made four different experiments which were carried out at the temperature of 120˚C, 140˚C, 160˚C and 180˚C for 24 h. The microstructure and morphology of the as-prepared products were investigated by SEM, as shown in Figure 1. Figure 1(a) depicts the SEM image of erythrocyte-like Cd2Ge2O6 prepared at 120˚C for 24 h. The SEM image demonstrates that the as-prepared Cd2Ge2O6 are composed of nanoparticles with an average size of about 30 nm. The inset shows the high magnified TEM image of the nanoparticles. At higher preparation temperatures, these small particles grow into rod-shaped Cd2Ge2O6. The
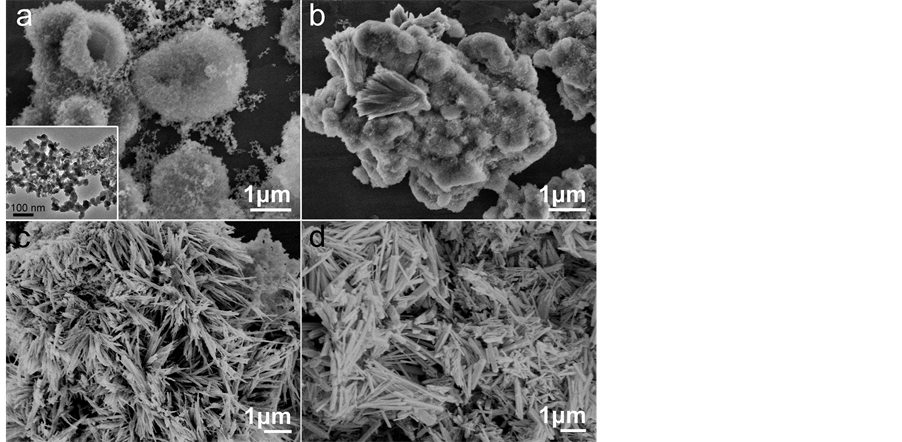
Figure 1. SEM images of the as-synthesized products that were carried out at (a) 120˚C; (b) 140˚C; (c) 160˚C and (d) 180˚C for 24 h. The inset is the high magnified TEM image of the nanoparticles.
product prepared at 140˚C is shown in Figure 1(b), the Cd2Ge2O6 particles getting larger in size and several short nanorods with 70 - 300 nm in width and 1 μm to 2 μm in length can be observed. The Cd2Ge2O6 prepared at 160˚C (Figure 1(c)) contains a large quantity of short nanorods and nanospheres. At the 180˚C, the Cd2Ge2O6 product is completely composed of nanorod-shaped nanostructure with the length ranging from hundreds nanometers to several micrometers, as shown in Figure 1(d). These results indicate that the reaction temperature has an important effect on the morphology of the Cd2Ge2O6 nanostructures. We observed that compared with the Cd2Ge2O6 synthesized at low temperature, the products synthesized at 180˚C were more prone to form short nanorods structures. Therefore, the high temperature leads to the growth of nanorods but not the nanoparticles.
In order to further explore other parameters that might make impacts on the morphology of the products, we examined the role of surfactant in the synthesis of Cd2Ge2O6 nanostructure. 0.5 mmol surfactant was completely dissolved in the GeO2 aqueous solution before following dropwise adding of Cd(CH3COO)2 aqueous solution. The microstructure and morphology of the as-prepared products synthesized at 180˚C for 24 h were investigated by SEM, as shown in Figure 2. The Cd2Ge2O6 disc-like microstructures prepared with EDA (Figure 2(a)) are constructed by massive nano-plates and each plate grows in a radial way from the center. As shown in Figure 2(c), the as-prepared product with the existing of the SDBS is composed of abundant Cd2Ge2O6 flower-like superstructures with a relatively good dispersion. High magnification SEM image reveals that the as-prepared hierarchical microstructures are constructed by many nanoparticles. Compared with above two types of superstructures, Cd2Ge2O6 products synthesized with PVP and CTAB (Figure 2(b) and Figure 2(d)) were completely composed of short nanorods structures. Apparently, Cd2Ge2O6 product prepared with CTAB was more prone to form relative long and uniform nanorods structures.
Different cadmium sources were tested to study their effects on the synthesis. When Cd(CH3COO)2·2H2O was replaced by CdO, the final products were comprised of a large quantity of Cd2Ge2O6ultralong nanowires with a length of 10 - 30 μm. Figure 3(a) and Figure 3(c) reveal the morphology of the as synthesized Cd2Ge2O6 by adding CTAB as surfactant and the reaction was carried out at the molar ratio of GeO2 to CdO of 2:1 or 1:1 at 180˚C for 24 h, respectively. The high-magnification SEM images (Figure 3(b) and Figure 3(d)) show that the diameters of the Cd2Ge2O6 nanowires are 50 - 300 nm. Besides, we noticed that the surface of nanowires carried out at the molar ratio of GeO2 to CdO of 1:1 is not as smooth as the other one made at the molar ratio of 2:1.
