Open Journal of Inorganic Chemistry
Vol. 2 No. 4 (2012) , Article ID: 23412 , 7 pages DOI:10.4236/ojic.2012.24012
Spectroscopic and molecular structure characterization of the bis(2-aminophenol)(5,10,15,20-tetraphenylporphyrinato) cobalt(II) complex
![]()
1Laboratoire de Physico-chimie des Matériaux, Faculté des Sciences de Monastir, Université de Monastir, Monastir, Tunisia
2Laboratoire de Chimie de Coordination, CNRS UPR 8241, Toulouse, France
Email: HUhnasri1@gmail.comUH, HUHabib.Nasri@fsm.rnu.tnU
Received 14 July 2012; revised 20 August 2012; accepted 10 September 2012
Keywords: meso-tetraphenylporphyrin cobalt(II); 2-aminophenol cobalt(II) complex; X-ray molecular structure
ABSTRACT
The reaction of the cobalt(II) meso-tetraphenylporphyrin (TPP) starting material with an excess of 2- aminophenol (Hon) in organic solvents, yields the cobalt(II) porphyrin species [CoII(TPP)(Hon)2] (1). This compound has been characterized by UV-vis, IR, MSI MS and 1H NMR spectroscopy. The UV-vis data and especially the proton NMR results, for the isolated product, indicated that complex 1 is a Co(II) mesoporphyrin derivative. The X-ray molecular structure of the title compound bis(2-aminophenol) (tetraphenyl-porphyrinato) cobalt(II) has been determined. This structure is the first one reported of a metalloporphyrin with a 2-aminophenol axial ligand species. The central metal is hexacoordinated by the four nitrogen atoms of the pyrrole rings and the nitrogen atoms of the two Hon trans axial ligands.
1. INTRODUCTION
Complexes with noble metal such as rhenium and technetium have been used for decades in medicine specially as potential radiotherapeutic agents for cancer [1] and many cobalt(II) phthalocyaninetetrasodiumsulfonate has been found to enhance the rate of oxidation of 2-aminophenol with dioxygen in water to 2-amino-phenoxazin- 3-one [2]. Thus, the main goal of the present work is to prepare a metalloporphyrin complex with much cheaper metal in particular transition metals such as cobalt, which is coordinated to a 2-aminophenol (Hon) axial ligand [3]. Such species can eventually replace the rhenium and technetium as potential radiotherapeutic agents and also can be used as catalysts in many organic reactions. It is noteworthy that no structures of 2-aminophenol Co(II) porphyrin derivative were reported up to day. The only reported cobalt(II) with a substituted 2-aminophenol is the polymer{[CoII(m2-AT)]}n(where m2-AT = m2-3-amino- (S)-tyrosinato) [4]. In order to gain more insight into the physico-chemistry properties of this new Co(II)-porphyrin derivative. We report in this paper the results of the spectroscopy and the X-ray molecular structure investigation on the 2-aminophenol cobalt(II) meso-tetraphenylporphyrin complex [CoII(TPP)(Hon)2].
2. EXPERIMENTAL
2.1. Materials and Methods
Ultraviolet-visible (UV-vis) spectra were measured on a SHIMADZU UV-2401 spectrometer and IR spectra were recorded from pure products using a PerkinElmer Spectrum 100 FT-IR equipped with a single-bounce diamond attenuated total reflectance (ATR) sampling accessory. Proton magnetic resonance spectra were measured at room temperature on a Bruker 300 Ultrashield spectrometer. Mass spectra were recorded on spectrometers MS/ MS API-365 (Perkin Elmer Sciex) equipped with electrospray source (ESI). All chemicals were purchased from SIGMA-ALDRICH Co. LLC. They were used as received without further purification except the dichloromethane which was distilled under CaH2 and freshly used.
Synthetic Methods
The free base 5,10,15,20-tetraphenylporphyrin and the corresponding cobalt(II) derivative [CoII(TPP)] were synthesized by literature methods [5,6].
2.2. Synthesis of [CoII(TPP)(Hon)2]
[CoII(TPP)] [6] (100 mg, 0.149 mmol) and (190 mg, 2.231 mmol) of 2-aminophenol in 25 mL of dichloromethane were stirred overnight at room temperature. The color of the solution turns from red-orange to dark-red and single crystals of the complex were prepared by slow diffusion of the hexanes into the CH2Cl2 solution. Typical yields were 55% - 65%. UV-vis [lmax (nm) in CH2Cl2, (loge)]: 434 (4.46), 521 (3.54), 555 (3.41). IR (KBr):n, cm-1 3411, 3385, 3354, 3329, 3298, 3027, 2324, 1888, 1811, 1622, 1595, 1500, 1440, 1378 , 1362, 1350, 1271, 1229, 1203, 1175, 1156, 1147, 1071, 1000, 993, 954, 928, 834, 796, 747, 701. 1H NMR (CDCl3): d, ppm, 16.07 (s, 8 H), 13.20 (s, 8 H), 9.97 (s, 8 H), 9.76 (s, 4 H), 5.30 (s, H), 3.70 (s, 2H). ESI(+) MS m/z calc for (C6H7NO) [M]+ 109.05, found 109.20; m/z calc for (C44H28N4Co) [M]+ 671.16, found 671.40; m/z calc for (C50H35CoN5O) [M-H]+ 779.21 found 779.50.
