International Journal of Organic Chemistry
Vol.3 No.2(2013), Article ID:32997,9 pages DOI:10.4236/ijoc.2013.32012
Design, Synthesis and Pharmacological Evaluation of New Nonsteroidal Anti-Inflammatory Derived from 3-Aminobenzothieno[2,3-d]pyrimidines
1Chemistry Department, Faculty of Science, Al-Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, KSA
2Photochemistry Department, National Research Centre, Cairo, Egypt
Email: *hendnagah2000@yahoo.com
Copyright © 2013 Hend Nagah Hafez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 29, 2013; revised May 7, 2013; accepted May 18, 2013
Keywords: Spirobenzothienopyrimidine; Triazolopyrimidine; Pyrazolopyrimidine; Analgesic; Anti-inflammatory; Ulcerogenic Effect
ABSTRACT
During the last few years, condensed thienopyrimidine derivatives have received considerable attention. The therapeutic importance of thienopyrimidines prompted us to synthesize some of spiro(benzothieno[2,3-d]pyrimidine-4-one) derivatives. Some of the novel benzothino-pyrimidine derivatives 3a, 9b, 10b, 11a, 11b, and 11c showed considerable potent anti-inflammatory and analgesic activity of superior G.I.T. safety profile in experimental rats in comparing to indomethacin and tramadol as reference drugs.
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most widely used therapeutics, primarily used for the treatment of pain and inflammation in arthritis for significant side effects of gastrointestinal lesions, bleeding, and nephrotoxicity. Therefore, the discovery of new safer anti-inflammatory drugs represents a challenging goal for such a research area, fused pyrimidines continue to attract considerable attention of researchers because of their great practical usefulness, primarily, due to a very wide spectrum of their biological activities. Thienopyrimidines occupy a special position among these compounds. Thienopyrimidine derivatives are characterized by a very broad spectrum of biological activities, such as anticancer [1-4], antiviral [5,6], antimicrobial [7-9], analgesic and anti-inflammatory [10-14] anticonvulsant [15,16], thymidine phosphorylase inhibitors [17], antiHIV [18], and antihistaminic [19]. The present work is an extension of our ongoing efforts towards the synthesis and evaluation of some new substituted thieno[2,3-d]- pyrimidine derivatives as analgesic and anti-inflammatory agents.
2. Results and Discussion
2.1. Chemistry
As a part of our continuing program on the synthesis of anti-inflammatory and analgesic compounds as therapeutics agents, we have earlier reported a series of heterocyclic moieties that have anti-inflammatory and analgesic activities [13,14]. Also, outlines the biological significance of one of the most important heterocyclic thienopyrimidine derivatives. This report deals with the synthesis and the pharmacological evaluation of a series of benzothieno[2,3-d]pyrimidines substituted at C-2 with various groups. The interaction of 5,5-dimethyl cyclohexane-1,3-dione with ethyl-cyanoacetate and sulfur metal in ethanol medium in the presence of diethylamine led to ethyl 2-amino-6,6-dimethyl-4-oxo-4,5,6,7-tetrahydro-1-ben zothiophene-3-carboxylate 1 [13]. The hydrazide compound 2 obtained by refluxing of ethylcarboxylate 1 with hydrazine hydrate in ethanol. The treatment of compound 2-amino-1-benzothiophene-3-carbo hydrazide derivative 2 with 5,5-dimethyl cyclo hexane- 1,3-dione, cyclohexanone and cyclo pentanone in basic medium (ethanol/pipredine) produced spiro[(3-aminobenzothieno[2,3-d]pyrimidine-4-one)] derivatives 3a-c (Scheme 1).
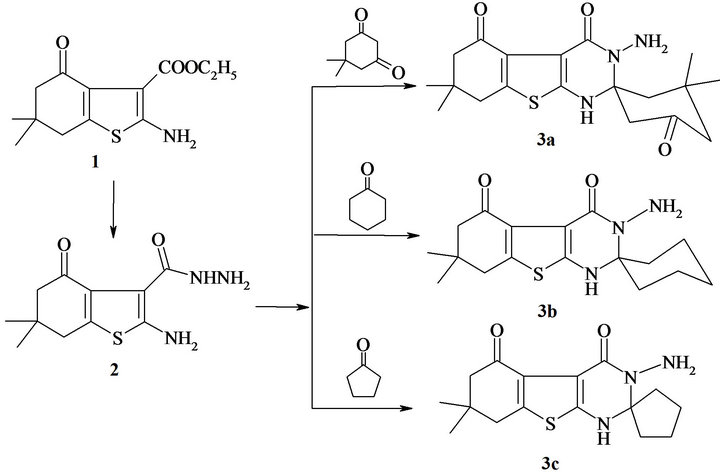
Scheme 1. Synthesis of spiro(3-amino-benzothieno[2,3-d] pyrimidine).
The treatment of ethylcarboxylate (1) with dimethylsulfate and carbon disulfide give (4) which on the treatment with hydrazinehydrate (99%) afforded 7, as shown in (Scheme 2). As a model experiment the alkylation of 5 was carried out by the reaction of 1 mol equiv of methyl iodide with the potassium salt (generated in situ by the reaction of 5 with alcoholic potassium hydroxide) generated the new 2-methylthiothieno[2,3-d]pyrimidines 6. Action of hydrazine hydrate on 3-amino-7,7-dimethyl-2- methylthio-3,6,7,8-tetrahydro [1] benzothineno[2,3-d]pyrimidine-4,5-dione (6) in ethanol afforded 2-hydrazino thino[2,3-d]pyrimidine4-one (7).
Structures of these compounds were supported by spectral data such as IR, NMR, mass and elemental analyses. Compound 7 could be considered as a starting material for the synthesis of new polynuclear heterocycles such as pyrazolobenzothinopyrimidine, imidazolobenzothienopyrimidine and triazolobenzothienopyrimidine derivatives. Thus, heating compound 7 with aliphatic acids, namely, formic and acetic acid, resulted in the formation 4-amino-8,9-dihydrobenzothieno[3,2-e][1, 2,4]triazolo [3,4-a]pyrimidine-5,6-dione (8a,b).
Compound 7 was heated under reflux in glacial acetic acid for 8 hrs with potassium thiocyanate to afford 1,4- diamino-8,8-dimethyl-8,9-dihydro[1]benzothieno[3,2-e][1, 2,4]triazolo[4,3-a]pyrimidine-5,6-(4H,7H)-dione (8c). When compound 7 was heated under reflux with α-haloketones, namely, chloroacetone or 2-bromo aceto-phenone in dry xylene, it yielded the respective 3,4-diamino-2,8,8- trimethyl-3,4,8,9-tetrahydro[1]benzothieno[3,2-e]imidazo[1,2-a]pyrimidine-5,6-(3H,7H)-dione (9a). 3,4-Diamino-8,8- dimethyl-2-phenyl-3,4,8,9-tetrahydro[1] benzothieno- [3,2-e]imidazo[1,2-a] pyrimidine-5,6-(3H,7H)-dione (9b).
Compound 7 reacted with β-diketone to form 2-(1- pyrazolyl)-derivatives. Thus, heating compound 7 with pentane-2,4-dione and/or 3-chloropentane-2,4-dione, yielded 10a,b respectively (Scheme 3). On the other hand, fusion of compound 6 with morpholine, N-methyl piprazine and piprazine in sand bath at 180˚C, yielded 3- amino-(morpholinyl/methylpiperazin/ and or piperazinyl) benzothienopyrimidine-4,5-dione 11a-c (Scheme 4).