XRD was examined to identify the structure for the Cd2Ge2O6 product obtained from the hydrothermal conditions of 180˚C for 24 h using CTAB as surfactant. As Figure 4 shows, according to the standard value [Joint Committee on Powder Diffraction Standards (JCPDS) file card No. 43-0468], all the reflection peaks can be indexed to a pure monoclinic phase of Cd2Ge2O6 and there are no other characteristic peaks from impurities [14]

Figure 2. SEM images of the Cd2Ge2O6 products synthesized at 180˚C for 24 h with (a) EDA; (b) PVP; (c) SDBS and (d) CTAB added as surfactant.
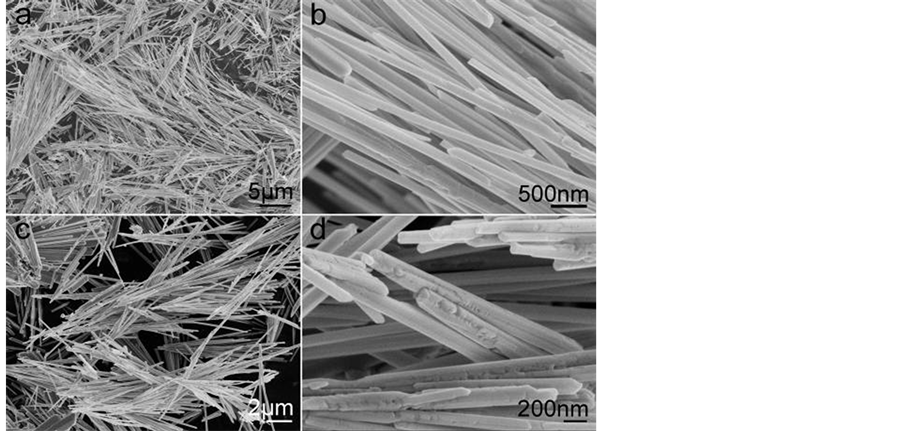
Figure 3. SEM images of the as-synthesized products with the molar ratio of GeO2 to CdO of (a) 2:1 and (b) 1:1. CTAB was added as surfactant.
[17] . The strong and sharp diffraction peaks indicate good crystallinity of the product. Additional evidence of the formation of Cd2Ge2O6 came from the energy dispersion X-ray analysis (EDS). Figure 5(a) shows a SEM image of the Cd2Ge2O6 nanowires synthesized at 180˚C for 24 h with the molar ratio of GeO2 to CdO of 2:1. The section in the yellow rectangle is taken for data collection. Figure 5(b) shows the energy dispersion X-ray spectrum of the as-prepared product. The peaks of Cd, Ge and O are easily found. Quantitative analysis shows that the molar ratio of Cd/Ge/O is 1:0.98:2.58, which is close to the stoichiometry in Cd2Ge2O6.
In the above hydrothemral system, surfactants play a significant role in facilitating the nucleation and growth of various Cd2Ge2O6 nanostructures, including nanorods, nanoparticles, nanowires and erythrocyte/flower/disclike superstructures. At the initial period, H2GeO3 forms from the reaction of GeO2 and H2O. Then spherical nanoparticles spontaneously occur in the supersaturated solution via the hydrothermalreaction between Cd (CH3COO)2 or CdO and H2GeO3. Small nanoparticles may be activated and proceeded to assemble larger nanocrystals by a self assembled growth process so as to minimize the surface energies [18] . Then the spherical na-

Figure 4. XRD pattern of the as-synthesized Cd2Ge2O6 product prepared using GeO2 and CdO as the precursors.