2.3. X-Ray Diffraction
A dark purple crystal of the cobalt(II) 2-aminophenol porphyrin derivative with approximate dimensions of 0.44 × 0.40 × 0.27 mm3 was mounted under inert perfluoropolyether at the tip of glass fiber and cooled in the cryostream of an Oxford Diffraction XCALIBUR.
The data were collected at 180 K using the monochromatic Mo Ka radiation (l = 0.71073). Lattice parameters were obtained by least-squares fit to the optimized setting angles of the 4447 collected unique reflections in the full theta range data collectioon 3.02˚ < q < 26.02˚. Intensity data were recorded using w scans. Data reduction was done using CrysAlisPro, Oxford Diffraction Ltd. [7]. Empirical absorption correction using sphereeeeeecal harmonics, implemented in SCALE3 ABSPACK scaling algorithm. The minimum and the maximum transmission factors values are 0.8592 and 1.000, respectively. The structure was solved by direct methods using SIR-2004-1.0 [8] and refined by full-matrix least squares on |F|2 using the SHELXL - 97 program [9]. The asymmetric unit of the structure contains half [CoII(TPP)(Hon)2] molecule where the Co(II) is located on an inversion center. The two hydrogens of the NH2 group and the H of the OH group of the axial ligand were found in the difference Fourier map and were included in the refinement using restraints (N-H = 0.92(1) Å; O-H = 0.85(1) Å) with Uiso(H) = 1.2Ueq(N3, O1). In the last refinement cycles, the H of the NH2 group were fixed and treated as riding on their parent N atom. The H attached to O1 was kept refining. The positions of H atoms attached to C atoms were calculated and they were treated as riding on their parent C atoms with C-H = 0.95Å and Uiso(H) = 1.2 Ueq(C). Refinement was then carried on to convergence with anisotropic thermal parameters for all non hydrogen atoms. Crystal data and experimental parameters used for the intensity data collection are summarized in Table 1. Complete crystallographic details are available from the CCDC (see Supporting information section).
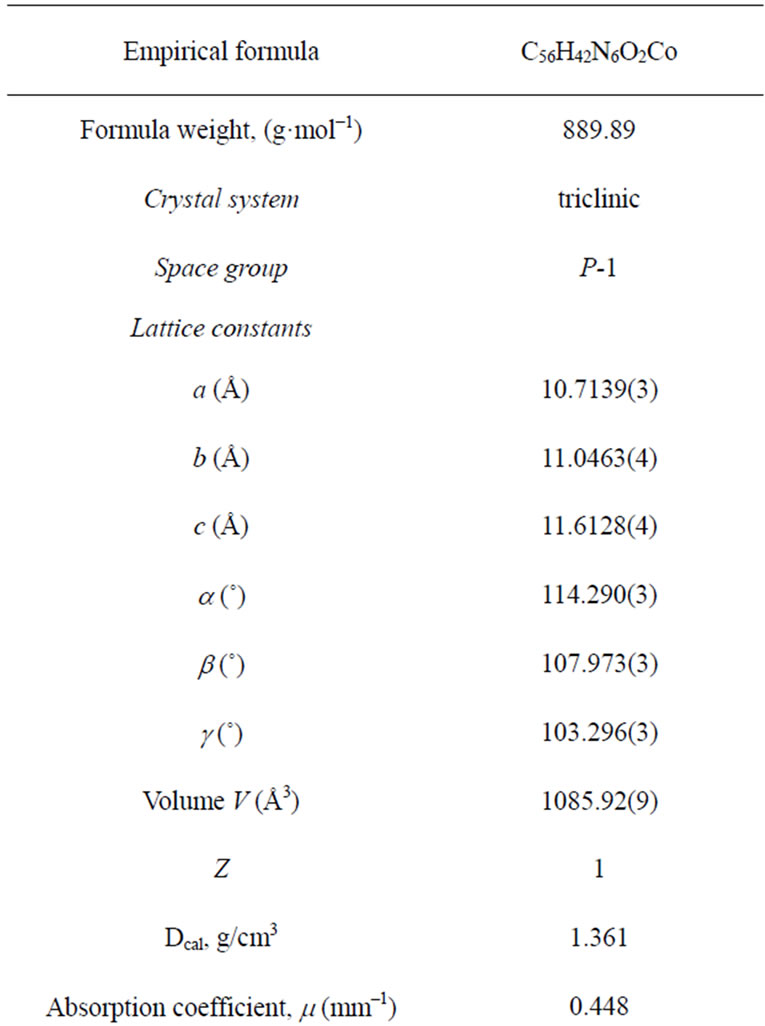

Table 1. Crystal data and refinement parameters for [CoII(TPP) (Hon)2].