2.2. Biological Evaluation
2.2.1. Anti-Inflammatory Effect
The anti-inflammatory activity of fourteen of the newly synthesized compounds 3a, 3b, 3c, 5, 6, 7, 8a, 8c, 9b, 10a, 10b, 11a, 11b, 11c were evaluated by applying carrageenan-induced paw edema bioassay in rats [20] using
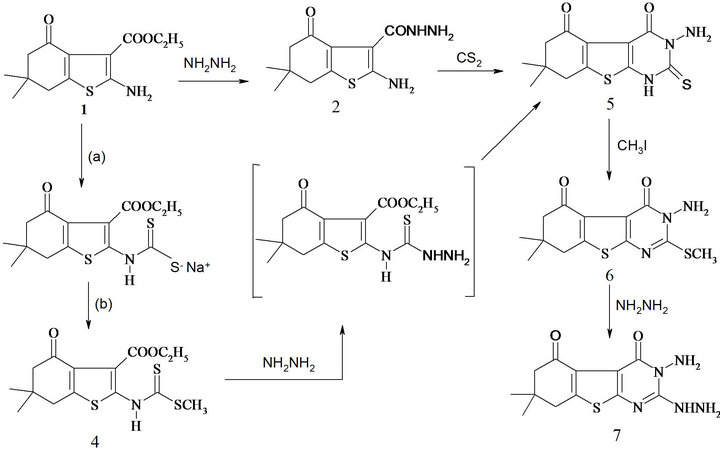
Scheme 2. Synthesis and reaction of benzothinenopyrimidine.
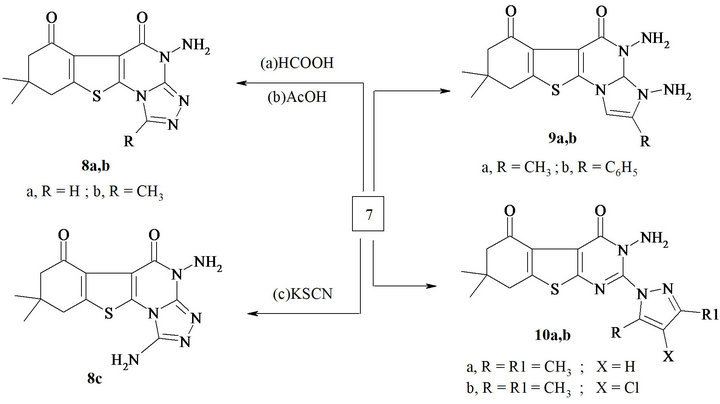
Scheme 3. Synthesis of triazolo and imidazolo pyrimidine.
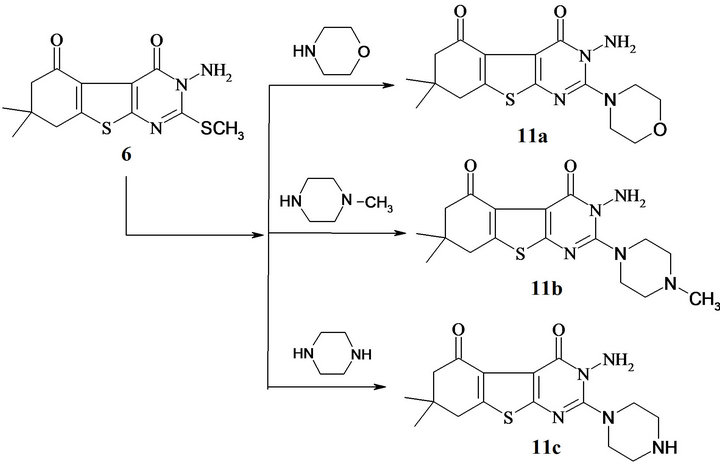
Scheme 4. Synthesis of 3-amino-(2-alkylamino)benzothienopyrimidine-4,5-dione.
indomethacin as a reference standard. Results were expressed as mean ± S.E. Difference between vehicle control and treatment groups were tested using one way ANOVA followed by the least significant difference (L.S.D.). Methods of statistical analysis were done according to Armitage et al. [21].
According to Table 1, administration of many of tested compounds 60 min prior to carrageenan injection at dose of 9 mg/kg b wt caused significant inhibition of paw edema response. Compounds 3a, 3b, 11a and 11c caused significant decreases in paw edema after 2, 3, 4 h after drug administration, while 9b and 10b gave their response after 2 h of administration and continued to the third hour. Compounds 8c and 11b showed the effect only after 2 h but compounds 3c, 5, 7 significantly decreased the paw edema after 4 h post administration.
2.2.2. Analgesic Activity
The analgesic activity of the fourteen derivatives was also evaluated by applying Hot plate test [22] using tramadol as a standard reference. Results were expressed as mean ± S.E. Difference between vehicle control and treatment groups were tested using one way ANOVA followed by the least significant difference (L.S.D.).
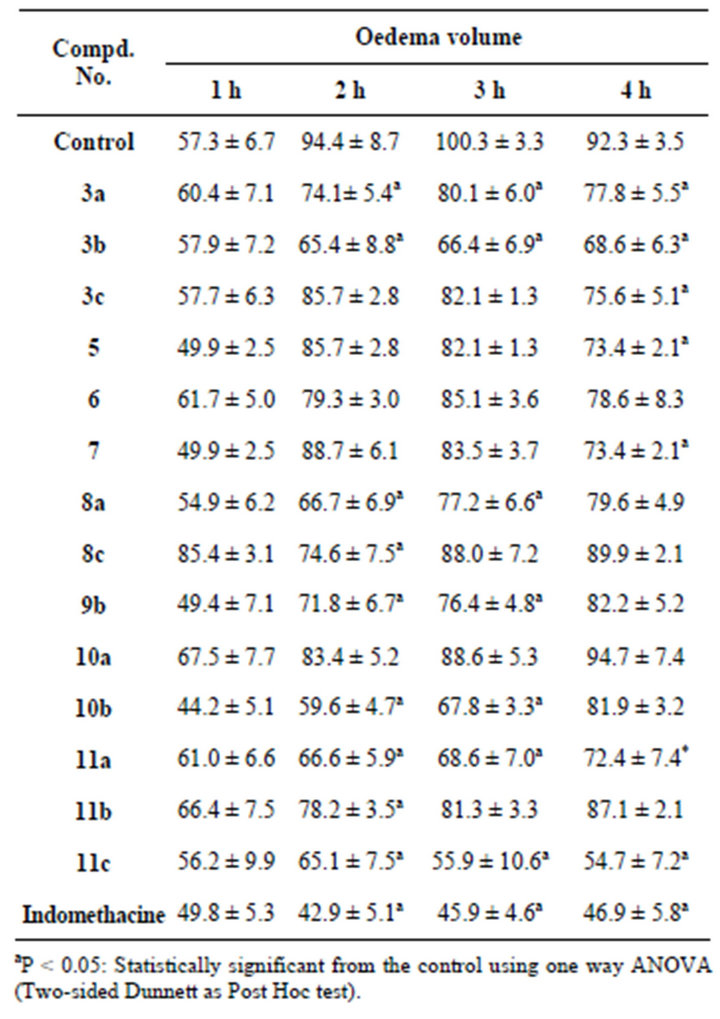
Table 1. Anti-inflammatory effect.
Methods of statistical analysis were done according to Armitage et al. [21].
According to Table 2, compounds 3a, 8a, 10a, 11a and 11c showed significant analgesic activity higher than that obtained by Tramadol 1 h and 2 h post administration. Compounds 8c, 10b and 11b exhibited significant analgesic activity higher than or slightly equipotent to Tramadol only after 2 h of administration. Compounds 3b and 5 exhibited the analgesic effect after 1 h of administration only. Compounds 3c, 6, 7 and 9b have no analgesic activity Thus, it can be concluded that, compounds 3a, b, 5, 8a, c, 10a, b, 11a-c have significant analgesic activity and compound 11a is the most potent compound.
