Open Journal of Molecular and Integrative Physiology
Vol.1 No.3(2011), Article ID:8344,7 pages DOI:10.4236/ojmip.2011.13006
Heart and ventilatory measures in crayfish during copulation
Department of Biology, University of Kentucky, Lexington, USA.
E-mail: rlcoop1@email.uky.edu
Received 23 August 2011; revised 25 September 2011; accepted 5 October 2011.
Keywords: Crayfish; Copulation; Heart Rate; Autonomic
ABSTRACT
Monitoring heart rate (HR) and ventilatory rate (VR) during defined sensory stimuli and during aggressive and submissive social interactions has provided additional information of a crayfish’s physiological state which is not achieved by behavioral observations. In this study, the HR and VR of crayfish were monitored before, during and after the act of copulation in both heterosexual partners. The female crayfish abruptly reduces HR and VR during copulation but the male maintains HR and VR. After separation from copulation the female HR and VR are elevated, potentially paying back the O2 debt. The tight relationship with HR and VR in direction of change indicates a potential neural coupling. These physiological changes in cardiac and respiratory systems suggest an autonomic-like regulation of HR and VR. How these neuronal functions are driven and regulated remains to be determined. Olfactory cues from the female to the male during copulation may be reduced by the reduction in VR in the female. These studies offer experimental paradigms for future neuronal and pharmacological investigations into autonomic regulation of HR and VR as well as the neural circuitry involved.
1. INTRODUCTION
It is well known that physiological assessment of an animal’s state of being allows greater insights into the functional aspect of the animal than purely behavioral observations. This is particular the case for autonomic regulation where the animal does not have to cognitively control the system. A means in which to easily and readily measure an aspect of the autonomic system in relation to respiration and cardiac function is to directly measure ventilation rate (VR) and heart rate (HR) [1]. Earlier studies using crayfish had demonstrated that VR and HR rapidly change (<3 sec) during an environmental alteration such as a pebble drop in a small holding aquaria when the animal is quiescent [2]. In addition, it was shown that a crayfish during social interactions which would be scored by observing behaviors as nonresponsive to aggressive posturing by an opponent indeed were very responsive in physiological terms with dramatic increases in HR and VR [2-4]. The cardiac and respiratory systems are ones that have evolved to serve extremely important function for survival of a species that experiences predation, so it is expected that such rapid responses would be observed in crayfish [1].
There are relatively few investigations into whole animal autonomic function in invertebrates in spite of very early studies dating back to 1927 in arthropods in general [5-7]. Later studies focused on particular neuronal paths of regulation in cardiac and ventilatory function in crustaceans [8-13] and in relation to whole animal function [1,14-18]. There are some studies where HR and VR are used specifically in terms of a bioindex for correlating autonomic function during social interactions or environmental changes in crustaceans [2-4, 19-26]. Here we extend this field of investigation by examining the effects on HR and VR in crayfish during the voluntary act of copulation for both males and females.
As for mammals [27,28], one would expected some degree of autonomic regulation in crustaceans in regards to HR and VR for males and females during the act of copulation. Observations which occurred serendipitously by mistakenly wiring a female crayfish for accessing male-male social interactions in a previous study [2] revealed unexpected results. We therefore chose to further address the regulation of HR and VR in males and females during mating, however we have found it extremely a rare occurrence for copulation to occur in contained environments during observation while both partners were wired for monitoring HR and VR. In recording from over 50 different pairings for numerous hours we were able to obtain five cases, over a span of 7 years, in which HR and VR were monitored during the act of copulation.
These studies are significant in knowing the ability how an individual alters physiological functions to differences in autonomic regulation of voluntary acts such as during reproduction of a species when visual behavioral observations are misleading for such internal physiological alterations.
Portions of this study were previously presented only in abstract form [29,30].
2. MATERIALS AND METHODS
2.1. Animals
Procambarus clarkii (10 - 12 cm body length) were obtained from a commercial supplier, Atchafalaya Biological Supply Co. (Raceland, LA). The animals were housed in an aquatic facility within our regulated-temperature laboratory (19˚C - 20˚C). They were kept in individual tanks and fed fish food pellets weekly until the time of experimentation.
2.2. Recording Procedures
Similar methods were used as previously described [2-4]. In brief, two insulated iridium/platinum wires (diameter 0.005 inches and with the coating 0.008 inches) or stainless steel wires were used (A-M systems, Inc., Carlsburg, WA). The four leads were thread into a larger diameter plastic tubing so that the crayfish would not expose the wire when chewing on the leads. This was necessary when holding animals for several days as to accommodate the animals to the recording leads glued on their carapace (Figure 1(A)). Wires were glued by cyanoacrylate ester and accelerator (HobbyTown USA, Lexington, KY). The use of this rapid drying glue reduced handling stress of the animals which is known to have an effect on HR [4,31]. The placement of the recording leads for obtaining the VR and HR are as reported earlier [2-4] and has been shown in video format that is freely accessible on the Internet [19].