3. RESULS AND DISCUSSION
3.1. UV-Visible, IR and Mass Spectrometry
The electronic spectra of complex 1 and the [CoII(TPP)] starting compound are represented in Figure 1. The Soret bands of the related species [CoII(TpivPP)(OAc)]− [10], and other Co(II) meso-porphyrin derivatives (Table 2), are in the range [438 - 441 nm]. This data confirm complex creation of a new Co(II)-meso-porphyrin coordination compound by displaying red shifted Soret band of complex 1. The IR spectrum of [CoII(TPP)(Hon)2] (Figure 2) shows the existence of the coordinated 2-aminophenol ligand. Thus, the frequencies of the O-H(Hon) and the N-H(NH2) stretching are 3386 and 3332 cm−1 respectively.
The ESI MS experiments recorded in the positive ion mode show the presence of the three fragments: [Hon]+ with a m/z value of 190.20, the [CoII(TPP)]+ and the [CoII(TPP)(on)]+ with a m/z values of 671.40 and 779.50 respectively. For the latter fragment, it corresponds to [M-H]+ where the deprotonation of the OH group occurs on one 2-aminophenol axial ligand.
3.2. Proton NMR Spectroscopy
The paramagnetic starting material [CoII(TPP)] species (with the ground state configuration 3d7) presents downfield chemical shifts of the b-pyrrole protons (Hb-pyrr) = 15.75 ppm) as shown in Figure 3. For the diamagnetic cobalt(III) porphyrin derivatives (with the ground state configuration 3d6), the b-pyrrole protons resonate in the normal regions of the free base TPP porphyrin (8.1 ppm < δ(Hb-pyrr) < 9.1 ppm) [11,12] (Table 3). Complex 1
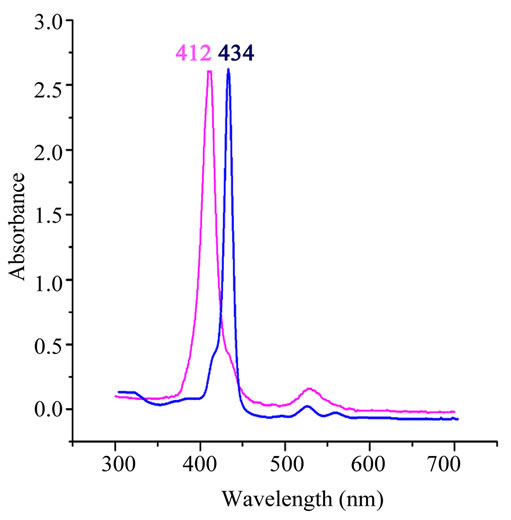
Figure 1. UV-vis spectra of [CoII(TPP)] starting material (pink line) and [CoII(TPP)(Hon)2] (blue line). The spectra were taken in CH2Cl2 at room temperature.
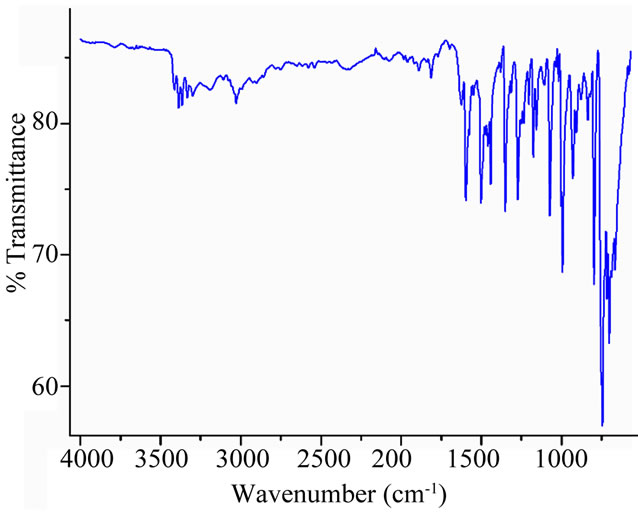
Figure 2. IR spectrum complex [CoII(TPP)(Hon)2] (solid neat).