2.2.3. Ulcerogenic Effect
The ulcerogenic effect of the most active anti-inflammatory and analgesic derivatives 3a, b, 8a, 10a, 11a, c was evaluated [23]. According to Table 3, it has been found that, compounds 3a, b, 8a, 11c have very little ulcerogenic effect with in comparison to indomethacin. Interestingly, compound 11a exhibited no ulcerogenic effect in all of the experimental animals. On the other hand compound 10a resulted in ulcer lesions in many of
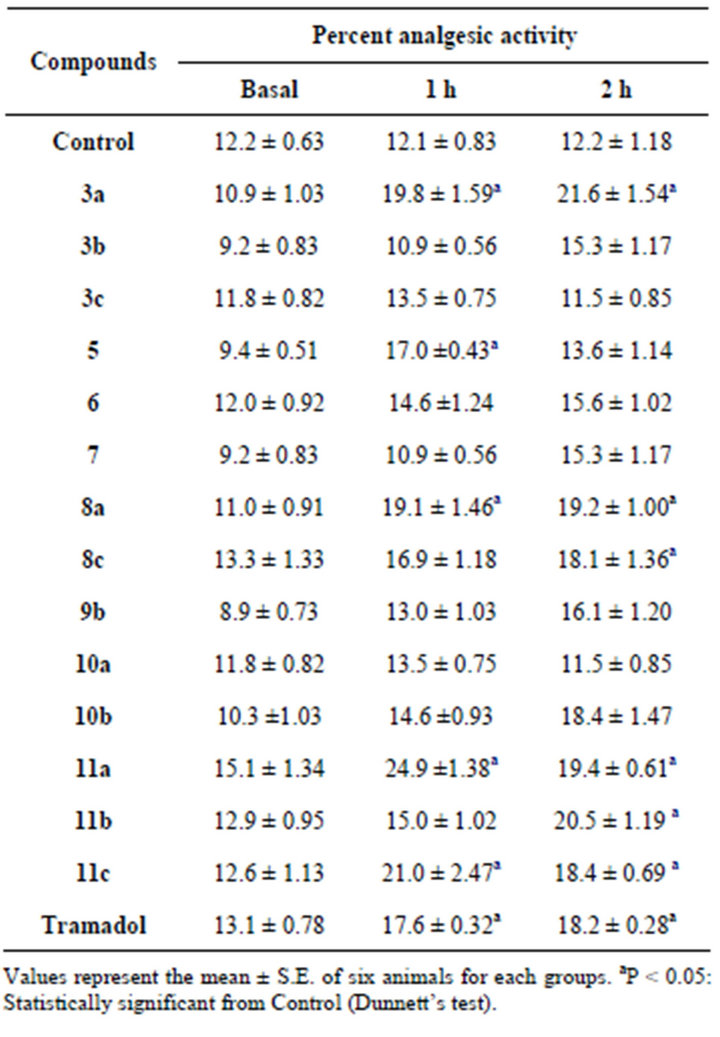
Table 2. Analgesic effect.
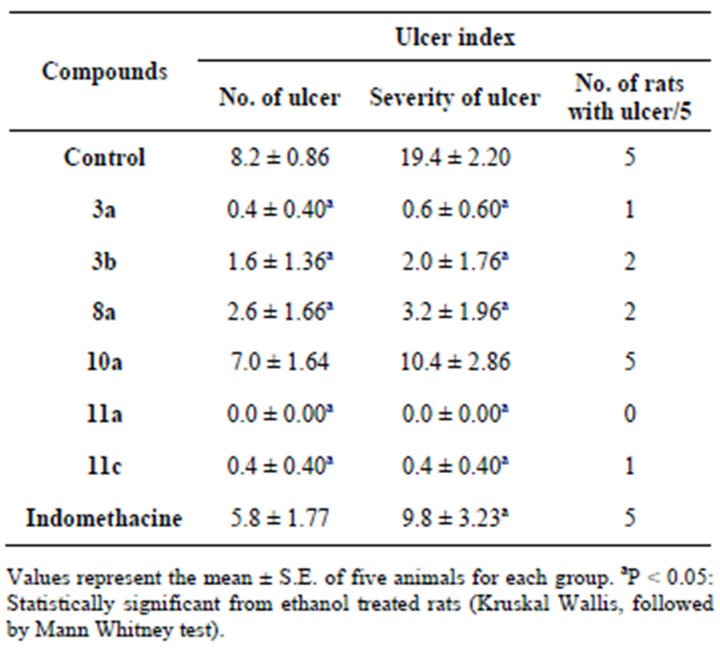
Table 3. Ulcerogenic effect.
the experimental rats. Therefore, the potential medicinal value of these compounds as anti-inflammatory and analgesic agents, that they have better safety margin than indomethacin on gastric mucosa.
3. Conclusion
Different fourteen compounds were evaluated as antiinflammatory and analgesic agents in experimental animals. It has been found that the compounds 3a, 3b, 8a, 10a, 11a, 11c exhibited the dual pharmacological activities with superior gastrointestinal safety profile when compared to indomethacin except 10a which resulted in ulcer lesions in many of the experimental rats. Surprisingly, compound 11a exhibited no ulcerogenic effect in all of the experimental animals. Thus, it can be concluded that spirobenzothienopyrimidine moiety, phenylpyrazolo-thinopyrimidine, morphonyl and piperazinylthinopyrimidine ring systems are important for both anti-inflammatory and analgesic activity of potent safety margin profiles towards gastrointestinal tract.
4. Experimental
4.1. Chemistry
Melting points were determined on the Electrothermal 9100 melting point apparatus (Electrothermal, UK) and were uncorrected. The IR spectra (KBr) were recorded on an FT-IR NEXCES spectrophotometer. The 1H NMR spectra were measured with a Jeol ECA 500 MHz (Japan) in DMSO-d6 or CDCl3 and chemical shifts were recorded in d ppm relative to TMS. Mass spectra (EI) were run at 70 eV with a Finnigan SSQ 7000 spectrometer. The purity of the compounds was checked on Aluminium plates coated with silica gel (Merck). The pharmacological evaluations of the products were carried out in Pharmacological Unit Pharmacology Department (NRC, Cairo, Egypt).
Synthesis of ethyl 2-amino-6,6-dimethyl-4-oxo-4,5, 6,7-tetrahydro-1-benzothiophene-3-carboxylate (1). A mixture of 5,5-dimethyl cyclohexane-1,3-dione (10 m mol), ethylcyano-acetate (10 m mol), sulfur (10 m mol) and diethylamine (15 m mol) was heated under stirring in absolute ethanol for 4 h, then leave the mixture for 24 h at 0˚C. The formed solid was collected by filtration, washed with ethanol (20 mL), dried and crystallized from absolute ethanol, as white crystals in a 72% yield, m.p. 168˚C - 170˚C; IR (cm−1, n); 3420 (br, NH), 3042, 2917 (CH alkyl), 1720, 1706 (2C=O); 1H NMR (DMSO-d6): δ 0.82 (s, 3H, CH3), 0.93 (s, 3H, CH3), 2.12 (d, J = 17.0 Hz, 2H, CH2), 2.33 (d, J = 17.0 Hz, 2H, CH2), 1.58 (t, 3H, CH3) 4.08 (q, 2H, CH2), 7.73 (br, 2H, NH2, D2O exchangeable); Its MS (m/z) 267 (M+, 67%); C13H17NO3S (267.3); Requires (Found): C, 58.40 (58.38); H, 6.41 (6.37); N, 5.23 (5.20).