Impedance detectors (UFI, model 2991) were used, which allowed HR and VR to be monitored as a measure of dynamic resistance. These signals were recorded to a computer via a Powerlab/4s interface (ADInstruments, Australia). All events were measured and calibrated with the Chart software (version 7, ADInstruments, Australia) with an acquisition rate set at 4 kHz - 10 kHz. The HR and VR were determined by direct measures with a window discriminator which measured a running average of an instantaneous events. The values were then converted to beats per minute (BPM).
Dim white lighting was used during these experimental paradigms. The individual behaviors as well as the interactions of some crayfish interactions were taken using a CCD camera (Toshiba, model IK-537A) fitted with a zoom lens (Pentax TV, zoom 8 - 48 mm) and a video cassette recorder (Panasonic, time lapse SVHS, model AG6T20).
The mating behaviors were conducted in various ways over the years. In some cases the animals were both placed in the same tank with a divider which allowed water to circulate between the sides for one or two days prior to pairing. In other cases, novel introduction of a pair was used.
2.3. Statistical Analysis
Nonparametric analysis (Rank sum) in the direction of change of HR and VR within each individual was used. Five samples with a consistent directional change reveals P < 0.05, at > 95% confidence).
3. RESULTS
The HR and VR measures were obtained from numerous heterosexual pairing of crayfish over a 7 year period starting in 2004. Some of the pairings resulted in antagonistic interactions and were halted to spare the crayfish from injury. Other pairings were repeated over a series of days without any interest shown, by behavioral observation, among the pairs for copulation. In some instances the male crayfish appeared to mount a female crayfish and turn her over which appeared to be positioning her for mounting but persistent resistance of the female would ward off the male. We had no success in predetermining if a copulation would occur. This was a time consuming process to place the recording leads on crayfish and monitor video interactions for hours on playback of the VHS tapes. In order to expedite the process of locating pairs of interest, screening crayfish by placing heterosexual pairs in small containers was performed during this past year. This allowed one to determine if crayfish would social interact in what appeared to be nonaggressive interactions. Thus, these pairs were then implanted with recording leads for HR and VR and allowed 1 day to recover prior to recording the HR and VR.
