Open Journal of Molecular and Integrative Physiology
Vol.2 No.1(2012), Article ID:17231,4 pages DOI:10.4236/ojmip.2012.21002
Determinants of airway hyperresponsiveness—Balance of tonic and phasic contractility of airway smooth muscles of lobular bronchioles
![]()
Respiratory Division, Department of Internal Medicine, Itami City Hospital, Hyogo, Japan
Email: in1007@poh.osaka-med.ac.jp
Received 17 September 2011; revised 13 November 2011; accepted 12 December 2011
Keywords: Airway Hyperresponsiveness; Stochastic Model of Airway Smooth Muscle; Ising-Type of Antimagetic Interactions; Percolation Process; Phasic and Tonic Contractions
ABSTRACT
Airway hyperresponsiveness (AHR) is a characteristic feature of asthma, and generally correlates with severity of asthma. Understanding the protection mechanism against excessive airway narrowing and how it breaks down is fundamental to solving the problem of asthma. In this paper we have proposed a stochastic modeling the airway smooth muscle bundle for reproducing AHR such as an increased sensitivity of the airways to an inhaled constrictor agonist, a steeper slope of the dose-response curve, and a greater maximal response to agonist. A large number N of contractile muscle cells was assumed to repeat themselves in between contraction and relaxation asynchronously. Dynamic equilibrium of statistic physics was applied to the system of ASM bundle. Thus, the relation of dose to response of a piece of ASM bundle was described by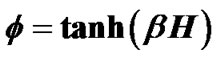 , where β was Boltzman factor and H represented energy of contraction induced by constrictor agents. Each of adjacent pair contractile cells was assumed to have Ising-type of antimagnetic interactions of preference energy J (for the condition of contraction-relaxation) between them. A motion equation for a piece of ASM bundle was described by
, where β was Boltzman factor and H represented energy of contraction induced by constrictor agents. Each of adjacent pair contractile cells was assumed to have Ising-type of antimagnetic interactions of preference energy J (for the condition of contraction-relaxation) between them. A motion equation for a piece of ASM bundle was described by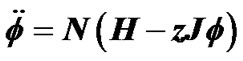 , which explained existence of combined tonic and phasic contractions. Based on observations of Venegas et al. [4], airway responsiveness was assumed to be assessable by total volume of the ventilation defects (TVD) of 13NN PET-CT images. Interactions via propagation of Ca ion waves between ASM bundles would cause perco- lation probability by
, which explained existence of combined tonic and phasic contractions. Based on observations of Venegas et al. [4], airway responsiveness was assumed to be assessable by total volume of the ventilation defects (TVD) of 13NN PET-CT images. Interactions via propagation of Ca ion waves between ASM bundles would cause perco- lation probability by  along the tree, then the relation of dose (βH) to TVD was described by
along the tree, then the relation of dose (βH) to TVD was described by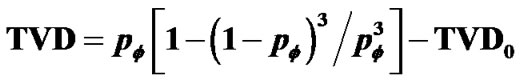 . TVD0 represented the protection mechanism against excessive airway narrowing, which was determined by the ratio of amplitudes between tonic and phasic contractions, thus the balance of amplitudes between tonic and phasic contractions of peripheral lobular smooth muscles would be the determinant of AHR.
. TVD0 represented the protection mechanism against excessive airway narrowing, which was determined by the ratio of amplitudes between tonic and phasic contractions, thus the balance of amplitudes between tonic and phasic contractions of peripheral lobular smooth muscles would be the determinant of AHR.
1. INTRODUCTION
The initial observation that bronchoconstriction occurs more rapidly in asthmatics when compared to non-athmathics after exposure to a constrictor agonist was made in 1921 [1]. Tiffeneau and Beauvallet were the first to describe the use of acetylcholine inhalation tests to determine the degree of airway responsiveness in asthmatic patients in 1945 [2]. Now the methods for measuring airway hyperresponsiveness (AHR) have been standardized and are widely accepted [3].