Synthesis of 2-amino-6,6-dimethyl-4-oxo-4,5,6,7- tetrahydro-1-benzothiophene-3-carbohydrazide (2). A suspension of dry compound 1 (10 m mol) in hydrazine hydrate (80%) (5 mL) was stirred under gentle reflux. The insoluble solid dissolved within 10 min with evolution of hydrogen sulfide to form a clear solution. After 0.5 h. when the solid product started separating out, heating was continued for 8 h. The reaction mixture was then allowed to cool to room temperature. The solid was filtered, washed with ethanol, dried and crystallized from dioxane; as yellow crystals in a 85% yield, m.p. 210˚C - 213˚C; IR (cm−1, n): 3400 (br, NH), 1715, 1690 (2C=O); 1H NMR (DMSO-d6): δ 0.95 (s, 3H, CH3), 1.00 (s, 3H, CH3), 1.96 (d, J = 16.9 Hz, 2H, CH2), 2.14 (d, J = 17.0 Hz, 2H, CH2), 4.35 (br, 2H, NH2), 7.63 (br, 2H, NH2), 8.93 (br, 1H, NH) (NH, 2NH2, D2O exchangeable); Its MS (m/z), 253 (M+, 29%); C11H15N3O2S (253.3); Requires (Found): C, 52.15 (52.13); H, 5.96 (5.92); N, 16.58 (16.53).
Synthesis of Spiro(benzothieno[2,3-d]pyrimidine-4- one) (3a-c). General procedure: A mixture of compound 2 (10 m mol) with 5,5-dimethyl cyclohexane-1,3- dione/or cyclohexanone/and or cyclopentanone was refluxed in basic medium formed from (ethanol/ pipredine). The solid that separated upon cooling was filtered off and crystallized from appropriate solvent to produced (3a-c).
Synthesis of 3-amino-5’,5’,7,7-tetramethyl-7,8-dihydro-1H,3’H-spiro[1-benzothieno[2,3-d]pyrimidine-2, 1’-cyclohexane]-3’,4,5(3H,6H)-trione (3a). A mixture of compound 2 (10 m mol) with 5,5-dimethyl cyclohexane-1,3-dione (10 m mol) was heated under reflux in (ethanol/ pipredine) for 8 h. The solid that separated upon cooling was filtered off and crystallized from dimethylformamide, as orange powder, in a 70% yield; m.p. 250˚C - 252˚C; IR (cm−1, n): 3300 (br, NH’s), 1718, 1710, 1686 (3C=O); 1H NMR (DMSO-d6): δ, 0.84 (s, 3H, CH3), 0.87 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.20 (s, 3H, CH3), 1.43 (d, 2H, CH2), 1.78 (d, 2H, CH2), 1.97 (d, 2H, CH2), 2.28 (d, 2H, CH2) 2.33 (d, 2H, CH2), 7.90 (br, NH), 8.83 (br, 1H, NH); (2NH, D2O exchangeable); 13C NMR (DMSO-d6): δ, 190.3, 187.1 (2CO), 164.6 (CO amide), 141.6, 139.5, 124.7, 114.2 (thiophene carbons), 72.54 (spiro head), 51.42, 50.09, 44.23, 43.29, 41.09 (5CH2), 38.79 (C(CH3)2), 28.98, 28.24, 27.57, 26.32 (4CH3); Its MS (m/z), 375 (M+, 68 %); C19H25N3O3S (375.4); Requires (Found): C, 60.77 (60.73); H, 6.71 (6.69); N, 11.19 (11.15).
Synthesis of 3-amino-7,7-dimethyl-7,8-dihydro-1Hspiro[1-benzothieno[2,3-d]pyrimidine-2,1’-cyclohexane]-4,5-(3H,6H)-dione (3b). It was obtained from the reaction of 2 (10 m mol) with cyclohexanone (10 m mol) as yellow powder crystallized from dimethylformamide, in a 65 % yield, m.p. 260˚C - 263˚C; IR (cm−1, n): 3430 (br, NH), 1711, 1690 (2C=O); 1H NMR (DMSO-d6): δ 0.89 (s, 3H, CH3), 0.95 (s, 3H, CH3), 2.12 (d, 2H, CH2), 2.33 (d, 2H, CH2), 2.53-3.05 (m, 10H, 5CH2), 8.00 (br, 1H, NH), 9.07 (br, 1H, NH) (2NH, D2O exchangeable); 13C NMR (DMSO-d6): δ, 189.2 (CO), 165.8 (CO amide), 141.8, 139.2, 123.9, 113.8 (thiophene carbons), 72.09 (spiro head), 51.02, 44.27 (2CH2), 40.58 (C(CH3)2), 40.29 - 37.89 (cyclohexane carbons), 29.03, 28.19, (2CH3); Its MS (m/z), 333 (M+, 57%); C17H23N3O2S (333.4); Requires (Found): C, 61.23 (61.20); H, 6.95 (6.91); N, 12.60 (12.57).
Synthesis of 3-amino-7,7-dimethyl-7,8-dihydro-1Hspiro[1-benzothieno[2,3-d]pyrimidine-2,1’-cyclopentane]-4,5-(3H,6H)-dione (3c). It was obtained from the reaction of 2 (10 m mol) with cyclopentanone (10 m mol) as white powder crystallized from dimethylformamide in a 67 % yield; m.p. 235˚C - 237˚C; IR (cm−1, n); 3285 (br, NH), 1721, 1673 (2C=O); 1H NMR (DMSO-d6): d 0.91 (s, 3H, CH3), 0.97 (s, 3H, CH3), 2.17 (m, 4H, 2CH2), 2.43 (d, 2H, CH2), 2.71 - 2.75 (m, 2H, CH2), 2.84 - 2.95 (m, 2H, CH2), 2.99 - 3.33 (m, 2H, CH2); Its MS (m/z), 319 (M+, 36%); C16H21N3O2S (319.4); Requires (Found): C, 60.16 (60.12); H, 6.62 (6.59); N, 13.15 (13.11).
Synthesis of ethyl 6,6-dimethyl-2-{[(methylthio) carbon thioyl]amino}-4-oxo-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylate (4). To a vigorously stirred solution of ethyl 2-amino-benzothiophene-3-carboxylate (1) (20 m mol) in dimethylsulfoxide (10 mL) at room temperature, carbon disulfide (25 m mol) and aqueous sodium hydroxide (1.2 mL, 20 mol solution) were added simultaneously over 0.5 h. the stirring was continued for further 30 min. Dimethylsulfate (20 m mol) was added drop wise to the reaction mixture with stirring at 5˚C - 10˚C, it was further stirred for 2 h. and poured into ice-water, the solid obtained was filtered, dried and crystallized from ethanol as yellow powder; in 87% yield, m.p. 120˚C - 122˚C; IR (cm−1, n): 3260 (br, NH), 1727, 1668 (2C=O); 1H NMR (DMSO-d6): d 1.00 (s, 3H, CH3), 1.04 (s, 3H, CH3), 1.89 (t, 3H, CH3) 2.19 (m, 4H, 2CH2), 3.89 (q, 2H, OCH2), 8.70 (br, NH); Its MS (m/z), 357 (M+, 21%); C15H19NO3S3 (357.4); Requires (Found): C, 50.39 (50.36); H, 5.35 (5.33); N, 3.91 (3.88).