Prior to pairing crayfish the HR and VR were obtained for baseline values. When a crayfish walks or has excessive movements both HR and VR will increase as shown in previous studies [2-4]. Thus an average reading over a period of relatively inactive state within a 30 minute period prior to pairing of the crayfish was taken for a baseline values. The HR and VR for the male and female were monitored during the copulatory act and notes were recorded directly on the computer file (Figure 1(B)). Thus, the onset and duration of copulation and physical separation were recorded (Figure 2). Each successful pairing resulted in various durations in copulation. In one case a repeated copulation occurred after 10 minutes of a physical separation with similar results
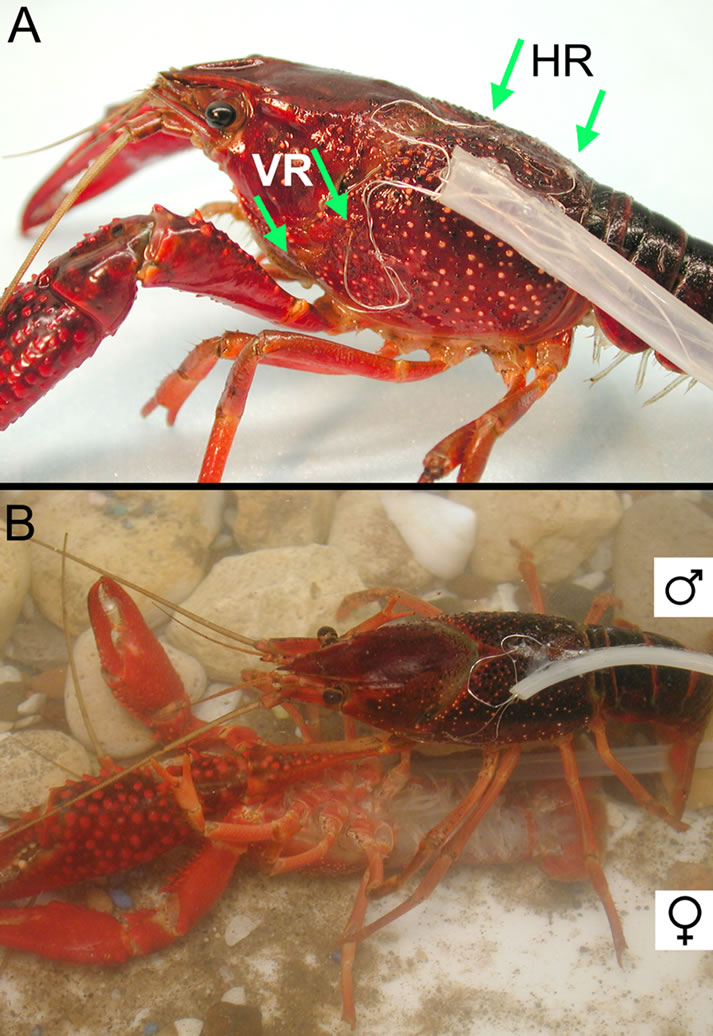
Figure 1. (A) Placement of the recording wires for monitoring the heart (HR) and ventilatory (VR) rates from a crayfish (Procambarus clarkii.). On the dorsal carapace, arrows indicate placement of the two wires which span the rostralcaudal axis of the heart to monitor heart rate. On the lateral side, two arrows indicate placement of the two wires which span the scaphognathite (SG) (i.e. prebranchial chamber) to monitor any change in the dynamic resistance in the water flow within the chamber. Wires are held in place with a drop of glue (cyanoacrylate ester) and accelerator (HobbyTown USA, Lexington, KY). The wires are placed in the plastic tubing and the base of the tubing is glued to the carapace. (B) Copulating position with the male on top and the female on bottom. The female’s chelae are normally held extended by the male. In the figure even one chela of the male is able to maintain the female in position.
in HR and VR responses for both the male and female. When a steady HR and VR was observed during copulation these values were recorded as the responses. If an entire minute during the copulation without any cardiac or ventilatory response detected, a zero was recorded. These instances are noted in Table 1. Snapshots of the recorded traces for HR and VR in a pair during baseline, copulation and postcopulation are depicted in Figure 3.
The first pair noted in Table 1 was a pair that showed repeated copulations. Only the first copulatory action was analyzed for alterations in HR and VR in order to compare with the other crayfish pairings. In addition, the initial baseline values were not obtained prior to the second act of copulation; however, the same trend was