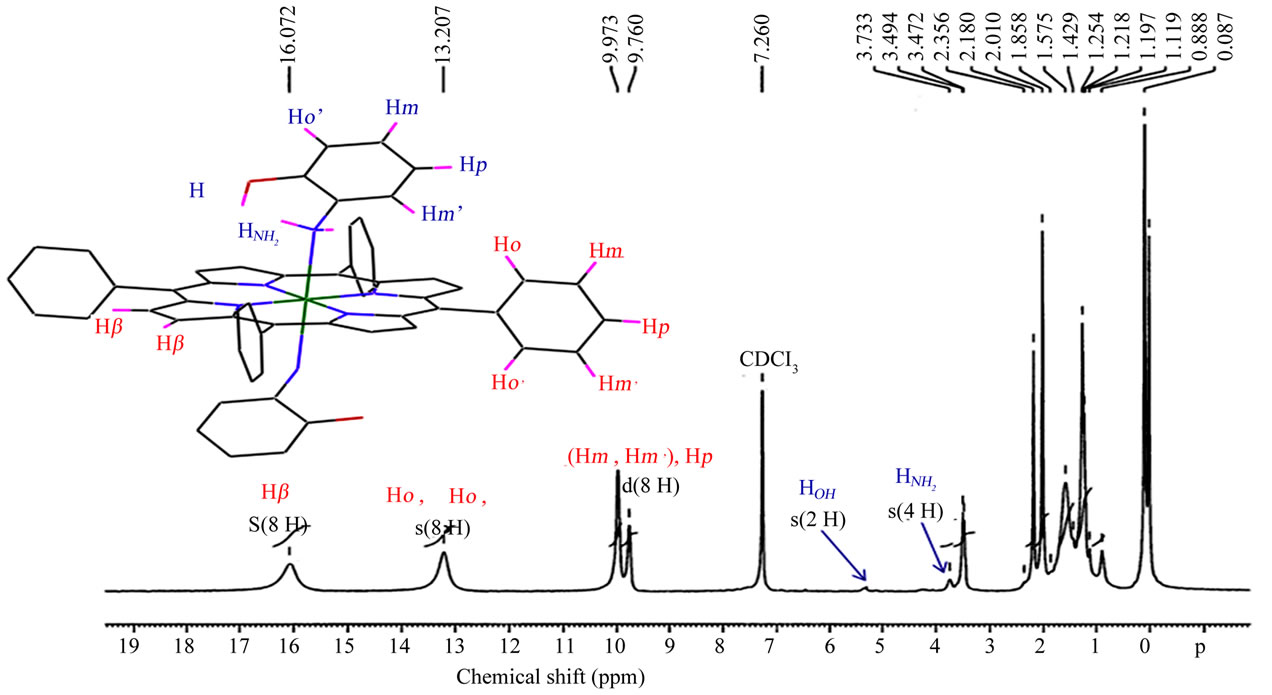
Figure 3. Proton NMR spectra of the [CoII(TPP)(Hon)2] complex taken in CDCl3 solution at room temperature.
presents a peak at 16.07 attributed to the b-pyrrole protons. This is an indication that our derivative is a paramagnetic cobalt(II) meso-porphyrin species [13]. The 1H NMR peaks for the NH2 and the OH protons of the two Hon ligands appear at δ ≈ 3.7 ppm and δ ≈ 5.3 ppm respectively.
3.3. Crystal Structure
The structure of [CoII(TPP)(Hon)2] compound was determined at 180 K and Figure 4 is an ORTEP diagram [15] of this complex. The Co(II) is coordinated to thefour nitrogens of the porphyrin ring and the nitrogen at oms of the NH2 groups of the two 2-aminophenol trans axial ligands. Selected bond distance (Ǻ) angles (deg) for the [CoII(TPP)(Hon)2] complex are represented in Table 4.The preference for the amino nitrogen is understanddable since Co(II) would have a lower affinity for the more electronegative oxygen donor atom, which prefers
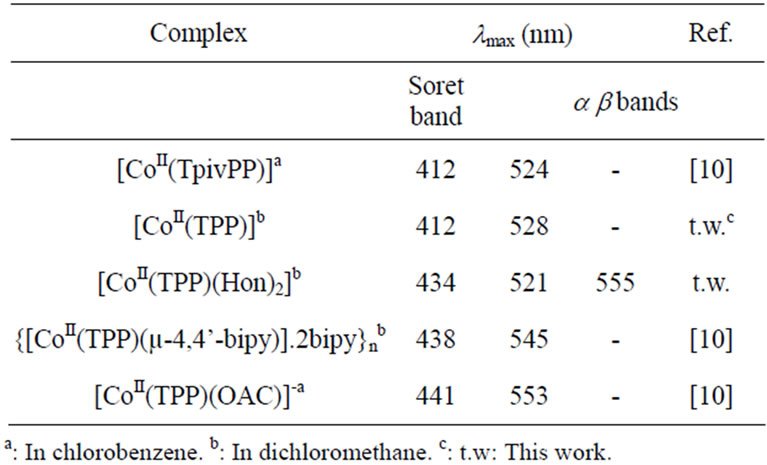
Table 2. Electronic spectra data for selected cobalt(II) mesoporphyrins.