Synthesis of 3-amino-7,7-dimethyl-2-thioxo-1,2,3, 6,7,8-hexahydro[1]benzothieno[2,3-d]pyrimidine-4,5- dione (5). To a solution of 4 (10 m mol) in ethanol 30 mL was treated with hydrazine hydrate (10 m mol, 99%) and refluxed on a water bath until the methyl-mercaptan evolution ceases (8 h). After cooling, the solid obtained was filtered, dried and recrystallized from ethanol/acetone mixture as brown crystals; in 85% yield, m.p. 180˚C - 183˚C; IR (cm−1, n): 3410 (brs, NH’s), 1714, 1666 (2C=O); 1H NMR (DMSO-d6): d 0.99 (s, 3H, CH3), 1.02 (s, 3H, CH3), 2.13 (m, 4H, 2CH2), 8.87, 9.53 (brs, 2NH’s); 13C NMR (DMSO-d6): δ, 188.3 (C=O), 174.4 (CS), 165.4 (CO amide), 140.2, 139.1, 124.5, 114.3 (thiophene carbons), 50.31, 42.21 (2CH2), 38.94 (C(CH3)2), 29.34, 27.42 (2CH3); Its MS (m/z), 295 (M+, 52%); C12H13N3O2S2 (295.3); Requires (Found): C, 48.79 (48.76); H, 4.43 (4.40); N, 14.22 (14.20).
The second way for preparation. To a warmed ethanolic sodium hydroxide solution (0.40 g in 50 mL ethanol), compound 2 (10 m mol), and carbon disulfide (excess 5 mL) were added. The mixture was heated under reflux for 15 h. The reaction mixture was allowed to cool to 0˚C, the deposited precipitate was filtered off, washed by water (20 mL), dried, and crystallized from ethanol/acetone mixture as brown crystals; in 68% yield, m.p. 180˚C - 183˚C.
Synthesis of 3-amino-7,7-dimethyl-2-(methylthio)- 3,6,7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidine-4, 5-dione (6). To a warmed ethanolic KOH solution prepared by dissolving (10 m mol) of KOH in 50 mL (ethanol) was added each of compound 5 (10 m mol), the heating was continued for 30 min and the mixture was allowed to cool to room temperature, and the proper methyliodide (12 m mol) was added. The mixture was stirred under reflux for 5 h, then cool to room temperature, poured into cold water (100 mL). The solid product precipitated was filtered off washed with 100 mL water. The product was dried and crystallized from dioxane as a yellow powder, in yield 85%, m.p. 205˚C - 207˚C, IR (cm−1, n): 3415 (brs, NH’s), 1718, 1668 (2C=O); 1H NMR (DMSO-d6): d 0.98 (s, 3H, CH3), 1.01 (s, 3H, CH3), 2.11 (m, 4H, 2CH2), 2.34 (s, 3H, SCH3), 8.85, 9.52 (brs, 2NH’s); Its MS (m/z), 309 (M+, 38%); C13H15N3O2S2 (309.4); Requires (Found): C, 50.46 (50.44); H, 4.88 (4.85); N, 13.58 (13.54).
Synthesis of 3-amino-2-hydrazino-7,7-dimethyl-3,6, 7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidine-4,5- dione (7). A suspension of compound 6 (10 m mol) in hydrazine hydrate (99%, 20 ml) was stirred under reflux for 10 h. The reaction mixture was allowed to cool to room temperature. The solid precipitated was filtered off, washed with ethanol, dried and crystallized from dimethylformamide to produce 7 as white powder in 90% yield; m.p. 278˚C - 280˚C, IR (cm−1, n): 3455 (brs, NH’s), 1721, 1669 (2C=O); 1H NMR (DMSO-d6) ppm: d 1.02 (s, 3H, CH3), 1.13 (s, 3H, CH3), 2.17 (m, 4H, 2CH2), 8.95, 9.52 ( brs, NH’s); 13C NMR (DMSO-d6): δ, 189.4 (C=O), 165.7 (CO amide), 161.4 (C-2, pyrimidine), 142.1, 139.3, 125.1, 115.6 (thiophene carbons), 51.32, 43.27 (2CH2), 39.71 (C(CH3)2), 28.98, 27.57 (2CH3); Its MS (m/z), 293 (M+, 52 %); C12H15N5O2S (293.3); Requires (Found): C, 49.13 (49.10); H, 5.15 (5.13); N, 23.87 (23.84).
Synthesis of 4-amino-8,8-dimethyl-8,9-dihydro[1] benzothieno[3,2-e][1,2,4]triazolo[4,3-a]pyrimidine-5, 6-(4H,7H)-dione (8a). A mixture of 7 (10 m mol) and formic acid (10 mL) and 2 mL of concentrated hydrochloric acid was heated under reflux for 8 h. The reaction mixture was allowed to cool to room temperature and was poured into water (100 mL). The solid formed was collected by filtration, washed with ethanol (20 mL), dried and crystallized from DMF as an orange powder in 80% yield; m.p. 255˚C - 257˚C, IR (cm−1, n): 3423 (br, NH), 1712, 1665 (2C=O); 1H NMR (DMSO-d6): d 0.99 (s, 3H, CH3), 1.02 (s, 3H, CH3), 2.12 (m, 4H, 2CH2), 6.31 (s, 1H, triazol proton), 9.52 ( br, NH); Its MS (m/z), 303 (M+, 44%); C13H13N5O2S (303.3); Requires (Found): C, 51.47 (51.44); H, 4.31 (4.29); N, 23.09 (23.07).
Synthesis of 4-amino-1,8,8-trimethyl-8,9-dihydro[1] benzothieno[3,2-e][1,2,4]triazolo[4,3-a]pyrimidine-5, 6-(4H,7H)-dione (8b). A mixture of 7 (10 m mol) and glacial acetic acid (50 mL) was stirred under reflux for 10 h (TLC). The reaction mixture was allowed to cool to room temperature an was then poured into water (100 mL). The solid formed was collected by filtration, washed with ethanol (20 mL), dried, and crystallized from dioxane as an pale yellow powder in 85 % yield; m.p. 260˚C - 261˚C, IR (cm−1, n): 3435 (brs, NH’s), 1717, 1680 (2C=O); 1H NMR (DMSO-d6) ppm: d 1.00 (s, 3H, CH3), 1.05 (s, 3H, CH3), 2.13 (m, 4H, 2CH2), 2.45 (s, 3H, CH3), 9.40 ( br, NH); 13C NMR (DMSO-d6) ppm: δ, 187.9 (CO), 164.6 (CO amide), 163.8 (C-triazoloe), 160.4 (C-2, pyrimidine), 141.5, 139.1, 124.5, 114.7 (thiophene carbons), 52.21, 44.07 (2CH2), 40.61 (C(CH3)2), 29.42, 28.05, 27.89 (3CH3); Its MS (m/z), 317(M+, 41%); C14H15N5O2S (317.3); Requires (Found): C, 52.98 (52.96); H, 4.76 (4.74); N, 22.06 (22.03).
Synthesis of 1,4-diamino-8,8-dimethyl-8,9-dihydro- [1]benzothieno[3,2-e][1,2,4]triazolo[4,3-a]pyrimidine- 5,6-(4H,7H)-dione (8c). A mixture of 7 (10 m mol) and potassium thiocyanate (15 m mol) was heated under reflux in glacial acetic acid (30 mL) for 8 h. The reaction mixture was allowed to cool to room temperature and was poured into water. The precipitate formed was collected by filtration, dried and crystallized from ethanol/dioxane (2:1) as a yellow powder in 80% yield. m.p. 210˚C - 212˚C, IR (cm−1, n): 3450 (brs, NH’s), 1721, 1665 (2C=O); 1H NMR (DMSO-d6): d 0.99 (s, 3H, CH3), 1.02 (s, 3H, CH3), 2.13 (m, 4H, 2CH2), 9.10, 9.80 ( brs, 2NH); Its MS (m/z), 318 (M+, 32%); C13H14N6O2S (318.3); Requires (Found): C, 49.04 (49.02); H, 4.43 (4.40); N, 26.40 (26.38).