Figure 2. The HR and VR measures for a pair of crayfish throughout a copulatory encounter. The values are averages every minute during the baseline, copulation and postcopulation with comments in the observed behaviors.
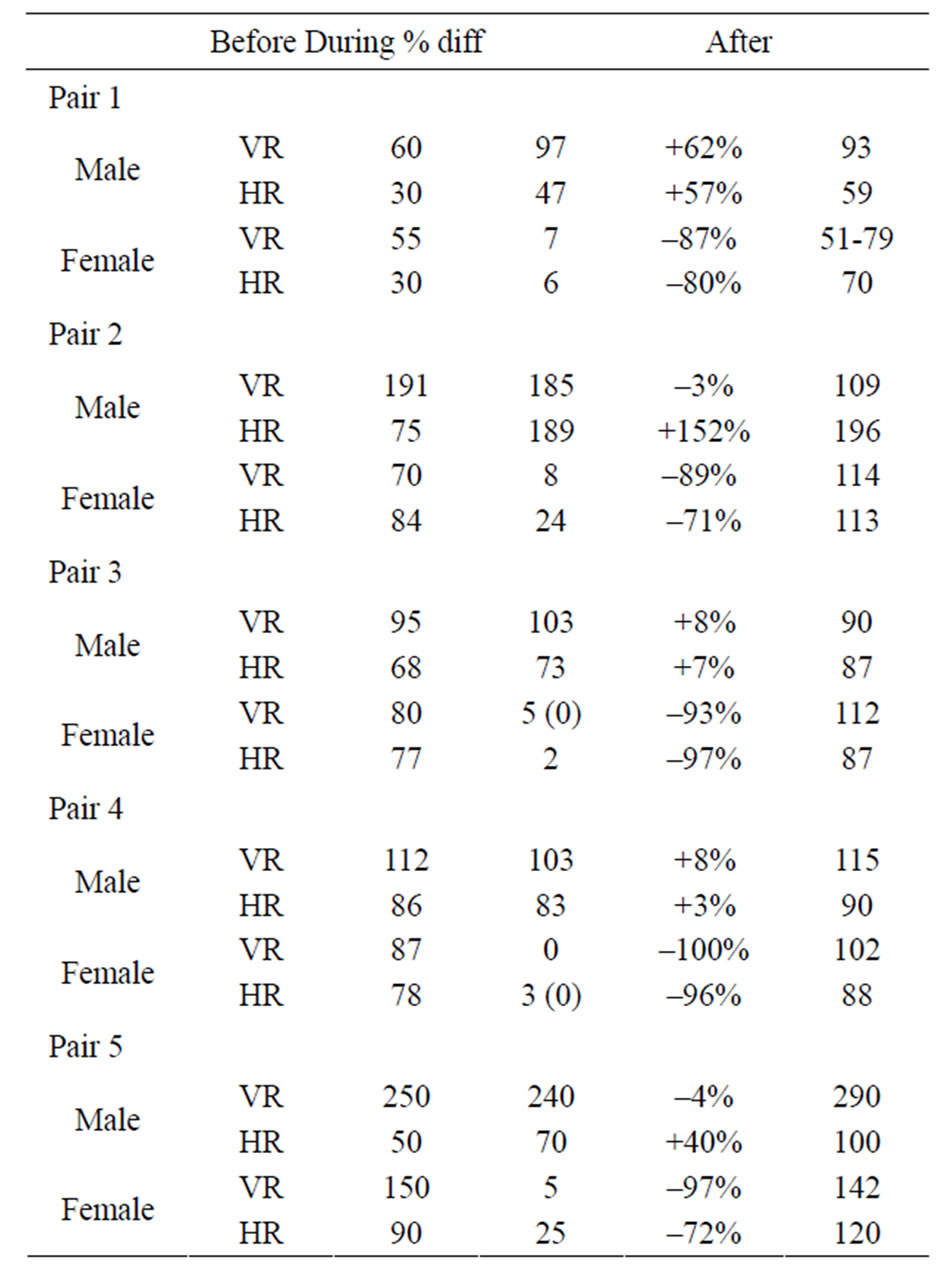
Table 1. Average beats per minute of HR and VR before, during and after copulation for the male and female crayfish.
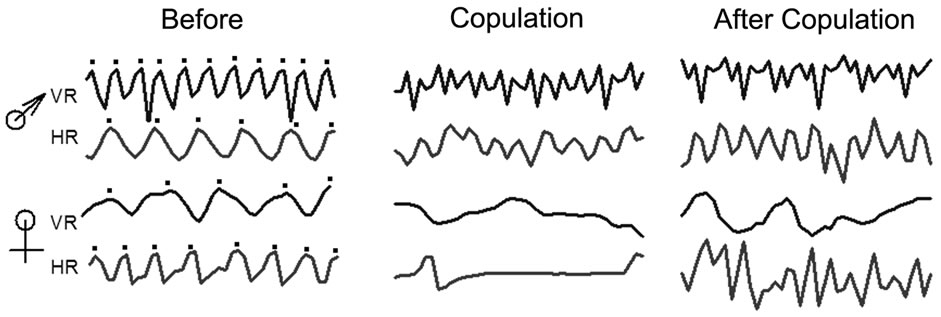
Figure 3. Representative traces in the recordings for VR and HR of the male and female before, during copulation and postcopulation. Note the female dramatically decreases HR and VR during copulation. The dots above the first column of traces indicates each deflection within the trace that is used to count a response.
observed with HR and VR reduction in the female without a substantial alteration in the male.
The other pairs showed similar trends in the female reducing HR and VR during the copulatory act (N = 5, P < 0.5, Table 1). The males did not alter their HR or VR in a consistent manner during copulation. Some males slightly increase in HR and VR while others had slight reductions. The changes in the HR and VR for the males during the steady phase of copulation were less in magnitude than the alterations normally obtained during an aggressive interaction or during walking or climbing the sides of the holding tank [2-4].
In all five pairings the female maintained a higher VR and HR after copulation was over (P < 0.5, Table 1). These recordings were obtained when the female was standing still. Recordings were also obtained from the males after the copulation was complete and when they appeared in a non-active state of walking; however, the rates did not show a consistent trend as those obtained for the females (Table 1).
The duration of copulation varied from 8 minutes to 20 minutes among the pairs. We attempted to correlate the duration of the heightened HR and VR during postcopulation for the female with the duration of the reduced HR and VR during copulation, but the intermittent movements (e.g., walking and defensive posturing to the male) of the female afterwards precluded obtaining reliable values unrelated to the movements. Speculation from intermittent recordings in the postcopulatory period suggests that the longer the female had an reduced HR and VR that heightened responses were prolonged.
