Experimental Study of a Solar Adsorption Refrigeration Unit, Factorial Analysis
Copyright © 2012 SciRes. SGRE
132
REFERENCES
[1] J. A. Edmonds, D. L. Wuebles and M. J. Scott, “Energy
and Radiative Precursor Emissions,” International Con-
ference on Alternative Energy Sources, Miami, 14-16 De-
cember 1987.
[2] F. Zigler, “Recent Development and Future Prospects of
Sorption Heat Pump Systems,” International Journal of
Thermals, Vol. 38, No. 3, 1999, pp. 191-208.
doi:10.1016/S1290-0729(99)80083-0
[3] E. B. Miller “The Development of Silica Gel Refrigera-
tion,” Refrigeration Engineering, Vol. 17, No. 4, 1929, pp.
103-108.
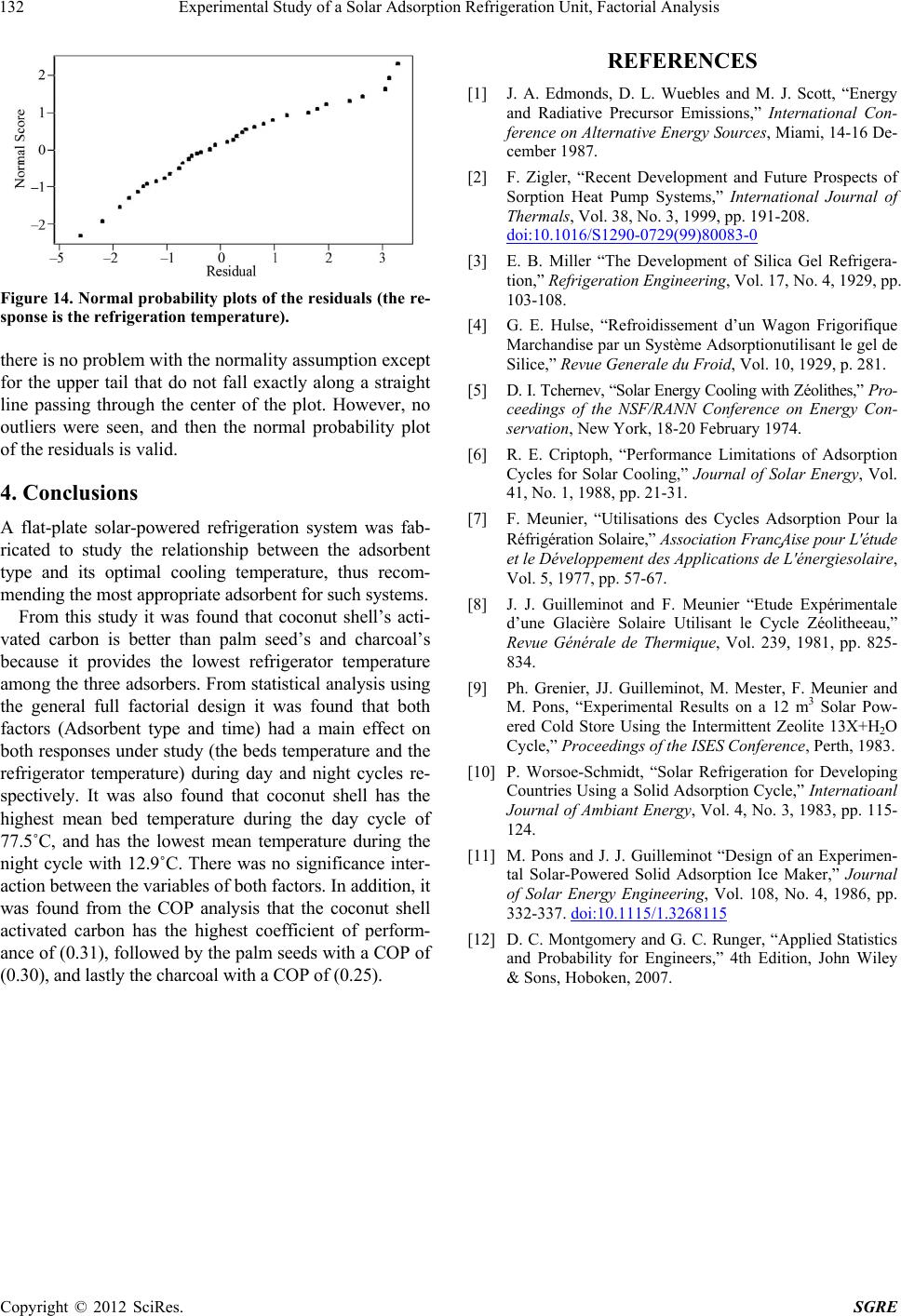
Figure 14. Normal probability plots of the residuals (the re-
sponse is the refrigeration temperature). [4] G. E. Hulse, “Refroidissement d’un Wagon Frigorifique
Marchandise par un Système Adsorptionutilisant le gel de
Silice,” Revue Generale du Froid, Vol. 10, 1929, p. 281.
