Journal of Encapsulation and Adsorption Sciences
Vol.3 No.1(2013), Article ID:29419,3 pages DOI:10.4236/jeas.2013.31002
Lipid Encapsulated Phenolic Compounds by Fluidization
Russell Research Center, United States Department of Agriculture, Agricultural Research Service, Athens, USA
Email: Ronald.Holser@ars.usda.gov
Received January 18, 2013; revised February 20, 2013; accepted March 1, 2013
Keywords: Capsule; Ferulic Acid; Fluidizer; Nanoparticle; Stability
ABSTRACT
Phenolic compounds exhibit antioxidant and antimicrobial activities with applications as functional food and feed additives. Ferulic acid, a phenolic compound present in grain crops and lignocellulose biomass, was encapsulated with saturated triglycerides using a laboratory fluidizer. Stability of the encapsulated ferulic acid particles was evaluated over a 3 month storage period. Laser light scattering and fluorescence spectroscopy were used to characterize particles. Loss of ferulic acid from particles was measured by emission spectra. Results showed no significant changes in particle diameter, 717.6 nm ± 28.4 nm, or loss of ferulic acid from lipid particles during storage. This combination of renewable materials, physical processing techniques, and nondestructive analytical methods promotes sustainable agriculture.
1. Introduction
Phenolic compounds have numerous health benefits and have emerged as a functional food and animal feed additive [1-5]. Current sources of phenolic compounds include the commodity grains such as corn, oat, and wheat, but they may also be obtained from low-cost agricultural residues [6-9]. This includes biomass feedstock used for bioethanol production. Removal of antimicrobial and antifungal phenolic compounds leads to increased conversion of the cellulosic substrate to ethanol. Separation and recovery of these phenolic compounds provide a valuable co-product and improve process efficiency. Such compounds may be encapsulated to inhibit degradation, preserve bioactivity, and extend the shelf life of formulated products [10-12].
Saturated lipids are useful as capsule materials and have demonstrated their advantages for the encapsulation and delivery of therapeutic agents [13,14]. Tripalmitin and tristearin are solid at room temperature but melt above 50˚C and mix with phenolic compounds. As the lipids cool and solidify the phenolics are trapped within the solid lipid particle. Blends of lipid compounds or even structured lipids can be used to obtain specific melting behavior for critical applications. The lipid material provides a barrier and facilitates product formulation. However, compatibility between the lipid coatings and the encapsulated material can influence product performance with loss of the bioactive compound from the particle over time.
Ferulic acid and coumaric acid are low molecular weight phenolic compounds that exhibit antioxidant and antimicrobial properties. They are prevalent in grain crops and biomass and are potential food and feed additives. These compounds absorb strongly in the ultraviolet (UV) region which provides a rapid spectroscopic measurement (Figure 1). For this study ferulic acid was encapsulated with saturated lipids and the stability of the resulting particles was evaluated over 3 months. Spectroscopic methods were used to detect loss of ferulic acid from the lipid particles while changes in particle size were measured by light scattering techniques.
2. Experimental
2.1. Materials
Ferulic acid, 98% pure, was obtained from Sigma-Aldrich (St. Louis, MO, USA). Tripalmitin and tristearin standards, 99% pure, were purchased from Nu-Check Prep, Inc. (Elysian, MN, USA). Tween 80 was obtained from Fisher Scientific (Fair Lawn, NJ, USA). Deionized water, 18 micro siemens, was produced from laboratory distilled water.
2.2. Preparation
Lipid particles were prepared by melting a mixture of 50 mg tripalmitin, 50 mg tristearin, and 10 mg of ferulic acid for 2 minutes at 75˚C. Tween 80 (0.5 wt% aq), 4 mL, was quickly added to the mixture and homogenized for 90 seconds at 20,000 RPM with a tissue homogenizer (Omni, Waterbury, CT, USA). This emulsion was then processed by a Microfluidics laboratory fluidizer model 110-s (Newton, MA, USA). The material was recycled
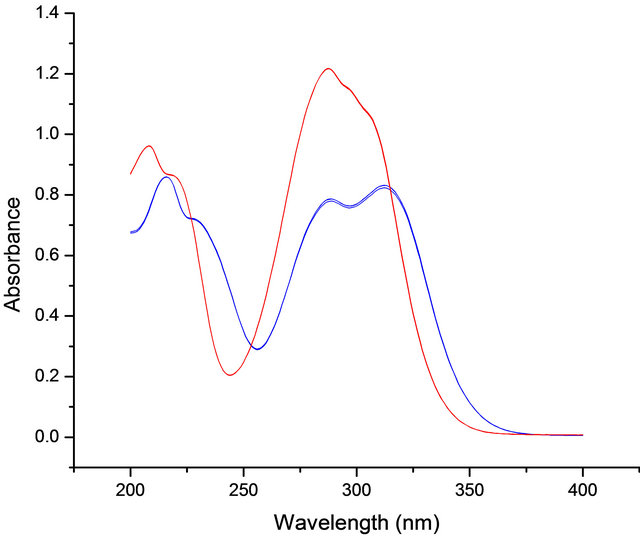
Figure 1. UV spectra of ferulic acid (blue) and p-coumaric acid (red).