The reduction in HR and VR of the female occurred rapidly after she was rolled on her back and chelipeds pinned above her by the male. HR and VR start to increase within a minute in the female prior to separation of the pair. During the initial increase in the rates no obvious movements were able to be observed in the pair. Upon struggling of the female the HR and VR rise substantially in the female followed by the pair separating. No significant alteration was noted for the male during this time until movements (walking away) from the interaction occurred.
4. DISCUSSION
In this study we showed that during copulation of heterosexual pairs of crayfish HR and VR substantially decrease and then increase during postcopulation in females but not in males. The alterations in HR and VR in the females are coupled in the direction of change which suggests a common neural regulatory center during the voluntary act of copulation. The cessation or near cessation of VR in the female we postulate to be a means to reduce any olfactory cues from the female to the closely opposed male while she is in a very vulnerable position to bodily harm of her exposed soft ventral abdomen. A plausible explanation for the reduced HR is to conserve metabolic demand while VR is reduced.
From various pairings, it is noted that crayfish do not appear to be actively engaged in mating behavior within our captive conditions. Perhaps the environment is not conducive to males and/or females. Housing stress may alter the females endocrine responses and possible production or release of sex pheromones. In the crayfish, Pacifastacus leniusculus (Dana) it was shown that exposure to water conditioned by mature females induces the male even if the cue is associated with an inanimate object [32]. In the Signal crayfish the release of chemical cues occurs during the breeding season by mature females [32].
In water, secretions from one crayfish can act as olfactory cues to signal its presence to another crayfish. This leads to clearly visible behavioral changes such as avoidance responses [33]. Whole animal behaviors based on olfaction cues in crustaceans are well documented [33-37]. In fact, it was shown that currents occur in the water separating two crayfish when in close face to face interaction [38] which would then be optimal for olfactory cues [39-41]. These currents are thought to be generated by gill currents induced by the crayfish themselves, likely to aid in assessing each other by olfaction since the crayfish actively flick their antennules during this time. Oxygen uptake in decapod crustaceans occurs across the gills that are situated in a narrow branchial chamber. For renewal of the water near gills, these animals use a very efficient pumping system consisting of two specialized appendages, scaphognathites (SG), one on each side, at the anterior ends of the branchial chambers. The SG which represents strongly modified second maxilla, is a blade like structure which moves from a fully levitated (dorsal) to a fully depressed (ventral) position, drawing water across the gills by its rhythmic movements [24]. The rhythm and force of the SG is the mechanism that generates the gill current. Since the female reduces the VR (i.e., SG beating) during the time of copulation we speculate that she is reducing any potential olfactory cues to the male. It is conceivable that with reduced oxygenation that one approach to conserve oxygen in the body is to reduce metabolic rate. This could be linked to the dramatic reduction in HR of the females during copulation. The females appear to be very still and cooperative during the copulation; however, they also appear to be the one that decides when copulation is to end, with the male obliging.
We have found no report of heart rate decreasing during copulation in any other animal species. If there is no reduction in heart rate and blood pressure response during the parasympathetic phase (e.g., engorgement of erectile tissue) of sexual arousal in mammals, this might suggest a separation in vagal and other parasympathetic neural circuitry during this physiological phase in mammals.
The coupling of the reduced VR and HR during copulation and increased rate postcopulation for the female crayfish suggests that the neural circuitry controlling these organs is tightly regulated. The nerves in the crayfish from the suboesophageal hemiganglion (SOG) carry the impulses to the muscles that control the SG. Likewise the adult decapod crustacean heart is neurogenic which requires neural impulses to initiate each beat. The cardiac ganglion is closely associated within the heart tissue [5,9,42,43]. The crayfish heart ganglion receives neural innervation from the suboesophageal ganglion [44-47]. The drive to the motor neurons for the cardiac and ventilator muscles is likely driven by a central command since they do parallel in the excitability of these distinctly different muscle groups [16,25,48]. The mechanisms in neuromodulator on such a circuitry would be of interest to know if modulation could be distinctly different on the drive to one or the other systems or if the higher command center is the primary site of action.