Synthesis of diamino(methyl or pheynel)benzothieno imidazopyrimidine dione (9a,b). General procedure: A mixture of compound 7 (10 m mol) and chloroacetone or 2-bromoacetophenone (10 m mol) was heated under reflux for 12 h in dry xylene (30 mL). The solid that separated upon cooling was filtered off and crystallized from appropriate solvent to produce 9a, b.
Synthesis of 3,4-diamino-2,8,8-trimethyl-3a,4,8,9- tetrahydro[1]benzothieno[3,2-e]imidazo[1,2-a]pyrimidine-5,6-(3H,7H)-dione (9a). Compound 9a was obtained from compound 7 (10 m mol) and chloroacetone (10 m mol) as white crystals crystallized from ethanol in 69% yield; m.p. 249˚C - 251˚C, IR (cm−1, n): 3440 (brs, NH’s), 1715, 1669 (2C=O); 1H NMR (DMSO-d6): d 0.98 (s, 3H, CH3), 1.01 (s, 3H, CH3), 2.10 (m, 4H, 2CH2), 2.23 (s, 3H, CH3), 5.70 (s, 1H, imidazol proton), 6.31 (s,1H, pyrimidine proton), 9.60, 9.85 (brs, 2NH); 13C NMR (DMSO-d6): δ, 188.3 (CO), 163.5 (CO amide), 158.4, 154.6 (2C-imidazol), 140.9, 138.7, 123.6, 113.9 (thiophene carbons), 65.7 (C-2, pyrimidine), 53.19, 43.57 (2CH2), 41.17 (C(CH3)2), 29.51, 27.85, (2CH3); Its MS (m/z), 332 (M+, 38%); C15H19N5O2S (333.4); Requires (Found): C, 54.02 (54.00); H, 5.74 (5.72); N, 21.01 (21.00).
Synthesis of 3,4-diamino-8,8-dimethyl-2-phenyl-3a, 4,8,9-tetrahydro[1]benzothieno[3,2-e]imidazo[1,2-a] pyrimidine-5,6-(3H,7H)-dione (9b). Compound 9b was obtained from compound 7 (10 m mol) and 2-bromoacetophenone (10 m mol) as a yellow powder crystallized from ethanol in 75% yield; m.p. 262˚C - 264˚C, IR (cm−1, n): 3425 (brs, NH’s), 1718, 1675 (2C=O); 1H NMR (DMSO-d6): d 1.02 (s, 3H, CH3), 1.08 (s, 3H, CH3), 2.11 (m, 4H, 2CH2), 5.46 (s, 1H, imidazol proton), 6.09 (s,1H, pyrimidine proton), 7.09 (m, 2H, phenyl proton), 7.34 (m, 3H, phenyl proton), 8.95, 9.60 ( brs, 2NH’s); Its MS (m/z), 395 (M+, 42%); C20H21N5O2S (395.4); Requires (Found): C, 60.73 (60.70); H, 5.35 (5.33); N, 17.71 (17.69).
Synthesis of 3-amino pyrazolyl benzothieno[2,3-d] pyrimidine-4,5-dione (10a,b). General procedure: A mixture of compound 7 (10 m mol) and β-diketone (10 m mol) in absolute ethanol (30 mL) was stirred under reflux for 5 h. The reaction mixture was allowed to cool to 0˚C for 3 h, the precipitate was filtered off, dried and crystallized from an appropriate solvent to produce 10a,b.
Synthesis of 3-amino-2-(3,5-dimethyl-1H-pyrazol-1- yl)-7,7-dimethyl-3,6,7,8-tetrahydro[1]benzothieno[2,3- d]pyrimidine-4,5-dione (10a). Was obtained from 7 (0.01 mol) with pentan-2,4-dione (0.01 mol) as gray powder from dioxane in 63% yield; m.p. 200˚C - 202˚C, IR (cm−1, n) 3410 (br, NH), 1723, 1667 (2C=O); 1H NMR (DMSO-d6): d 0.99 (s, 3H, CH3), 1.03 (s, 3H, CH3), 2.15 (m, 4H, 2CH2), 2.26 (s, 3H, CH3), 2.85 (s, 3H, CH3), 6.03 (s, 1H, pyrazolyl proton), 9.60 ( brs, NH); 13C NMR (DMSO-d6): δ, 187.9 (CO), 164.6 (CO amide), 159.8 (C-2, pyrimidine), 148.1, 144.2, 143.86 (pyrazol proton), 142.3, 139.5, 124.2, 114.6 (thiophene carbons), 53.08, 45.14 (2CH2), 39.92 (C(CH3)2), 29.42, 28.05, 22.19, 22.03 (4CH3); Its MS (m/z), 357 (M+, 34%); C17H19N5O2S (357.4); Requires (Found): C, 57.12 (57.10); H, 5.35 (5.33); N, 19.59 (19.58).
Synthesis of 3-amino-2-(4-chloro-3,5-dimethyl-1Hpyrazol-1-yl)-7,7-dimethyl-3,6,7,8-tetrahydro[1]benzo thieno[2,3-d]pyrimidine-4,5-dione (10b). Was obtained from 7 with 3-chloropentan-2,4-dione (10 m mol) as a light yellow powder crystallized from ethanol in 77% yield; m.p. 210˚C - 212˚C, IR (cm−1, n): 3400 (br, NH), 1715, 1668 (2C=O); 1H NMR (DMSO-d6): d 0.96 (s, 3H, CH3), 1.01 (s, 3H, CH3), 2.12 (m, 4H, 2CH2), 2.23 (s, 3H, CH3), 2.78 (s, 3H, CH3), 9.48 (brs, NH); Its MS (m/z), 391 (M+, 25%), (M+ + 1, 19%); C17H18Cl N5O2S (391.8); Requires (Found): C, 52.10 (52.09); H, 4.62 (4.60); N, 17.87 (17.86).
Synthesis of 3-amino(morpholinyl/methylpiperazin/ and or piperazinyl)-benzothienopyrimidine-4,5-dione (11a-c). General procedure: A mixture of compound 6 (10 m mol) fused with morphline/methylpiprazine/and or piprazine (15 m mol) in sand bath at 180˚C for 3 h. The reaction mixture was allowed to cool to room temp., and then add 20 mL of ethanol the precipitate was filtered off, dried and crystallized from an appropriate solvent to produce 11a-c.
Synthesis of 3-amino-7,7-dimethyl-2-morpholin-4- yl-3,6,7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidine-4,5-dione (11a). It was obtained from 6 with morpholine (15 m mol) as a brown powder crystallized from dioxane in 74 % yield; m.p. 237˚C - 240˚C, IR (cm−1, n): 3410 (br, NH), 1720, 1675 (2C=O); 1H NMR (DMSO-d6): d 0.98 (s, 3H, CH3), 1.03 (s, 3H, CH3), 2.10 (m, 4H, 2CH2), 3.24 (t, 4H, morpholinyl 2NCH2, J = 5.0 Hz), 3.67 (t, 4H, morpholinyl 2OCH2, J = 4.98 Hz), 9.45 (brs, NH); 13C NMR (DMSO-d6): δ, 188.7 (CO), 166.3 (CO amide), 163.6 (C-2, pyrimidine), 141.4, 139.7, 124.5, 114.3 (thiophene carbons), 66.54, 47.09 (4C, O(CH2)2, N(CH2)2) 51.35, 43.65 (2CH2), 40.19 (C(CH3)2), 29.67, 28.03 (2CH3); Its MS (m/z), 348 (M+, 34%); C16H20N4O3S (348.4); Requires (Found): C, 55.15 (55.12); H, 5.78 (5.74); N, 16.08 (16.05).