there is no problem with the normality assumption except
for the upper tail that do not fall exactly along a straight
line passing through the center of the plot. However, no
outliers were seen, and then the normal probability plot
of the residuals is valid.
[5] D. I. Tchernev, “Solar Energy Cooling with Zéolithes,” Pro-
ceedings of the NSF/RANN Conference on Energy Con-
servation, New York, 18-20 February 1974.
[6] R. E. Criptoph, “Performance Limitations of Adsorption
Cycles for Solar Cooling,” Journal of Solar Energy, Vol.
41, No. 1, 1988, pp. 21-31.
4. Conclusions
[7] F. Meunier, “Utilisations des Cycles Adsorption Pour la
Réfrigération Solaire,” Association Franc
̧
Aise pour L'étude
et le Développement des Applications de L'énergiesolaire,
Vol. 5, 1977, pp. 57-67.
A flat-plate solar-powered refrigeration system was fab-
ricated to study the relationship between the adsorbent
type and its optimal cooling temperature, thus recom-
mending the most appropriate adsorbent for such systems. [8] J. J. Guilleminot and F. Meunier “Etude Expérimentale
d’une Glacière Solaire Utilisant le Cycle Zéolitheeau,”
Revue Générale de Thermique, Vol. 239, 1981, pp. 825-
834.
From this study it was found that coconut shell’s acti-
vated carbon is better than palm seed’s and charcoal’s
because it provides the lowest refrigerator temperature
among the three adsorbers. From statistical analysis using
the general full factorial design it was found that both
factors (Adsorbent type and time) had a main effect on
both responses under study (the beds temperature and the
refrigerator temperature) during day and night cycles re-
spectively. It was also found that coconut shell has the
highest mean bed temperature during the day cycle of
77.5˚C, and has the lowest mean temperature during the
night cycle with 12.9˚C. There was no significance inter-
action between the variables of both factors. In addition, it
was found from the COP analysis that the coconut shell
activated carbon has the highest coefficient of perform-
ance of (0.31), followed by the palm seeds with a COP of
[9] Ph. Grenier, JJ. Guilleminot, M. Mester, F. Meunier and
M. Pons, “Experimental Results on a 12 m3 Solar Pow-
ered Cold Store Using the Intermittent Zeolite 13X+H2O
Cycle,” Proceedings of the ISES Conference, Perth, 1983.
[10] P. Worsoe-Schmidt, “Solar Refrigeration for Developing
Countries Using a Solid Adsorption Cycle,” Internatioanl
Journal of Ambiant Energy, Vol. 4, No. 3, 1983, pp. 115-
124.
[11] M. Pons and J. J. Guilleminot “Design of an Experimen-
tal Solar-Powered Solid Adsorption Ice Maker,” Journal
of Solar Energy Engineering, Vol. 108, No. 4, 1986, pp.
332-337. doi:10.1115/1.3268115
[12] D. C. Montgomery and G. C. Runger, “Applied Statistics
and Probability for Engineers,” 4th Edition, John Wiley
& Sons, Hoboken, 2007.
(0.30), and lastly the charcoal with a COP of (0.25).