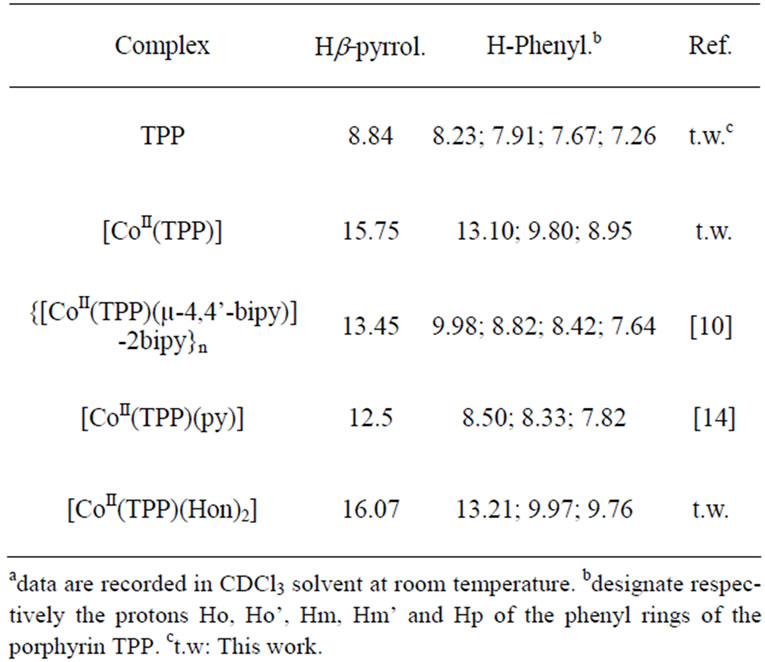
Table 3. 1H NMRa data for the free-base TPP porphyrin and a selected cobalt(II) tetraphenylporphyrin complexes.
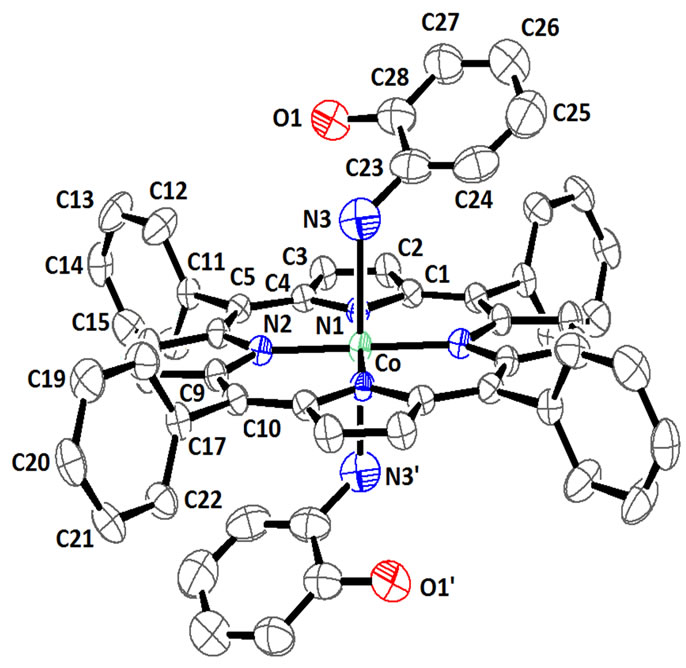
Figure 4. ORTEP drawing [15] of [CoII(TPP)(Hon)2] complex showing thermal ellipsoids at 50% probability level. The H atoms have been omitted for clarity.
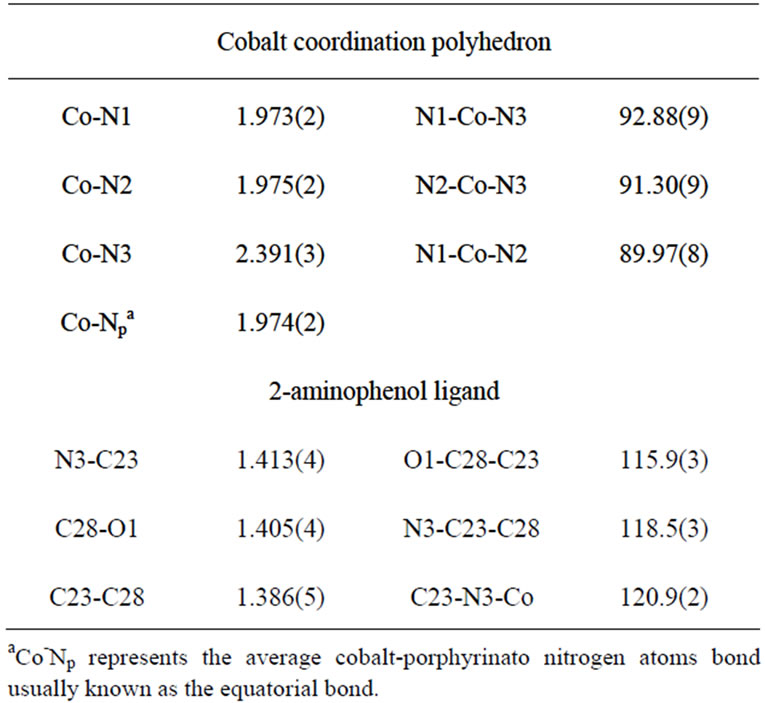
Table 4. Selected bond distances (Ǻ) and angles (deg) for the [CoII(TPP)(Hon)2] complex.