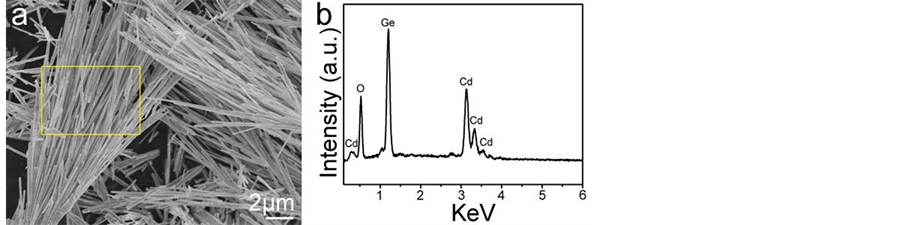
Figure 5. (a) A SEM image of the Cd2Ge2O6 nanowires. The section in the yellow rectangle was taken for data collection; (b) Energy dispersion X-ray spectrum of the as-prepared product.
noparticles serve as the nuclei for the growth of the Cd2Ge2O6 nanocrystals through an “Ostwald ripening” process [19] -[21] . With adding surfactants, such as EDA, PVP, SDBS and CTAB, the Cd2Ge2O6 nanocrystals further grow and finally result in the formation of nanowires and erythrocyte/flower/disc-like superstructures.
4. Conclusion
In summary, we have synthesized various Cd2Ge2O6 nanostructures, including nanorods, nanoparticles, nanowires and erythrocyte/flower/disc-like superstructures which can be achieved by simply tuning the hydrothermal reaction temperature, surfactants, and Cd precursor. These morphologies can be simply controlled by only selecting the reactants and controlling experimental conditions with excellent reproducibility. These studies of the Cd2Ge2O6 nanostructures reveal that temperature is a crucial parameter to tune the morphologies from nanoparticles to nanorods. High temperature would lead to high aspect ratio of nanorods. By adding various surfactants, different nanostructures such as flower/disc-like nanosticks could be obtained. Replacing Cd(CH3COO)2·2H2O with CdO as the precusor results in the formation of ultralong nanowires with CTAB as surfactant. Molar ratio of GeO2 to CdO was demonstrated as an important factor to influence the surface smoothness of nanowires. Since the properties rely on the structure of materials firmly, it is believed that the simple hydrothermal route may be the useful route to synthesize variable germanate nanostructures for various applications.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21171035 and 51302035), the Key Grant Project of Chinese Ministry of Education (Grant No. 313015), the PhD Programs Foundation of the Ministry of Education of China (Grant Nos. 20110075110008 and 20130075 120001), the National 863 Program of China (Grant No. 2013AA031903), the Science and Technology Commission of Shanghai Municipality (Grant No. 13ZR1451200), the Fundamental Research Funds for the Central Universities, the Program Innovative Research Team in University (IRT1221), the Shanghai Leading Academic Discipline Project (Grant No. B603), and the Program of Introducing Talents of Discipline to Universities (No. 111-2-04).
References
- Liu, G.Z., Zheng, S.T. and Yang, G.Y. (2007) In2Ge6O15(OH)2(H2dien): An Open-Framework Indate Germanate with One-Dimensional 12-Ring Channels. Angewandte Chemie-International Edition, 46, 2827-2830. http://dx.doi.org/10.1002/anie.200604921
- Hogan, M.J., Brinkman, A.W. and Hashemi, T. (1998) Humidity-Dependent Impedance in Porous Spinel Nickel Germanate Ceramic. Applied Physics Letters, 72, 3077-3079. http://dx.doi.org/10.1063/1.121546
- Bayya, S.S., Chin, G.D., Sanghera, J.S. and Aggarwal, I.D. (2006) Germanate Glass as a Window for High Energy Laser Systems. Optics Express, 14, 11687-11693. http://dx.doi.org/10.1364/OE.14.011687
- Song, R.Q., Xu, A.W. and Yu, S.H. (2007) Layered Copper Metagermanate Nanobelts: Hydrothermal Synthesis, Structure, and Magnetic Properties. Journal of the American Chemical Society, 129, 4152-4153. http://dx.doi.org/10.1021/ja070536l
- Pei, L.Z., Zhao, H.S., Tan, W., Yu, H.Y., Chen, Y.W., Zhang, Q.F. and Fan, C.G. (2009) Low Temperature Growth and Characterizations of Single Crystalline CuGeO3 Nanowires. CrystEngComm, 11, 1696-1701. http://dx.doi.org/10.1039/b900837n