AHR can be demonstrated in almost all patients with current symptomatic asthma. Using the standardized method, asthmatic subjects generally have a provocation concentration of methacholine or histamine causing a 20% fall in the forced expiratory volume in one second (FEV1.0) PC20 of <8 mg/mL. Most non-asthmatic subjects will have a PC20 of >16 mg/mL. The severity of AHR generally correlates with the severity of asthma. Many different factors have been suggested to be involved in causing AHR.
AHR is complex in its mechanisms. One component of AHR is the acute transient and reversible AHR, which is closely associated with effects of acute airway inflammation. Although the link to airway inflammation is strong, the precise mechanism between airway inflamemation and AHR are not entirely clear. The second, more persistent component of AHR appears related to the chronicity of the disease and likely the chronic effects of airway inflammation leading to multitude of structural airway changes known as airway remodeling.
There is currently a major controversy among the molecular and cellular biologists and experts in the lung mechanics. The experts in lung mechanics feel that the disease will be not controlled until we understand the mechanics of excessive airway narrowing that characterizes asthma. From the point of view of a scientist trying to understand the reasons of abnormal airway narrowing in asthma, it is striking that airway smooth muscle (ASM) is capable of shortening to the extent that all airways within the lung could be completely closed. However, in the normal lungs the degree of narrowing cannot be provoked. Normal lungs are protected against excessive airway narrowing. A plateau develops on the bronchial dose response curve so that even an increase in dose of smooth muscle agonists of several orders of magnitude fails to produce an increased response. This protective mechanism is lost in asthma.
Venegas and his colleagues demonstrated the functional images of airflow obstruction as the ventilation defects (VD) during the test of AHR, which would reflect uneven constrictions of lobular bronchioles [4]. Brown and Mitzner suggested the plateau of the doseresponse curve reflects uneven or limited aerosol delivery to the airways [5]. Another possibility is that mechanisms exist that act to limit the extent of muscle shortening in the health breathing lung, whereas these mechanisms become compromised in asthmatic lung. In this study, we have proposed another mechanism by a stochastic dynamic model of ASM to explain the protection mechanism against excessive airway narrowing in healthy lung and lost in asthmatic lung as well.
2. MODELING OF AIRWAY SMOOTH MUSCLE BUNDLE
2.1. Statistic Physics of Airway Smooth Muscle (ASM) Contractions
A large number of ASM cells compose the bronchial bundle (the ASM bundle) as a major contractile component of the bronchial tree. A piece of the ASM bundle with the degree of contraction f was assumed to be composed of N cells which consist of m cells in contraction and n cells in relaxation (N = n + m) , then two relations between the degree of contraction and the number of smooth muscles in contraction or relaxation were described as follows,
 (1)
(1)
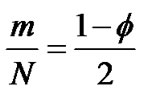 (2)
(2)
Each ASM cell was assumed to repeat itself asynchronously in between relaxation (σ = –1) and contraction (σ = +1). According to statistic physics of equilibrium [6], we would be able to find a ASM cell in the condition of σ by probability P(σ) as follows,
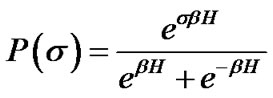 (3)
(3)
where H was the field energy inducing contraction of f, and β was Boltzman’s thermal factor. Thus, for the ASM bundle with the degree of contraction f and the field energy H, we obtained the equation as follows (Figure 1),
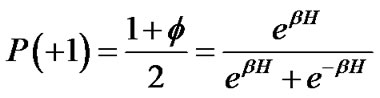
or
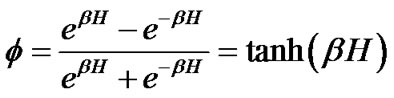 (4)
(4)
2.2. Ising-Type of Antimagnetic Interactions between Adjacent Pair Cells
Adjacent pair cells were assumed to have an energetic preference J to be in the different conditions (σi, σj) = (+1, –1) or = (–1, +1) (we called this Ising-type of antimagnetism interactions). The internal energy U of the piece of ASM bundle was able to be defined as follows,
 (5)
(5)
where S of 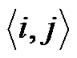 indicated summation of adjacent pair cells across the whole piece of ASM bundle. If each ASM cell repeated itself asynchronously in between relaxation and contraction even in the piece of ASM bundle with the degree of contraction f, we would be able to describe the internal energy
indicated summation of adjacent pair cells across the whole piece of ASM bundle. If each ASM cell repeated itself asynchronously in between relaxation and contraction even in the piece of ASM bundle with the degree of contraction f, we would be able to describe the internal energy  consisting of adjacent pair cells i and j in the probability of
consisting of adjacent pair cells i and j in the probability of 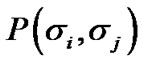 as
as

Figure 1. Standardized dose-response curve of ASM bundle.