ionic interaction with the metal. In the Cambridge Structural Database (CSD, version 5.33 of November 2011[16]) few structures of transition-metal-Hon structures werereported among them only one structure of a Co(II) substituted 2-aminophenol ligand was mentioned [4]. For all these published structures the metal ion is either bonded only to the nitrogen of the amino group or to the NH2 group and the deprotonated oxygen (Table 5). Figure 5 illustrates the geometry of the transition metal (M) andthe amino group of one coordinated Hon ligand. The CoII-NH2(Hon) bond length for 1 [ 2.391(3) Å] is slightly longer than the one of the related CoII-bis[2-m2-3amino-(S)-tyrosinato] polymer derivative [4] with values of 2.311(7) and 2.258(7) Å. The only reported structures of complexes with metal coordinating Hon ligand are those with the rhenium transition metal [17,18] (Table 6) where the M-NH2 distance is in the range [2.048(4)- 2.270(4) Å]. The CI–NH2 and CII-OH of the Hon ligand for complex 1 and the reported complexes are within the normal values: [1.40 - 1.45 Å] and [1.35 - 1.39 Å] respectively. The CI-N-M angle is 120.9(2)˚ for our species and the related Co(II) derivatives are in the range [115.4˚ - 121.0˚]. The complex [CoII(TPP)(Hon)2] presents an intramolecular hydrogen-bond (N3-H…..OI) with a distance of 2.677(5) Å which falls in the range [2.639(5) - 2.788(3) Å] for related species (Table 5).It has been noticed [19] for cobalt-porphyrin complexes that ruffling of the porphyrin core always results in a shortening of the porphyrin equatorial cobalt pyrrole nitrogen M-Np bonds. Thus, for the very ruffled structure [CoII(TPP)] [6], the Co-Np bond length value is 1.923(4) Å. while the practically planar porphyrin core of the dimer
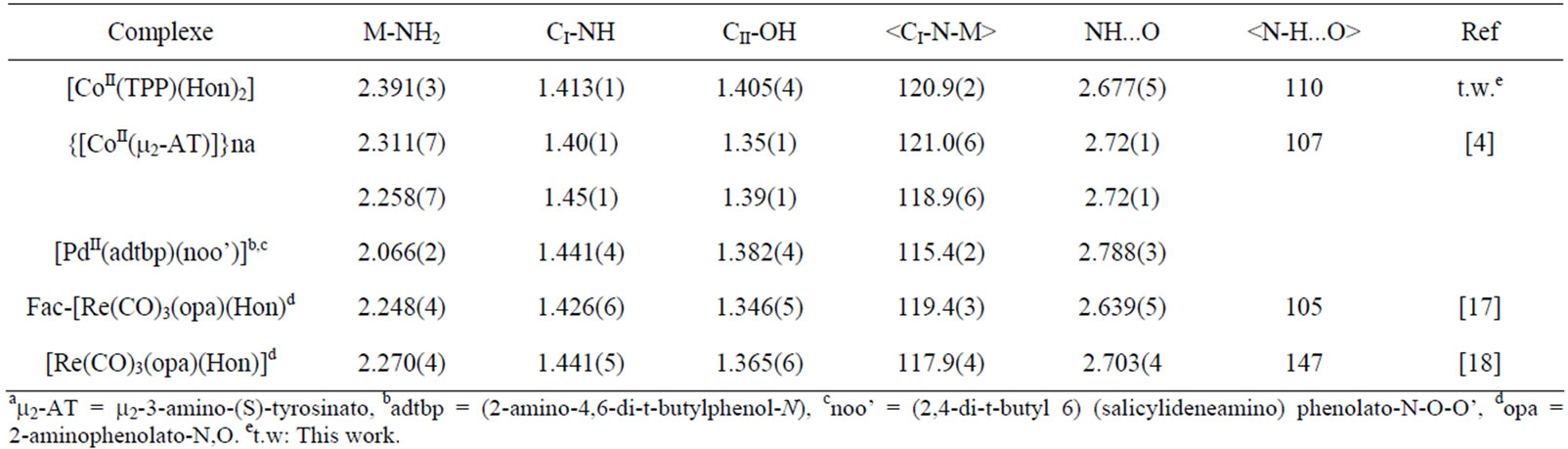
Table 5. Selected bond lengths (Å) and angles (˚) for several 2-aminophenol and substituted 2-aminophenol complexes.
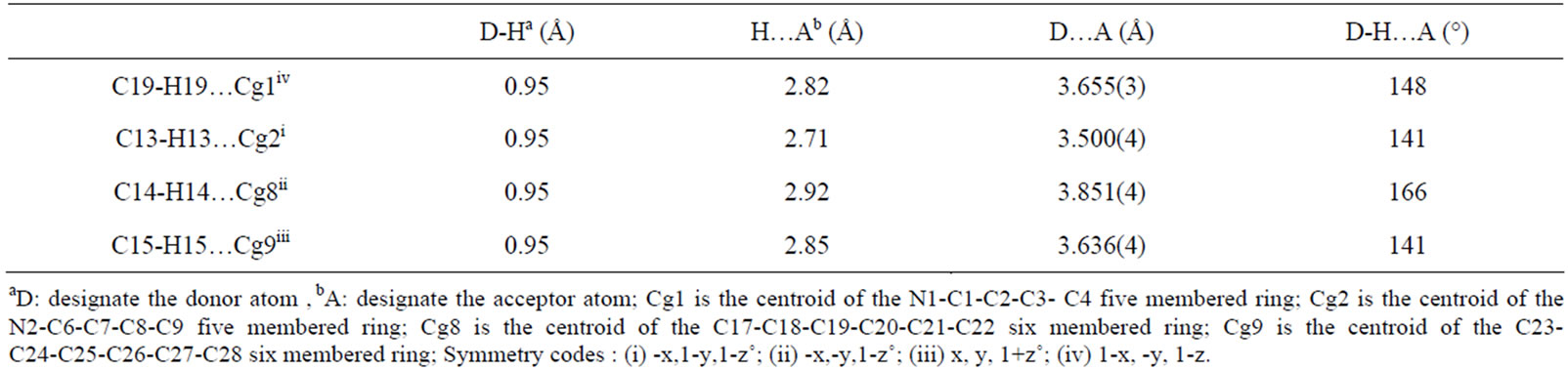
Table 6. Selected intermolecular hydrogen interactions.