three times to produce the final product.
2.3. Analysis
Ultraviolet spectra were collected with a Perkin Elmer model Lambda 2s UV/VIS spectrometer controlled by UV Winlab v 2.7 software (Waltham, MA, USA). Samples were placed into quartz cuvettes and scanned from 200 nm to 400 nm at 240 nm/sec using a slit width of 2 nm. Emission spectra were collected with a Perkin Elmer model LS-55 luminescence spectrometer controlled by FL Winlab v 4.0 software (Waltham, MA, USA). Samples were placed into quartz cuvettes and excited at 377 nm. Emission was scanned from 400 - 450 nm. A spectral bandwidth of 5 nm was set for both excitation and emission. Spectra were processed with The Unscrambler X software, Camo Software, Inc. (Woodbridge, NJ, USA). Spectra were smoothed by the Savitzky-Golay method with second order polynomial and baseline corrected. Emission at 425 nm was used to detect ferulic acid. Particle size distributions were measured with a Nicomp model 380 ZLS particle size system (Santa Barbara, CA, USA). Measurements were taken at 23˚C using a 635 nm source and a scattering angle of 90˚. Samples were diluted 1:10 with deionized water. Zeta potential values were measured with the same instrument using the zeta module.
3. Results and Discussion
Lipid particles were prepared from an oil-in-water emulsion using a high pressure fluidizer to disperse the molten lipid mixture into the aqueous phase. This technique produced solid lipid particles that encapsulated the phenolic compound as the material cooled. The stability of the particles depends on several factors including the size
Table 1. Average values of emission intensity at 425 nm for encapsulated ferulic acid.
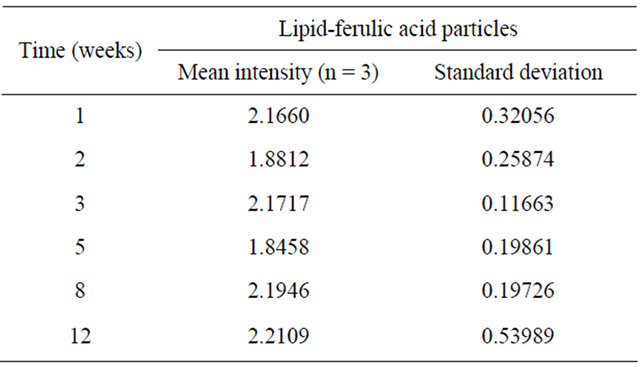
and surface charge of the particles which can be measured by light scattering and zeta potential, respectively. Weekly analysis of the lipid encapsulated ferulic acid particles showed the mean particle diameter of 717.6 nm ± 28.4 nm was unchanged after 92 days. The corresponding zeta potential measured 37.3 mV which is characteristic of stable emulsions. In addition to the need of the dispersed particles to remain suspended in the aqueous solution is the requirement that the ferulic acid not be expelled from the particle. Spectroscopic techniques offer a rapid and nondestructive approach to determine concentration changes in solution. Phenolic compounds display characteristic UV spectra which is useful for detection and quantitation. However, fluorescence techniques provide greater sensitivity. Ferulic acid produces a strong emission at 425 nm when excited at 377 nm. Therefore emission spectra were used to detect loss of ferulic acid from within the particle. Mean values of the intensity at 425 nm are listed in Table 1 along with the standard deviations of replicated measurements. The results of ANOVA indicated that mean intensity values were not significantly different at the 95% confidence level (p > 0.05) over the 3 month period.
4. Conclusion
These results show that lipid encapsulated ferulic acid particles prepared with saturated triglycerides were stable after 3 months storage at ambient conditions. These particles were prepared from an aqueous emulsion with commercially available fluidization equipment. Advantages of this approach to encapsulation include the use of renewable materials and physical rather than chemical processing. The only required solvent is water. The analytical techniques used are nondestructive. All of this is compatible with current principles of sustainability in agricultural and chemistry.