Synthesis of 3-amino-7,7-dimethyl-2-(4-methyl piperazin-1-yl)-3,6,7,8-tetrahydro[1]benzo-thieno[2,3-d]pyrimidine-4,5-dione (11b). It was obtained from 6 with methylpiprazine (15 m mol) as a yellow powder crystallized from DMF in 68% yield; m.p. 232˚C - 234˚C, IR (cm−1, n): 3390 (br, NH), 1715, 1669 (2C=O); 1H NMR (DMSO-d6): d 0.96 (s, 3H, CH3), 1.01(s, 3H, CH3), 2.09 (m, 4H, 2CH2), 2.30 (s, 3H, piperazinyl NCH3), 2.53 (brs, 4H, piperazinyl 2NCH2), 3.36 (brs, 4H, piperazinyl 2NCH2), 9.76 (brs, NH); 13C NMR (DMSO-d6): δ, 189.7 (CO), 164.9 (CO amide), 161.4 (C-2, pyrimidine), 142.3, 140.4, 124.6, 113.9 (thiophene carbons), 56.47, 46.18 (4C, N(CH2)2, N(CH2)2) 50.85, 42.93 (2CH2), 41.19 (C(CH3)2), 30.02, 29.01 (2CH3); Its MS (m/z), 361 (M+, 29%); C17H23N5O2S (361.4); Requires (Found): C, 56.48 (56.45); H, 6.41 (6.39); N, 19.37 (19.35).
Synthesis of 3-amino-7,7-dimethyl-2-piperazin-1- yl)-3,6,7,8-tetraahydro[1]benzothieno[2,3-d]pyrimidine-4,5-dione (11c). It was obtained from 6 with piprazine (15 m mol) as a pale yellow powder crystallized from DMF in 65% yield; m.p. 250˚C - 252˚C, IR (cm−1, n): 3400 (br, NH), 1713, 1667 (2C=O); 1H NMR (DMSO-d6): d 1.01 (s, 3H, CH3), 1.04 (s, 3H, CH3), 2.11 (m, 4H, 2CH2), 2.48 (brs, 4H, piperazinyl 2NCH2), 3.29 (brs, 4H, piperazinyl 2NCH2), 9.76, 10.15 (brs, NH’s); Its MS (m/z), 347 (M+, 38%); C16H21N5O2S (347.4); Requires (Found): C, 55.30 (55.28); H, 6.09 (6.06); N, 20.15 (20.13).
4.2. Biological Screening
4.2.1. Materials and Methods
Animals-adult rats of both sexes weighing 150 - 200 g and adult mice weighing 20 - 25 g were used in the experiments. Animals were housed under standardized conditions for light and temperature and received standard rat chow and tap water and libitum. Animals were randomly assigned to different experimental groups, each kept in a separate cage. All animal procedures were performed after approval from the Ethics committee of the National Research Center and in accordance with the recommendations for the proper care and use of laboratory animals (NIH publication No. 85-23, revised 1985).
4.2.2. Antiinflammatory Testing
The carrageenan rat paw edema model of inflammation was used to evaluate the anti-inflammatory properties of the tested compounds. Rats were randomly assigned to the treatment groups and sterile carrageenan lambda (100 ul of a 1% solution in saline) was injected sub-planter into right hind paw of the rat. Carrageenan caused visible redness and pronounced swelling that was well developed by 4 h and persisted for more than 48 h. Right hind paw was measured with a planimeter [24,25] before, and at 1, 2, 3 and 4 h after carrageenan injection. All the tested compounds were dissolved in DMSO then injected i.p. (9 mg/ kg b wt).The control animals were injected (i.p.) with appropriate volume of DMSO. The standard drug was indomethacin (10 mg/kg b wt). Different compounds or indomethacin were given 1 hr before carrageenan injection.
4.2.3. Analgesic Testing
The hot-plate test was performed on mice by using an electronically controlled hot-plate (Ugo Basile, Italy) heated to 52˚C, for possible centrally mediated analgesic effect of the drugs. Fourteen groups of rats were given vehicle and/or the different compounds and the last group received tramadol (20 mg/kg b wt) 60 min prior to testing. Latency to lick a hind paw or jumping [26] was recorded sequentially before and at 1, 2 h post treatment.
4.2.4. Ulcerogenic Effects
Groups of five male Wistar rats with a weight between 150 and 175 g are used. They are starved 48 h prior to drug administration. The test compounds are administered orally in 10 mL/kg as aqueous suspension. Doses which are highly active in the activity (9 mg/kg) are chosen and used. The animals are sacrificed after 7 h. Stomachs are removed and placed on saline soaked filter paper until inspection. A longitudinal incision along the greater curvature is made with fine scissor. The stomach is inverted over the index finger and the presence or the absence of gastric irritation is determined. The presence of a single or multiple lesions (erosion, ulcer or perforation) is considered to be positive [23]. The number of ulcers and the occurrence of hyperemia is noted (determine ulcer index).
5. Acknowledgements
The authors are thankful to the Al-Imam Mohammad Ibn Saud Islamic University (IMSIU), Faculty of Science, for providing laboratory facilities, Micro-analytical Centre, and the Pharmacological Unit National Research Centre, for microanalyses and pharmacological screening of the compounds.
REFERENCES
- A. J. Folkes, K. Ahmadi, W. K. Alderton, S. Alix, S. J. Baker, G. Box, I. S. Chuckowree, P. A. Clarke, P. Depledge, S. A. Eccles, L. S. Friedman, A. Hayes, T. C. Hancox, A. Kugendradas, L. Lensun, P. Moore, A. G. Olivero, J. Pang, S. Patel, G. H. Pergl-Wilson, F. I. Raynaud, A. Robson, N. Saghir, L. Salphati, S. Sohal, M. H. Ultsch, M. Valenti, H. J. A. Wallweber, N. C. Wan, C. Wiesmann, P. Workman, A. Zhyvoloup, M. J. Zvelebil and S. J. Shuttleworth, “The Identification of 2-(1H-Indazol-4-yl)-6-(4- methanesulfonylpiperazin-1-yl-methyl)-4-morpholin-4- yl-thie-no[3,2-d]pyrimidine (GDC-0941) as a Potent, Selective, Orally Bioavailable Inhibitor of Class I PI3 Kinase for the Treatment of Cancer,” Journal Medicinal Chemistry, Vol. 51, No. 18, 2008, pp. 5522-5532.
- L. D. Jennings, S. L. Kincaid, Y. D. Wang, G. Krishnamurthy, C. F. Beyer, J. P. McGinnis, M. Miranda, C. M. Discafani and S. K. Rabindran, “Parallel Synthesis and Biological Evaluation of 5,6,7,8-Tetrahydrobenzothieno [2,3-d]pyrimidin-4(3H)-one Cytotoxic Agents Selective for p21-Deficient Cells,” Bioorganic & Medicinal Chemistry Letter, Vol. 15, No. 21, 2005, pp. 4731-4735.
- Y. D. Wang, S. Johnson, D. Powell, J. P. McGinnis, M. Miranda and S. K. Rabindran, “Inhibition of Tumor Cell Proliferation by Thieno[2,3-d]pyrimidin-4(1H)-one-based Analogs,” Bioorganic & Medicinal Chemistry Letter, Vol. 15, No. 16, 2005, pp. 3763-3766.
- T. Horiuchi, J. Chiba, K. Uoto and T. Soga, “Discovery of Novel Thieno[2,3-d]pyrimidin-4-yl Hydrazone-Based Inhibitors of Cyclin D1-CDK4: Synthesis, Biological Evaluation, and Structure-Activity Relationships,” Bioorganic & Medicinal Chemistry Letter, Vol. 19, No. 2, 2009, pp. 305-308.