Figure 5. Drawing showing the ion metal M coordinated to the amino group of one 2-aminophenol (Hon) axial ligand.
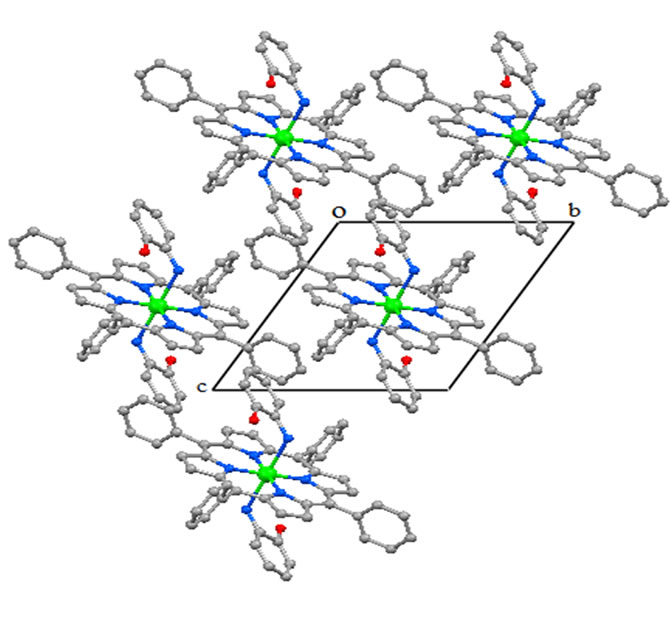
Figure 6. Drawing showing the packing in lattice of [CoII(TPP) (Hon)2], viewed down the a axis.
{[CoII(TPP)(µ-4,4’-bipy)].2bipy}n [10] presents a Co-Np distances of 1.985(5) and 1.993(1) Å. The Co-Np value for our complex [CoII(TPP)(Hon)2] is 1.974(2) Å which is an indication that 1 presents a slightly ruffled porphyrin core. On the other hand, the average displacements of the meso carbons above and below the porphyrin mean plan are small [−0.01 and 0.034 Å] which indicate a moderate ruffling.
The crystal structure of 1 is stabilized by intermolecular interactions type O-H.....Cg and C-H.....Cg where Cg is the centroid of pyrrole five member ring or phenyl six member ring. A selection of these intermolecular distances is summarized in Table 6. Figure 6 is a drawing showing the packing in lattice of [CoII(TPP)(Hon)2], viewed down the a axis.
4. SUPPORTING INFORMATION
Crystallographic data (excluding structure factors) for the [CoII(TPP)(Hon)2] compound have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC-878829. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223-336-033; or email: HUdeposit@ccdc.cam.ac.ukU
5. ACKNOWLEDGEMENTS
The authors gratefully acknowledge financial support from the Ministry of Higher Education and Scientific Research of Tunisia.
REFERENCES
- Gerber, T.I.A., Luzipo, D. and Mayer, P. (2004) The reaction of the cis-dioxorhenium(V) core with N,O-donor ligands: monoand bidentate coordination of 3-methyl- 2-aminophenol. Inorganica Chimica Acta , 357, 429-435. doi: 10.1016/j.ica.2009.02.037
- Hassenein, M., Abdo M., Gerges, S. and El-Khalafy, S. (2008) Study of the oxidation of 2-aminophenol by molecular oxygen catalyzed by cobalt(II) phthalocyaninetetrasodiumsulfonate in water. Journal of Molecular Catalysis A, 287, 53-56. doi: 10.1016/j.molcata.2006.11.045
- Abbreviations used: TPP: dianion of meso-tetraphenylporphyrin; Hon: 2-aminophenol (or ortho-aminophenol); OAc: acetato
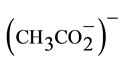 ; m2-AT: m2-3-amino-(S)-tyrosinato; adtpb: (2-amino-4,6-di-t-butylphenol-N); noo’(2,4-di-tbutyl-6) (salicylideneamino) phenolato-N-O-O’; d: opa2- aminophenolato-N,O; PhCN: benzonitrile; BAP: tetrabutylammonium perchlorate.