follows,
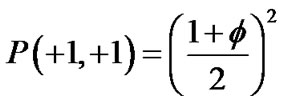 ,
, 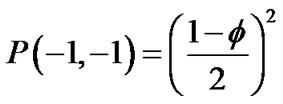
and

Then,
 (6)
(6)
If each cell was surrounded by z adjacent cells, the internal energy of the piece of ASM bundle with the degree of contraction f was able to be described by the following U(f),
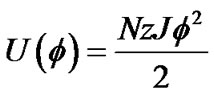 (7)
(7)
Thus, the total energy E of this piece of ASM bundle with the degree of contraction f under the contraction energy H was defined by the following equation,
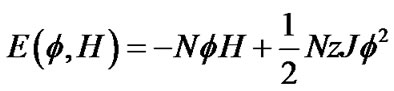 (8)
(8)
2.3. Percolation Probability along Bronchial Tree
Inhaled constricting agonists such as methacholine or histamine would induce local tonic contractions of ASM, which would proceed to bronchoconstriction as a whole. Through asynchronous calcium ion waves among ASM cells local tonic contractions of ASM bundle would propagate to both peripheral and central parts of bundle along the binary branching tree in a stochastic manner (Figure 2). Thus, when the probability of propagation at the bifurcation (the site probability) was assumed to be equal to p in the whole branching tree, it was possible to describe the percolation probability of tonus along the bronchial tree θ(p) as follows [8],
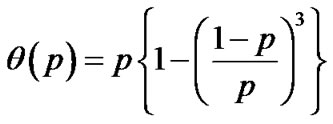 (9)
(9)
3. RESULTS
3.1. Combined Phasic and Tonic Contractions of the ASM Bundle
The differential equation for dynamics of ASM was obtained by use of Eq.8,
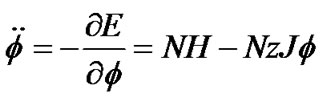 or
or
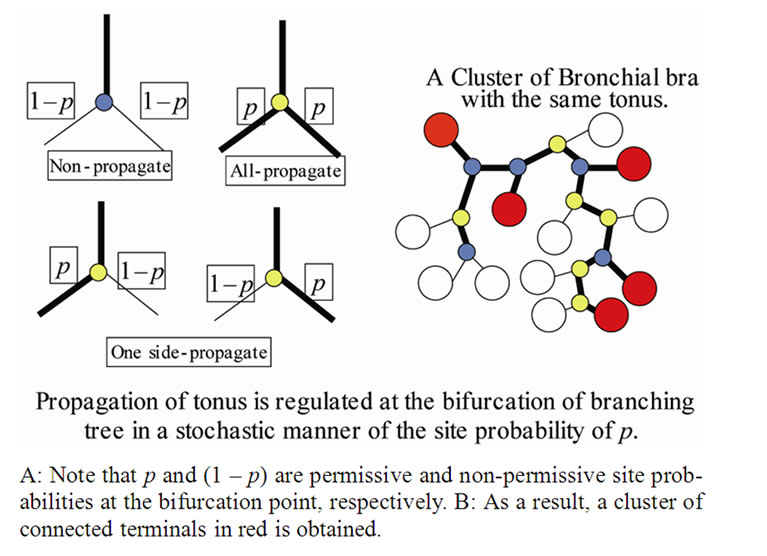
Figure 2. A stochastic process model of propagation of muscle tonus along a binary tree.