- A. Angell, C. McGuigan, L. G. Sevillano, R. Snoeck, G. Andrei, E. De Clercq and J. Balzarini “Bicyclic AntiVZV Nucleosides,” Bioorganic & Medicinal Chemistry Letter, Vol. 14, No. 10, 2004, pp. 2397-2399. doi:10.1016/j.bmcl.2004.03.029
- A. Brancale, C. Mcguigan, B. Algain, S. Pascal, R. Benhida, J. L. Fourrey, G. Andrei, R. Snoeck, E. De Clercq and J. Balzarini, “Bicyclic Anti-VZV Nucleosides: Thieno Analogues Retain Full Antiviral Activity,” Bioorganic & Medicinal Chemistry Letter, Vol. 11, No. 18, 2001, pp. 2507-2510.
- M. H. Bhuiyan. K. M. Rahman, K. Hossain, A. Rahim, I. Hossain and M. Abu Naser, “Synthesis and Antimicrobial Evaluation of Some New Thienopyrimidine Derivatives,” Acta pharmaceutica, Vol. 56, No. 4, 2006, pp. 441-450.
- R. V. Chambhare, B. G. Khadse, A. S. Bobde and R. H. Bahekar, “Synthesis and Preliminary Evaluation of Some N-[5-(2-Furanyl)-2-methyl-4-oxo-4H-thieno[2,3-d]pyrimidin-3-yl]carboxamide and 3-Substituted-5-(2-furanyl)-2- methyl-3H-thieno[2,3-d]pyrimidin-4-ones as Antimicrobial Agents,” European Journal of Medicinal Chemistry, Vol. 38, No. 1, 2003, pp. 89-100.
- S. Yousieff and B. E. Bayoumy, “1,3,4-Thiadiazolylthieno Pyrimidines and 1,3,4-Oxadiazolyl Thienopyrimidines for Antibacterial Activity,” Journal of Pharmaceutical Sciences, Vol. 31, No. 1-3, 1990, p. 67.
- V. Alagarsamy, S. Vijayakumar and V. R. Solomon, “Synthesis of 2-Mercapto-3-substituted-5,6-dimethylthieno[2,3-d]Pyrimidin-4(3H)-ones as New Analgesic, AntiInflammatory Agents,” Biomed Pharmacother, Vol. 61, No. 5, 2007, pp. 285-291. doi:10.1016/j.biopha.2007.02.008
- V. Alagarsamy, S. Meena, K. V. Ramseshu, V. R. Solomon, K. Thirumurugan, K. Dhanabal and M. Murugan, “Synthesis, Analgesic, Anti-Inflammatory, Ulcerogenic Index and Antibacterial Activities of Novel 2-Methylthio- 3-substituted-5,6,7,8-tetrahydrobenzo(b)thieno[2,3-d]pyrimidin-4(3H)-ones,” European Journal of Medicinal Chemistry, Vol. 41, No. 11, 2006, pp. 1293-1300.
- I. O. Donkor, L. I. Hui and S. F. Queener, “Synthesis and DHFR Inhibitory Activity of a Series of 6-Substituted- 2,4-diaminothieno[2,3-d]pyrimidines,” European Journal of Medicinal Chemistry, Vol. 38, No. 6, 2003, pp. 605- 611.
- H. N. Hafez and A. B. A. El-Gazzar, “Design and Synthesis of 3-Pyrazolyl-thiophene, Thieno[2,3-d]pyrimidines as New Bioactive and Pharmacological Activities,” Bioorganic & Medicinal Chemistry Letter, Vol. 18, No. 19, 2008, pp. 5222-5227.
- A. B. A. El-Gazzar, H. A. R. Hossein and H. N. Hafez, “Synthesis and Biological Evaluation of Thieno[2,3- d]pyrimidine Derivatives as Anti-inflammatory, Analgesic and Ulcerogenic Activity,” Acta Pharmaceutica, Vol. 57, No. 4, 2007, pp. 395-411. doi:10.2478/v10007-007-0032-6
- M. Santagati, M. Modica, A. Santagati, F. Russo and S. Spampinato, “Synthesis of Aminothienopyrimidine and Thienotriazolopyrimidine Derivatives as Potential Anticonvulsant Agents,” Die Pharmazie, Vol. 51, No. 1, 1996, pp. 7-11.
- M. R. Prasad, R. A. Raghuram, R. P. Shanthan, R. K. Subramanian, S. Meena and K. Madhavi, “Synthesis and Adenosine Receptor Binding Studies of Some Novel Triazolothienopyrimidines,” European Journal of Medicinal Chemistry, Vol. 43, No. 3, 2008, pp. 614-620.
- L. P. Melissal, C. G. Wayne, E. Tara, A. N. Jason, L. Patricia, C. J. José, D. Fernando and J. T. Robert, “Design of Novel N-(2,4-Dioxo-1,2,3,4-tetrahydro-thieno[3,2-d] pyrimidin-7-yl)-guanidines as Thymidine Phosphorylase Inhibitors, and Flexible Docking to a Homology Model,” Bioorganic & Medicinal Chemistry Letter, Vol. 13, No. 1, 2003, pp. 107-110.
- H. N. Hafez, H. A. Hussein and A. B. A. El-Gazzar, “Synthesis of Substituted Thieno[2,3-d]pyrimidine-2,4- dithiones and Their S-Glycoside Analogues as Potential Antiviral and Antibacterial Agents,” European Journal of Medicinal Chemistry, Vol. 45, No. 9, 2010, pp. 4026- 4034.
- C. J. Shishoo, V. S. Shirsath, I. S. Rathod, M. J. Patil and S. S. Bhargava, “Design Synthesis and Antihistaminic (H1) Activity of Some Condensed 2-(Substituted)arylaminoethylpyrimidine-4-(3H)-ones,” Arzneim Forsch, Vol. 51, No. 3, 2001, pp. 221-231.
- G. A. Winter, E. A. Rislfy and G. W. Nuss, “Carrageenininduced Edema in Hind Paw of Rat as an Assay for Antiinflammatory Drugs,” Proceedings of the Society for Experimental Biology and Medicine, Vol. 111, 1962, pp. 544-547.
- P. Armitage, “Statistical Methods in Medical Research,” 1st Edition, Blackwell Scientific Publ., Oxford, London, 1971.
- G. Woolfe and A. D. MacDonald, “The Evaluation of the Analgesic Action of Pethidine Hydrochloride,” Journal of Pharmacoogy and Experimental Therapeutics, Vol. 80, No. 3, 1944, pp. 300-307.
- A. E. Amr and M. M. Abdulla, “Synthesis and Antiinflammatory Activities of New Cyanopyrane Derivatives Fused with Steroidal Nuclei,” Archiv der Pharmazie Chemistry Life Science, Vol. 339, No. 2, 2006. pp. 88-95. doi:10.1002/ardp.200500209
- M. G. Obukowics, D. J. Walseh, W. J. Salsgiver, C. L. Martin-Berger, K. S. Chinn, K. L. Duffin, A. Ras and P. Needleman, “Novel, Selective Δ6 or Δ5 Fatty Acid Desaturase Inhibitors as Antiinflammatory Agents in Mice,” Journal of Pharmacoogy and Experimental Therapeutics, Vol. 287, No. 1, 1998, pp. 157-166.
- L. Meng, R. Mohan, B. H. B. Kwok, M. Elofssof, N. Sin and C. M. Crews, “Epoxomicin, a Potent and Selective Proteasome Inhibitor, Exhibits in Vivo Antiinflammatory Activity,” Proceedings of the National Academy of Science, USA, Vol. 96, No. 18, 1999, pp. 10403-10408.
- M. Eaton, “Common Animal Models for Spasticity and Pain,” Journal of Rehabilitation Research and Development, Vol. 40, No. 4, 2003, pp. 41-54. doi:10.1682/JRRD.2003.08.0041
NOTES
*Corresponding author.