It is unlikely the reduction in VR results in a rapid rise in PCO2 within the hemolymph of the crayfish to cause the heart to slow down in parallel since the HR is able to rapidly increase after a prolonged cessation of VR during the period of copulation. It is known that crayfish can sense and avoid water that is high in CO2 and that if the animal is exposed to water high in CO2 that both the HR and VR will rapidly decrease and stop [37]. However after being placed in aerated water from water saturated in CO2 the animal is slow to regain a normal HR and VR. A future study in monitoring PCO2/PO2 in the hemolymph during the copulatory act would help to substantiate if there are alterations induced during the reduced VR. The drive in the female to reduce her HR and VR would appear to be partly voluntary driven given that she positions herself in copulatory stance or wards off the attempts. Thus, if she follows through with copulation or submissiveness possibly an acute relaxation induced by hormonal or neural regulation might be induced; however, there is no evidence as of yet to determine the underlying physiological mechanisms in the reduction of heart and ventilatory rates during copulation. Since there is direct GABA innervation to the heart in several crustaceans [11,49-51] there might be a selective inhibitory drive to the heart overriding any excitatory input, thus leaving the cardiac ganglion to be the site of integration to the heart muscle directly. Likewise for the SG, in crabs there is direct excitatory and inhibitory drive to the muscles [52-54], but the innervation profile has not been addressed in crayfish. It is possible that central command as well as direct inhibitory innervation is responsible for the reduction in HR and VR in the female. The precise mechanisms remain to be addressed.
5. ACKNOWLEDGEMENTS
This work was supported in part by NSF-IBN-0131459 (RLC), REU-NSF supplement fellowship (MA) and University of KY research fellowship for high school students (RMC) as well as personal funds (RLC).
REFERENCES
- McMahon, B.R. (1995) Integrated neural and neurohormonal control of respiratory and circulatory function in crustaceans: Is there evidence for an “autonomic” control system? Verhandlungen der Deutschen Zoologischen Gesellschaft, 88, 87-101.
- Schapker, H., Breithaupt, T., Shuranova, Z., Burmistrov, Y. and Cooper, R.L. (2002) Heart rate and ventilatory correlative measures in crayfish during environmental disturbances & social interactions. Comparative Biochemistry and Physiology, 131A, 397-407. doi:10.1016/S1095-6433(01)00492-5
- Li, H., Listerman, L.R., Doshi, D. and Cooper, R.L. (2000) Use of heart rate to measure intrinsic state of blind cave crayfish during social interactions. Comparative Biochemistry and Physiology, 127A, 55-70. doi:10.1016/S1095-6433(00)00241-5
- Listerman, L.R., Deskins, J., Bradacs, H. and Cooper, R.L. (2000) Heart rate within male crayfish: Social interactions and effects of 5-HT. Comparative Biochemistry and Physiology, 125A, 251-263. doi:10.1016/S1095-6433(99)00180-4
- Alexandrowicz, J. S. (1932) The innervation of the heart of crustacea I. Decapoda. Quarterly Journal of Microscopical Science, 75, 181-249.
- Orlov, Y. (1927) Das magenganglion des fluβkrebses, ein beitrag zur vergleichenden histologis des sympathischen nervensystem. Zeitschrift fur Mikroskopisch—Anatomische Forschung, 8, 67-102.
- Zavarzin, A.A. (1941) Ocherki po evol’utsionnoj gistologii nervnoj sistemy (Essays on the evolutionary histology of the nervous system). In: Zavarzin, A.A. and Izbrannye, T., Eds., Moskva-Leningrad, Moscow, 1950.
- Welsh, J.H. and Maynard, D.M. (1951) Electrical activity of a simple ganglion. Federation Proceedings, 10, 145.
- Cooke, I.M. (1988) Studies on the crustacean cardiac ganglion. Comparative Biochemistry and Physiology, 91C, 205-218. doi:10.1016/0742-8413(88)90188-0
- Hartline, D.K. (1979) Pattern generation in the lobster (Panulirus) stomatogastric ganglion: Pyloric network simulation. Biological Cybernetics, 33, 223-236. doi:10.1007/BF00337411
- Maynard, D.M. (1961) Cardiac inhibition in decapod Crustacea. In: Nervous Inhibition. Florey, E., Ed., Oxford, 144-178.
- Pasztor, V.M. (1968) The neurophysiology of respiration in decapod Crustacea: The motor system. Canadian Journal of Zoology, 46, 585-596. doi:10.1139/z68-076
- Young, R.E. (1975) Neuromuscular control of ventilation in the crab Carcinus maenas. Journal of Comparative Physiology, 101, 1-37. doi:10.1007/BF00660117
- Larimer, J.L. and Tindel, J.R. (1966) Sensory modifications of heart rate in crayfish. Animal Behavior, 14, 239-245. doi:10.1016/S0003-3472(66)80078-7
- Guirguis, M.S. and Wilkens, J.L. (1995) The role of the cardioregulatory nerves in mediating heart rate responses to locomotion, reduced stroke volume and neurohormones in Homarus americanus. Biological Bulletin, 188, 179-185. doi:10.2307/1542083
- McMahon, B.R. and Wilkens, J.L. (1972) Simultaneous apnoea and bradycardia in the lobster Homarus americanus. Canadian Journal of Zoology, 50, 165-170. doi:10.1139/z72-025
- Shuranova, Z.P., Vekhov, A.V. and Burmistrov, Y.M. (1993) The behavioral reactions of fresh-water crayfish to sensory exposures: The autonomic components. Zhurnal Vyssheĭ Nervnoĭ Deiatelnosti Imeni I P Pavlova, 43, 1159-1169.