 (10a)
(10a)
If the field potential H is zero, motions of ASM are oscillations (the phasic contractions with frequency of ω = ). In general, dynamics of ASM bundles should be described by the combination of phasic and tonic contractions as follows,
). In general, dynamics of ASM bundles should be described by the combination of phasic and tonic contractions as follows,
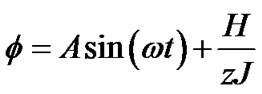 (10b)
(10b)
Tonic contractions are cancelled when the amplitude of A is larger than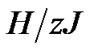 , and phasic ones are visible. Tonic contractions will be observed when A is less than
, and phasic ones are visible. Tonic contractions will be observed when A is less than 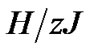 (Figure 3).
(Figure 3).
3.2. Constriction of Bronchial Tree as a Percolation of Tonic Contractions
If ASM bundles in adjacent pair branches appeared in the same contracted condition by the probability of P(+1, +1), the site probability of p was assumed to be P(+1, +1)
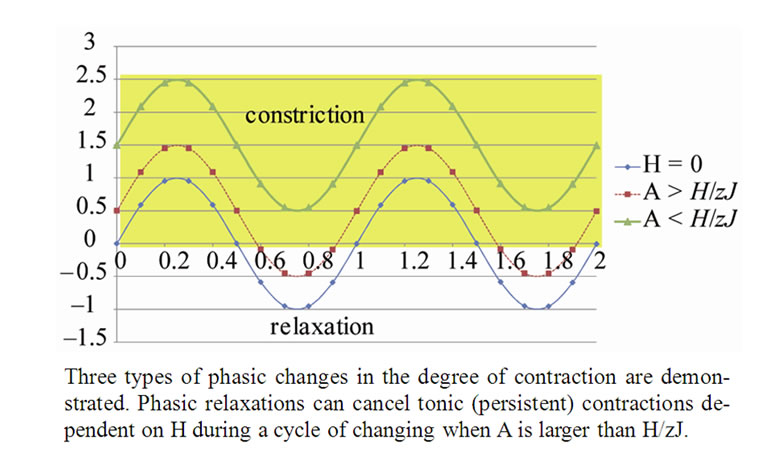
Figure 3. Balance of Combined Tonic and Phasic changes in the degree of contraction of ASM bundle at the Lobular bronchiole.