REFERENCES
- L. Yu, K. Zhou and J. W. Parry, “Inhibitory Effects of Wheat Bran Extracts on Human LDL Oxidation and free Radicals,” LWT—Food Science and Technology, Vol. 38, 2005, pp. 463-470.
- T. Sergent, J. Vanderstraeten, J. Winand, P. Beguin and Y. J. Schneider, “Phenolic Compounds and Plant Extracts as Potential Natural Anti-Obesity Substances,” Food Chemistry, Vol. 135, No. 1, 2012, pp. 68-73. doi:10.1016/j.foodchem.2012.04.074
- S. Khadem and R. J. Marles, “Monocyclic Phenolic Acids; Hydroxyand Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies,” Molecules, Vol. 15, No. 11, 2010, pp. 7985-8005. doi:10.3390/molecules15117985
- B. Baurhoo, C. A. Ruiz-Feria and X. Zhao, “Purified Lignin: Nutritional and Health Impacts on Farm Animals—A Review,” Animal Feed Science and Technology, Vol. 144, No. 3, 2008, pp. 175-184. doi:10.1016/j.anifeedsci.2007.10.016
- K. J. Jenkins, F. W. Collins and M. Hidiroglou, “Research Note: Efficacy of Various Flavonoids and Simple Phenolics in Prevention of Nutritional Myopathy in the Chick,” Poultry Science, Vol. 71, No. 9, 1992, pp. 1577- 1580. doi:10.3382/ps.0711577
- N. Okarter, C. S. Liu, M. E. Sorrells and R. H. Liu, “Phytochemical Content and Antioxidant Activity of Six Diverse Varieties of Whole Wheat,” Food Chemistry, Vol. 119, No. 1, 2010, pp. 249-257. doi:10.1016/j.foodchem.2009.06.021
- P. Soengas, M. E. Cartea, M. Francisco, T. Sotelo and P. Velasco, “New Insights into Antioxidant Activity of Brassica Crops,” Food Chemistry, Vol. 134, No. 2, 2012, pp. 725-733. doi:10.1016/j.foodchem.2012.02.169
- D. A. Vattem, Y. T. Lin and K. Shetty, “Enrichment of Phenolic Antioxidants and Anti-Helicobacter Pylori Properties of Cranberry Pomace by Solid-State Bioprocessing,” Food Biotechnology, Vol. 19, No. 1, 2005, pp. 51- 68. doi:10.1081/FBT-200049065
- C. Yi, J. Shi, J. Kramer, S. Xue, Y. Jiang, M. Zhang, Y. Ma and J. Pohorly, “Fatty Acid Composition and Phenolic Antioxidants of Winemaking Pomace Powder,” Food Chemistry, Vol. 114, No. 2, 2009, pp. 570-576. doi:10.1016/j.foodchem.2008.09.103
- A. M. Bakowska-Barczak and P. P. Kolodziejczyk, “Black Currant Polyphenols: Their Storage Stability and Microencapsulation,” Industrial Crops and Products, Vol. 34, No. 2, 2011, pp. 1301-1309. doi:10.1016/j.indcrop.2010.10.002
- P. Laine, P. Kylli, M. Heinonen and K. Jouppila, “Storage Stability of Microencapsulated Cloudberry (Rubus chamaemorus) Phenolics,” Journal of Agricultural and Food Chemistry, Vol. 56, No. 23, 2008, pp. 11251-11261. doi:10.1021/jf801868h
- Z. Fang and B. Bhandari, “Encapsulation of Polyphenols —A Review,” Trends in Food Science and Technology, Vol. 21, No. 10, 2010, pp. 510-523. doi:10.1016/j.tifs.2010.08.003
- R. H. Muller, W. Mehnert, J.-S. Lucks, C. Schwarz, A. Zur Muhlen, H. Weyhers, C. Freitas and D. Ruhl, “Solid Lipid Nanoparticles (SLN)—An Alternative Colloidal Carrier for System for Controlled Drug Delivery,” European Journal of Pharmaceutics and Biopharmaceutics, Vol. 41, 1995, pp. 62-69.
- M. Uner, “Preparation, Characterization and PhysicoChemical Properties of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Their Benefits as Colloidal Drug Carrier Systems,” Pharmazie, Vol. 61, 2006, pp. 375-386.