- Zhan, J.H., Bando, Y., Hu, J.Q., Yin, L.W., Yuan, X.L., Sekiguchi, T. and Golberg, D. (2005) Hollow and Polygonous Microtubes of Monocrystalline Indium Germanate. Angewandte Chemie International Edition, 45, 228-231. http://dx.doi.org/10.1002/anie.200502870
- Yan, C.Y., Zhang, T. and Lee, P.S. (2008) Single Crystalline Semi-Nanotubes of Indium Germanate. Crystal Growth & Design, 8, 3144-3147. http://dx.doi.org/10.1021/cg8004882
- Chen, R.G., Bi, J.H., Wu, L., Li, Z.H. and Fu, X.Z. (2009) Orthorhombic Bi2GeO5 Nanobelts: Synthesis, Characterization, and Photocatalytic Properties. Crystal Growth & Design, 9, 1775-1779. http://dx.doi.org/10.1021/cg800842f
- Liu, Q., Zhou, Y., Kou, J.H., Chen, X.Y., Tian, Z.P., Gao, J., Yan, S.C. and Zou, Z.G. (2010) High-Yield Synthesis of Ultralong and Ultrathin Zn2GeO4 Nanoribbons toward Improved Photocatalytic Reduction of CO2 into Renewable Hydrocarbon Fuel. Journal of the American Chemical Society, 132, 14385-14387. http://dx.doi.org/10.1021/ja1068596
- Yu, L., Zou, R.J., Zhang, Z.Y., Song, G.S., Chen, Z.G., Yang, J.M. and Hu, J.Q. (2011) A Zn2GeO4-Ethylenediamine Hybrid Nanoribbon Membrane as a Recyclable Adsorbent for the Highly Efficient Removal of Heavy Metals from Contaminated Water. Chemical Communications, 47, 10719-10721. http://dx.doi.org/10.1039/c1cc14159g
- Wang, T., Liu, Q., Li, G., Xu, K.B., Zou, R.J. and Hu, J.Q. (2014) Hydrothermal Control Growth of Zn2GeO4-Diethylenetriamine 3D Dumbbell-Like Nanobundles. CrystEngComm, 16, 3222-3227. http://dx.doi.org/10.1039/c3ce41604f
- Wang, N., Ding, J., Li, G.C. and Peng, H.R. (2010) Synthesis and Properties of PbGeO3 Nanostructures. Crystal Research and Technology, 43, 316-320. http://dx.doi.org/10.1002/crat.200900516
- Liu, Q., Zhou, Y., Tu, W.G., Yan, S.C. and Zou, Z.G. (2014) Solution-Chemical Route to Generalized Synthesis of Metal Germanate Nanowires with Room-Temperature, Light-Driven Hydrogenation Activity of CO2 into Renewable Hydrocarbon Fuels. Inorganic Chemistry, 53, 359-364. http://dx.doi.org/10.1021/ic402292a
- Huang, J.H., Ding, K., Wang, X.C. and Fu, X.Z. (2009) Nanostructuring Cadmium Germanate Catalysts for Photocatalytic Oxidation of Benzene at Ambient Conditions. Langmuir, 25, 8313-8319. http://dx.doi.org/10.1021/la9005345
- Pei, L.Z., Yang, Y., Pei, Y.Q. and Ran, S.L. (2011) Synthesis and Microstructural Control of Flower-Like Cadmium Germinate. Materials Characterization, 62, 1029-1035. http://dx.doi.org/10.1016/j.matchar.2011.08.001
- Pei, L.Z., Yang, Y., Pei, Y.Q., Yuan, C.Z., Duan, T.K. and Zhang, Q.F. (2011) Cd2Ge2O6 Nanowires Grown by a Simple Hydrothermal Route. Crystal Research and Technology, 46, 480-484. http://dx.doi.org/10.1002/crat.201100096
- Zhang, L., Cao, X.F., Chen, X.T. and Xue, Z.L. (2011) Fast Preparation and Growth Mechanism of Erythrocyte-Like Cd2Ge2O6 Superstructures via a Microwave-Hydrothermal Process. CrystEngComm, 13, 2464-2471.http://dx.doi.org/10.1039/c0ce00872a
- Zhang, D.F., Sun, L.D., Yin, J.L. and Yan, C.H. (2003) Low-Temperature Fabrication of Highly Crystalline SnO2 Nanorods. Advanced Materials, 15, 1022-1025. http://dx.doi.org/10.1002/adma.200304899
- Burlakov, V.M. (2006) Ostwald Ripening in Rarefied Systems. Physical Review Letter, 97, 155703.http://dx.doi.org/10.1103/PhysRevLett.97.155703
- Zhu, W.C., Zhu, S.L. and Xiang, L. (2009) Successive Effect of Rolling up, Oriented Attachment and Ostwald Ripening on the Hydrothermal Formation of Szaibelyite MgBO2(OH) Nanowhiskers. CrystEngCommun, 11, 1910-1919.http://dx.doi.org/10.1039/b905698j
- Xu, D., Liu, Z.P., Liang, J.B. and Qian, Y.T. (2005) Solvothermal Synthesis of CdS Nanowires in a Mixed Solvent of Ethylenediamine and Dodecanethiol. Journal of Physical Chemistry B, 109, 14344-14349. http://dx.doi.org/10.1021/jp051980i
NOTES

*Corresponding author.