as follows (Figure 4),
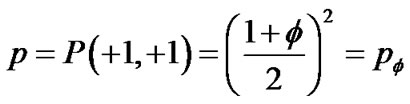 (11)
(11)
Thus, based on Eq.9 and Eq.11 tonic contractions of the ASM bundle in the whole bronchial tree was described by the percolation probability 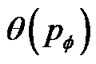 as follows,
as follows,
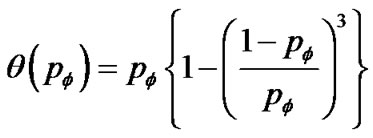 (12)
(12)
3.3. Determinants of Airway Hyperresponsiveness
Test of airway hyperresponsiveness (AHR) is designed for assessing the degree of tonic bronchoconstriction induced by inhaled methacholine or histamine. Bronchoconstriction is assessed by decrease in forced expiratory volume in one second (FEV1.0). Recent functional images of asthma by Venegas and his colleagues showed the ventilation defects (VD) as peripheral bronchiolar obstructions [4]. Total volume of VD (TVD) was proportional to decrease in FEV10, therefore the degree of bronchoconstriction was proportional to TVD. If TVD was caused by peripheral bronchiolar obstructions induced by inhaled constricting agonists, TVD would be expressed by the percolation probability 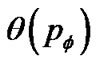 as follows,
as follows,
 (13)
(13)
The site probability 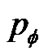 was described by the equation from Eq.4 as follows,
was described by the equation from Eq.4 as follows,
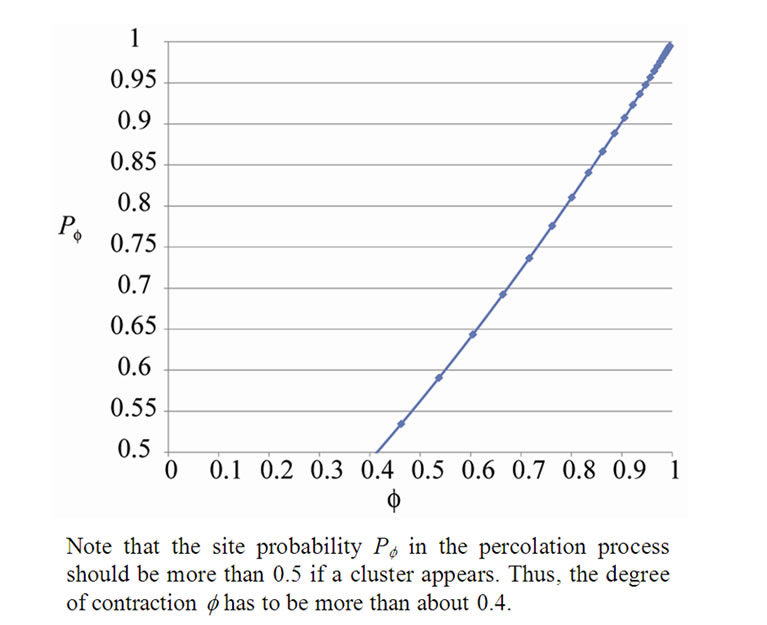
Figure 4. The relation of the degree of ASM contraction to the site probability.
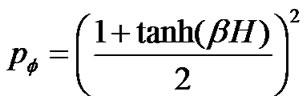 (14)
(14)
If the maximal degree of bronchoconstriction was measured by 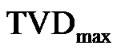 during the provocation test, the parameter TVD0 = 1 – TVDmax indicated the protection mechanisms of excessive airway narrowing. Thus, the test of provocation was described by Eqs.15a, b and c as follows,
during the provocation test, the parameter TVD0 = 1 – TVDmax indicated the protection mechanisms of excessive airway narrowing. Thus, the test of provocation was described by Eqs.15a, b and c as follows,
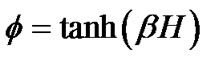 (15a)
(15a)
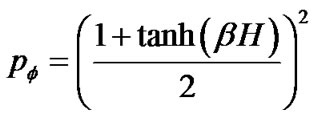 (15b)
(15b)
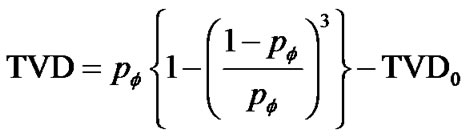 (15c)
(15c)
We computed f, pf, and TVD (at TVDmax of 0.2, 0.4, 0.7, 1.0) in the domain of between βH = 0 and βH = 3.0 according to Eqs.15a, b and c, and demonstrated the relationships between βH and TVD in Figure 5. Simulated these curves reproduced clearly properties seen in the bronchial dose response curves of the change in FEV1.0 vs baseline induced by increasing dose of a bronchoconstrictor stimulus (methacholine) in patients with mild, moderate and severe asthmatics vs healthy individuals [9].