; m2-AT: m2-3-amino-(S)-tyrosinato; adtpb: (2-amino-4,6-di-t-butylphenol-N); noo’(2,4-di-tbutyl-6) (salicylideneamino) phenolato-N-O-O’; d: opa2- aminophenolato-N,O; PhCN: benzonitrile; BAP: tetrabutylammonium perchlorate. - Gao, X.-Y., Xing, L.-X., Chen, Z.-F., Bao, Y.-C., Yang, J.-H., Wu, M.-F., Xiong, R.-G., You, X.-Z. and Fun, H.-K. (2003). Synthesis and crystal structure of a biologically relevant homochiral two-dimensional metal-organic herring-bone molecular grid: Bis(3-amino-(S)-tyrosinato) cobalt(II). Chinese Journal of Inorganic Chemistry, 19, 802.
- Alder, A.D., Longo, F.R, Finarelli, J.D., Gildmacher, J., Assour, J. and Korsakoff, L. (1967) A simplified synthesis for mesotetraphenyl porphine. Journal of Organic Chemistry, 32, 476-476. doi:10.1021/jo01288a053
- Mansour, A., Belkhiria , M.S., Daran, J.C. and Nasri, H. (2010) (5,10,15,20-tetraphenylporphyrinato) cobalt(II)- 18-crown-6 (1/1). Acta Crystallographica Section E, 66, m509-m510. doi: 10.1107/S1600536810012080
- Oxford Diffraction (2009). Chrysalis Promotions, Oxford Diffraction Ltd., Abingdon, Oxfordshire, England. doi: 10.1021/ic50166a056
- Burla, M.C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G.L., De Caro, L., Giacovazzo, C., Polidori, G. and Spagna ,R. (2005) SIR2004: An improved tool for crystal structure determination and refinement. Journal of Applied Crystallography, 38, 381-388. doi: 10.1107/S002188980403225X
- Sheldrick, G.M. (2008) A short history ofSHELX. Acta Crystallographica Section E, 64, 112-122. doi: 10.1107/S0108767307043930
- Mansour, A. and Nasri, H. (2012) “Synthesis, crystal structure and spectroscopic characterization of 1D Co(II) coordination polymers {[CoII(α,β,α,β-TpivPP)(µ-4,4’-bp y)2]}n and {[CoII(TPP)(µ-4,4’-bpy)2]}n with the (a,b,a,btetrakis(o-pivalamidophenyl)porphyrin) a,b,a,b-TpivPP) and the tetraphenylporphyrin (TPP). In press.
- Hoshino, M., Sonoki, H., Miyazaki,Y., Iimura, Y. and Yamamoto, K. (2000) Structure and photo chemistry of dicyanocobalt(III) tetraphenyl porphyrin. Photochromic reaction caused by photodissociation of axial ligand. Inorganic Chem istry, 39, 4850-4857.doi: 10.1021/ic000408x
- Shirazi, A. and Goff, H.M. (1982) Carbon-13 and proton NMR spectroscopy of fourand five-coordinate cobalt(II) porphyrins: Analysis of NMR isotropic shifts. Inorganic Chemistry, 21, 3420-3425. doi: 10.1021/ic00139a030
- La Mar, G. N. and Walker, F.A. (1973) Proton nuclear magnetic resonance and electron spin resonance investigation of axial solvation in planar, low-spin cobalt(II) porphyrin complexes. Journal of American Chemical Society, 95, 1790-1796. doi: 10.1021/ja00787a017
- Choi I-K., Lin, Y., Wei, Z. and Rayan, M.D. (1997) Reactions of hydroxylamine with metal porphyrins. Inorganic Chemistry, 36, 3113-3118.doi: 10.1021/ic9605783
- Farrugia, L.J. (1997) ORTEP-3 for windows a ver sion of ORTEP-III with a graphical user interface (GUI). Journal of Applied Crystallography, 30, 565. doi: 10.1107/S0021889897003117
- Allen, F.H. (2002) The Cambridge Structural Data base: A quarter of a million crystal structures and rising. Acta Crystallographica Section B, 58, 380-388. doi: 10.1107/S0108768102003890
- Mayer, P., Potgieter, K.C. and Gerber, T.I.A. (2010) Rhenium (I), (III) and (V) complexes of tridentate ONX (X=O, N, S)-donor Schiff bases. Polyhedron, 29, 1423-1430. doi: 10.1016/j.poly.2010.01.013
- Gerber, T.I.A., Betz, R., Booysen, I.N., Potgieter, K.C. and Mayer, P. (2011) Coordination of bidentate aniline derivatives to the fac-[Re(CO)3]+ core. Polyhedron, 30, 1739-1745.doi: 10.1016/j.poly.2011.04.019
- Iimura,Y., Sakurai, T. and Yamamoto, K. ( 1988) The crystal and molecular structure of aquachloro (meso-tetraphenylporphyrinato) cobalt(III). Bulletin of the Chemical Society of Japan, 61, 821-826. doi: 10.1246/bcsj.61.821