- Wilkens, J.L., Young, R.E. and DiCaprio, R.A. (1989) Responses of the isolated crab ventilatory central pattern generators to variations in oxygen tension. Journal of Comparative Physiology B, 159, 29-36. doi:10.1007/BF00692680
- Bierbower, S.M. and Cooper, R.L. (2009) Measures of heart and ventilatory rates in freely moving crayfish. Journal of Visualized Experimentation (JoVE), 32. http://www.jove.com/index/details.stp?id=1594 doi:10.3791/1594
- Burmistrov, Y.M. and Shuranova, Z.P. (1996) Individual features in invertebrate behavior: Crustacea. In: Abramson, C.I., Shuranova, Z.P. and Burmistrov, Y.M., Eds., Russian Contributions to Invertebrate Behavior, Praeger, Westport, 111-144.
- Cuadras, J. (1979) Heart rate and agonistic behavior in unrestrained crabs. Marine Behavior and Physiology, 6, 189‑196. doi:10.1080/10236247909378566
- Cuadras, J. (1980) Cardiac responses to visual detection of movement, mechanostimulation and cheliped imposed movement in hermit crabs. Comparative Biochemistry and Physiology A, 66, 113-1171.
- McMahon, B.R. and Wilkens, J.L. (1983) Ventilation, perfusion and oxygen uptake. In: Biology of Crustacea, Mantel, L. and Bliss, D., Eds., Academic Press, New York, 6, 289-372.
- Shuranova, Z.P., Burmistrov, Y.M. and Cooper, R.L. (2003) Activity of the ventilatory muscles in the crayfish. Comparative Biochemistry and Physiology, 134A, 461-469.
- Shuranova, Z.P., Burmistrov, Y.M., Strawn, J.R. and Cooper, R.L. (2006) Evidence for an autonomic nervous system in decapod crustaceans. International Journal of Zoological Research, 2, 242-283. doi:10.3923/ijzr.2006.242.283
- Wilkens, J.L. (1976) Neuronal control of respiration in decapod Crustacea. Federation Proceedings, 35, 2000-2006.
- Motofei, I.G. and Rowland, D.L. (2005) The physiological basis of human sexual arousal: Neuroendocrine sexual asymmetry. International Journal of Andrology, 28, 78-87. doi:10.1111/j.1365-2605.2004.00514.x
- Rampin, O. and Giuliano, F. (2000) Central control of the cardiovascular and erection systems: Possible mechanisms and interactions. American Journal of Cardiology, 1, 19-22. doi:10.1016/S0002-9149(00)00886-9
- Adami, M., Schapker, H., Breithaupt, T., Calosi, P., Bradacs, H. and Cooper, R.L. (2005) Heart and ventilatory measures in crayfish during social interactions. Neuroscience Meeting Planner, Society for Neuroscience, Atlanta. http://www.sfn.org/index.aspx?Paename=abstracts_ampublications
- Kolasa, J., Bierbower, S., Adami, M. and Cooper, R.L. (2006) Heart and ventilatory measures in crayfish during altered environments and social interactions. Neuroscience Meeting Planner, Atlanta, GA: Society for Neuroscience, Program No. 372.9. 2006. Online. http://www.sfn.org/index.aspx?Paename=abstracts_ampublications
- Wilkens, J.L., Mercier, A.J. and Evans, J. (1985) Cardiac and ventilatory responses to stress and to neurohormonal modulators by the shore crab Carcinus maenas. Comparative Biochemistry and Physiology, 82C, 337-343.
- Stebbing, P.D., Bentley, M.G. and Watson, G.J. (2003) Mating behaviour and evidence for a female released courtship pheromone in the signal crayfish pacifastacus leniusculus. Journal of Chemical Ecology, 29, 465-475. doi:10.1023/A:1022646414938
- Zulandt Schneider, R.A. and Moore, P.A. (2000) Urine as a source of conspecific disturbance signals in the crayfish procambarus clarkii. Journal of Experimental Biology, 203, 765-771.