4. DISCUSSION
Previously reported studies on modeling dynamics of the airway smooth muscles (ASM) have been concentrated on changes in dynamic properties of the sub-cellular
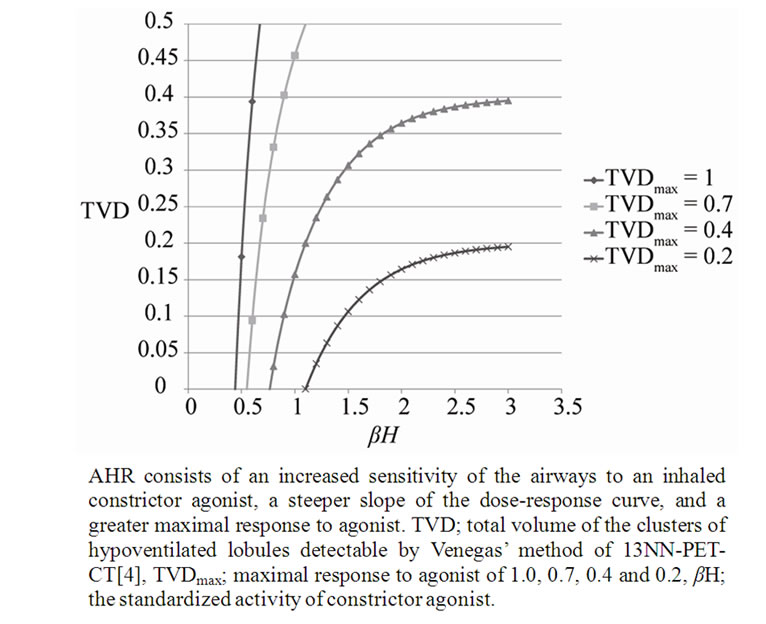
Figure 5. Dose-response curves simulating Airway Hyperresponsiveness (AHR).
molecular elements such as the acto-myosin cross links or the cytoskeleton [10,12]. Many of the biophysical phenomena were observed from the strip of ASM tissue which is composed of a large number of cells. All of the biophysical properties of ASM, such as active force generation and shortening velocity, are based on data from experiments on various strips of ASM tissues. However, no discussion has thought of the multicellularity of the experimentally prepared materials until today. What we proposed in this study was the modeling based on the multicellularity of airway smooth muscle bundle, thus we were able to take account of using the concept of statistic physics on this ASM system (see Eq.4).
There is a well-known statistic physical model of Ising for describing ferromagnetism of materials [6,7]. Ising model in this study was assumed to have antiferromagnetic type of interactions among contractile cells (i.e., the energy preference J was assumed in adjacent pair cells in different conditions such as contraction-relaxation). Then, the model for the ASM bundle we proposed revealed the existence of oscillations or phasic contractions. Recent studies on fetal lung explants have showed that airway peristalsis (AP) during morphogenesis, which appears in the first trimester of pregnancy and then increases in frequency towards birth (~1.0 cycle/min), is the spontaneous phasic propagation of waves of ASM contractility [12-15]. The existence of AP in fetal lungs would support the assumption of Ising-type of antiferromagnetic interactions among muscle cells.
In the healthy intact dog, ASM possesses sufficient force generating capacity to close all airways. But healthy animals or humans are challenged with inhaled contractile agonists in concentrations thought to be sufficient to activate the muscle maximally, resulting airway narrowing limited in extent. Brown and Mitzner [5] have suggested that the plateau of the dose-response curves reflects uneven or limited aerosol delivery to the airways. Venegas and colleagues [4] also demonstrated appearing of clusters of poorly ventilated pulmonary lobules (the ventilation defects, VD) in asthmatics at the induced airflow obstruction. The existence of VD suggested uneven tonic constriction of lobular bronchioles induced during airflow obstruction. However, we took another possibility of understanding the uneven bronchiolar constrictions. Based on the recent studies of calcium ion waves among muscle cells, we applied the stochastic process of transmission of ion waves along ASM cells to explain uneven constrictions of peripheral bronchioles. As the bronchial tree is a binary branching tree, the percolation probability 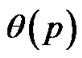 in Eq.9 is obtained logically and was used for explaining the unevenness of bronchiolar contractions. Bronchial thermoplasty represents an exciting new therapy for refractory asthma [16]. However, the mechanism for its effects is unclear. From our study, we propose the hypothesis that bronchial thermoplasty achieves its distal airway effects by change in the percolation probability. But, now we have no data about the site probability p. Then, in this study we proposed to take the probability of adjacent branches in same tonic constrictions of Eq.11 as p.
in Eq.9 is obtained logically and was used for explaining the unevenness of bronchiolar contractions. Bronchial thermoplasty represents an exciting new therapy for refractory asthma [16]. However, the mechanism for its effects is unclear. From our study, we propose the hypothesis that bronchial thermoplasty achieves its distal airway effects by change in the percolation probability. But, now we have no data about the site probability p. Then, in this study we proposed to take the probability of adjacent branches in same tonic constrictions of Eq.11 as p.
As described above, healthy animals or humans are challenged with inhaled contractile agonists in concentrations thought to be sufficient to activate the muscle maximally, resulting airway narrowing limited in extent. This is called the protection mechanism against excessive airway narrowing. Professor Peter Macklem [17] said “Understanding the protective mechanism and how it breaks down is, from the point of view of the physiologist, absolutely fundamental to solving the problem of asthma.” Many extramuscular factors have been proposed such as the cartilage of the large bronchi, the elasticity of the airway wall, elastic tethering forces conferred by contractile cells in the lung parenchyma [18,19], mechanical coupling of airway to the parenchyma by the peribronchial adventitia, and buckling of the airway epithelium and submucosa [20-22], but no one can explain sufficiently the protection mechanism against the excessive airway narrowing. According to Eq.10b, phasic relaxations of ASM at the lobular bronchioles are able to cancel tonic constrictions induced by a field energy of H when the amplitude A is larger than  as shown in Figure 3, which would act as disappearance of VD. If increase in VD indicates the excessive airway narrowing, this mechanism of cancelling of VD would be the protection mechanism against the excessive airway narrowing. Thus, in this study we have proposed that the phasic change in tonus of ASM bundles over tonic contractions at the peripheral bronchioles is the major role of protection against excessive narrowing of bronchioles. The potential H would be composed of many extramuscular factors including airway inflammation. Although the determinants of amplitude A are unknown at all, it is necessary to describe in detail the determinants of parameter A for understanding the pathophysiology of chronic persistent asthma.
as shown in Figure 3, which would act as disappearance of VD. If increase in VD indicates the excessive airway narrowing, this mechanism of cancelling of VD would be the protection mechanism against the excessive airway narrowing. Thus, in this study we have proposed that the phasic change in tonus of ASM bundles over tonic contractions at the peripheral bronchioles is the major role of protection against excessive narrowing of bronchioles. The potential H would be composed of many extramuscular factors including airway inflammation. Although the determinants of amplitude A are unknown at all, it is necessary to describe in detail the determinants of parameter A for understanding the pathophysiology of chronic persistent asthma.
AHR has been known to consist of the sensitivity and the reactivity [9]. The sensitivity is determined by the initial dose of inhaled methacholine or histamine for induction of bronchoconstriction, and the reactivity is the relative increase in the degree of bronchoconstriction at inhalations of higher incremental dose. The relationships between TVD and βH in Figure 4 showed correlated increases in the sensitivity and the reactivity among asthmatics, and the difference between asthmatics and normal lungs as well. These were explained by the difference in TVD0 which was determined by the ratio of H to A. Therefore, the conclusion of this study is that AHR are dependent upon the protection mechanism against the excessive airway narrowing, which is determined by balance of the phasic and tonic contraction-relaxation properties of ASM bundle under the level of potential energy consisting of many extramascluar factors including airway inflammation.