- Tierney, A.J. and Dunham, D.W. (1982) Chemical communication in the reproductive isolation of the crayfishes orconectes propinquus and orconectes virilis (Decapoda, Cambaridae). Journal of Crustacean Biology, 2, 544-548. doi:10.2307/1548094
- Zulandt Schneider, R.A., Schneider, R.W.S. and Moore, P.A. (1999) Recognition of dominance status by chemoreception in the red swamp crayfish, procambarus clarkii. Journal of Chemical Ecology, 25, 781-794. doi:10.1023/A:1020888532513
- Devine, D.V. and Atema, J. (1982) Function of chemoreceptor organs in spatial orientation of the Lobster, Homarus americanus: Differences and overlap. Biological Bulletin, 163, 144-153. doi:10.2307/1541504
- Bierbower, S.M. and Cooper, R.L. (2010) The effects of acute carbon dioxide on behavior and physiology in Procambarus clarkii. Journal of Experimental Zoology, 313A, 484-497. doi:10.1002/jez.620
- Bergman, D.A., Martin III, A.L. and Moore, P.A. (2005) The control of information flow by the manipulation of mechanical and chemical signals during agonistic encounters by crayfish, orconectes rusticus. Animal Behavior, 70, 485-496. doi:10.1016/j.anbehav.2004.11.021
- Atema, J. and Steinbach, M.A. (2007) Chemical communication and social behavior of the lobster, homarus americanus, and other decapod crustacea. In: Duffy, J.E. and Thiel, M., Eds., Evolutionary Ecology of Social and Sexual Systems: Crustaceans as Model Organisms, Oxford University Press, New York, 115-144. doi:10.1093/acprof:oso/9780195179927.003.0006
- Breithaupt, T. and Petra, J. (2003) Evidence for the use of urine signals in agonistic interactions of the American lobster. Biological Bulletin, 185, 318-323.
- Hill, A.M. and Lodge, D.M. (1999) Replacement of resident crayfishes by an exotic crayfish: The roles of competition and predation. Ecological Applications, 9, 678- 690. doi:10.1890/1051-0761(1999)009[0678:RORCBA]2.0.CO;2
- Cooke, I.M. (2002) Physiology of the crustacean cardiac ganglion. In: The Crustacean Nervous System, Wiese, K., Ed., Springer, Berlin, 45-88. doi:10.1007/978-3-642-56092-7_3
- Kuramoto, T. and Yamagishi, H. (1990) Physiological anatomy, burst formation, and burst frequency of the cardiac ganglion of crustaceans. Physiological Zoology, 63, 102‑116.
- Wiersma, C.A. and Novitski, E. (1942) The mechanism of nervous regulation of the crayfish heart. Journal of Experimental Biology, 19, 255-265.
- Taylor, E.W. (1970) Spontaneous activity in the cardioaccelerator nerves of the crayfish, astacus pallipes lereboullet. Comparative Biochemistry and Physiology, 33, 859-869. doi:10.1016/0010-406X(70)90034-4
- Field, L.H. and Larimer, J.L. (1975a) The cardioregulatory system of crayfish: Neuroanatomy and physiology. Journal of Experimental Biology, 62, 519-530.
- Field, L.H. and Larimer, J.L. (1975b) The cardioregulatory system of crayfish: The role of circumoesophageal interneurones. Journal of Experimental Biology, 62, 531-543.
- Young, R.E. (1978) Correlated activities in the cardiovascular nerves and ventilatory system in the Norwegian lobster, nephrops norvegicus (L.). Comparative Biochemistry and Physiology, 61A, 387-394. doi:10.1016/0300-9629(78)90052-X
- Florey, E. (1960) Studies on the nervous regulation of the heart beat in decapod Crustacea. Journal of General Physiology, 43, 1061-1081. doi:10.1085/jgp.43.6.1061
- Yazawa, T. and Katsuyama, T. (2001) Spontaneous and repetitive cardiac slowdown in the freely moving spiny lobster, panulirus japonicas. Journal of Comparative Physiology, 187A, 817-824. doi:10.1007/s00359-001-0252-z
- Wilkens, J.L. and Walker, R.L. (1992) Nervous control of the crayfish cardiac hemodynamics. Comparative Physiology, 11, 115-122.
- Mendelson, M. (1971) Oscillator neurons in crustacean ganglia. Science, 171, 1170-1173. doi:10.1126/science.171.3976.1170
- Simmers, A.J. and Bush, B.M.H. (1980) Non-Spiking neurons controlling ventilation in crabs. Brain Research, 197, 247-252. doi:10.1016/0006-8993(80)90453-9
- DiCaprio, R.A. (1989) Non-Spiking interneurons in the ventilatory central pattern generator of the shore crab, carcinus maenas. Journal of Comparative Neurology, 285, 83-106. doi:10.1002/cne.902850108