REFERENCES
- Alexander, H.I. and Paddock, R. (1921) Bronchial asthma: Response to pilocarpine and epinephrine. Archives of Internal Medicine, 27, 184-191. doi:10.1001/archinte.1921.00100080047004
- Tiffeneau, R. and Beauvallet, R. (1954) Epreuve de bronchoconstriction et de bronchodilation par aerosols. Bulletin de l’Académie de Médecine, 129, 165-168.
- ATS Board of Directors (2000) Guidelines for methacholine and exercise challenge testing-1999. American Journal of Respiratory and Critical Care Medicine, 161, 309- 329.
- Venegas, J.G., Winkler, T., Musch, G., Vidal-Melo, M.F., Layfield, D., Tgavalekos, N., Fischman, A.J., Callahan, R.J., Bellani, G. and Harris, R.S. (2005) Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature, 434, 777-782. doi:10.1038/nature03490
- Brown, R.H. and Mitzner, W. (1998) The myth of maximal airway responsiveness in vivo. Journal of Applied Physiology, 85, 2012-2017.
- Kittel, C. and Kroemer, H. (1980) Thermal Physics. 2nd Edition, Freeman, W.H. and Co., New York.
- Usui, T. (1990) Thermodynamics and statistical physics. Maruzen, Tokyo.
- Stauffer (1994) Introduction to Percolation Theory. 2nd Edition, UK/USA, 1994 (Translated in Japanese 2001, 48-62).
- O’Byrne, P.M. and Inman, M.D. (2003) Airway hyperresponsiveness. Chest, 123, 411S-416S. doi:10.1378/chest.123.3_suppl.411S
- Small, J.V. (1995) Structure-function relationships in smooth muscle: The missing links. Bioessays, 17, 785- 792. doi:10.1002/bies.950170908
- Small, J.V. and Gimona, M. (1998) The cytoskeleton of the vertebrate smooth muscle cell. Acta Physiologica Scandinavica, 164, 341-348. doi:10.1046/j.1365-201X.1998.00441.x
- McCray, P.B. Jr. (1993) Spontaneous contractility of human featal airway smooth muscle. American Journal of Respiratory Cell Molecular Biology, 8, 573-580.
- Schittny, J.C., Miserocchi, G. and Sparrow, M.P. (2000) Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. American Journal of Respiratory Cell Molecular Biology, 23, 11-18.
- Parvez, O., Voss, A.M., De Kok, M., et al. (2006) Bronchial muscle peristaltic activity in the fetal rat. Pediatric Research, 59, 756-761. doi:10.1203/01.pdr.0000219121.15634.d1
- Pandya, H.C., Innes, J., Hodge, T., et al. (2006) Spontaneous contraction of pseudoglandular-stage human airspaces is associated with presence of smooth muscle-α-acting and smooth muscle-specific myosin heavy chain in recently differentiated fetal human airway smooth muscle. Biology of the Neonate, 89, 211-219. doi:10.1159/000089797
- Cox, G., Thomson, N.C., Rubin, A.S., et al. (2007) Asthma control during the year after bronchial thermoplasty. The New England Journal of Medicine, 356, 1327- 1337. doi:10.1056/NEJMoa064707
- Macklem, P.T. (1998) The Mechanics of Breathing. American Journal of Respiratory and Critical Care Medicine, 157, S88-S94.
- Nagase, T., Moretto, A. and Ludwig, M.S. (1994) Airway and tissue behaviour during induced constriction in rats: Intravenous vs aerosol administration. Journal of Applied Physiology, 76, 830-838.
- Romero, P. and Ludwig, M.S. (1991) Maximal metacholine-induced constriction in rabbit lungs: Interactions between airways and tissues? Journal of Applied Physiology, 70, 1044-1050.
- Ding, D.J., Martin, J.G. and Macklem, P.T. (1987), Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. Journal of Applied Physiology, 62, 1324-1330.

