Paper Menu >>
Journal Menu >>
 American Journal of Analytical Chemistry, 2010, 1, 25-30 doi:10.4236/ajac.2010.11003 Published Online May 2010 (http://www.SciRP.org/journal/ajac) Copyright © 2010 SciRes. AJAC Kinetic Studies on Hexavalent Chromium Reduction Ankita Basu, Bidyut Saha* Department of Chemistry, University of Burdwan, Golapbag, India E-mail: b_saha31@rediffmail.com Received January 10, 2009; revised February 21, 2009; accepted February 23, 2009 Abstract Cr(VI) is a known human carcinogen. It is a wide spread environmental contaminant as it is extensively used in different industry. The kinetic study of reduction of Cr(VI) by a known organic substance, 1-butanol in micellar media have been studied spectrophotometrically. The reduction of Cr(VI) to Cr(III) occurs in a micro- heterogeneous system in cell cytoplasm. As micelles are considered to mimic the cellular membranes, the reduction process occurring in the micellar system is considered as a model to obtain insight in to the reduc- tion process prevailing in body systems. Micellar media is also a probe to establish the mechanistic paths of reduction of Cr(VI) to Cr(III) and the effects of some electrolytes common to a biological systems are stud- ied to establish the proposed reaction mechanism strongly. The overall reaction follows a first order depend- ency on substrate and hexavalent chromium and second order dependency on hydrogen ion. Suitable surfac- tant & suitable concentration of electrolyte enhance the rate of the reaction. Keywords: Pollution, Carcinogen, Cr(VI), Oxidation; Kinetics, Surfactant, Electrolyte 1. Introduction Water pollution by chromium is of considerable concern, as this metal has found widespread use in electro plating, leather tanning, metal finishing, nuclear power plant, tex- tile industries and chromate preparation [1]. The effluents from these industries contain Cr(III) and Cr(VI) at con- centrations ranging from ten to hundreds of mg/L [2]. The hexavalent form is 500 times more toxic than the trivalent [3]. Though chromium exists in nine valence states rang- ing from –2 to +6, Cr(III) and Cr(VI) are of major envi- ronmental significance because of their stability in the natural environment [4]. The chromate anion is highly soluble and therefore can overcome the cellular perme- ability barrier, entering via sulphate transport pathways since it bears structural similarity with [5-7]. It has been reported that hexavalent chromium causes lung can- cer, chromate ulcer, perforation of nasal septum and kid- ney damage in humans and it is also toxic to other organ- ism as well [8,9]. Chromium in its trivalent form is an essential micronutrient for many microorganisms, rela- tively insoluble in water. 2 4 SO A number of treatment methods for removal of metal ions from aqueous solutions have been reported mainly reduction, ion exchange, solvent extraction, reverse os- mosis, chemical precipitation and adsorption [10]. In the reduction followed by chemical precipitation method [11], Cr(VI) is reduced to Cr(III) first, then lime is added to precipitate chromium as hydroxide. In this paper, we studied kinetics and mechanism of Cr(VI) reduction to Cr(III) by an alcohol in presence of micelle and electrolyte because micelle [12-14] and elec- trolyte [15] substantiate the reaction mechanism. The outcome of such an exercise will certainly influence the detoxification methods. 2. Experimental 2.1. Materials and Reagents Butan-1-ol (AR, Merck, India), K2Cr2O7 (AR, BDH, In- dia), N-cetyl pyridinium chloride (CPC) (AR, SRL, In- dia), Sodium dodecyl sulphate (SDS) (AR, SRL, India), TX-100 (AR, SRL, India), NaCl (AR, Merck, India), NH4Cl (AR, Ranbaxy, India) and other chemicals used were of highest purity available commercially. Solutions were prepared in double distilled water. 2.2. Procedure and Kinetic Measurements Under the kinetic conditions, solutions of the oxidant and mixtures containing the known quantities of the sub- strate(s) (i.e., butan-1-ol) (under the conditions [S]T >> [Cr(VI)]T), acid and the other necessary chemicals were 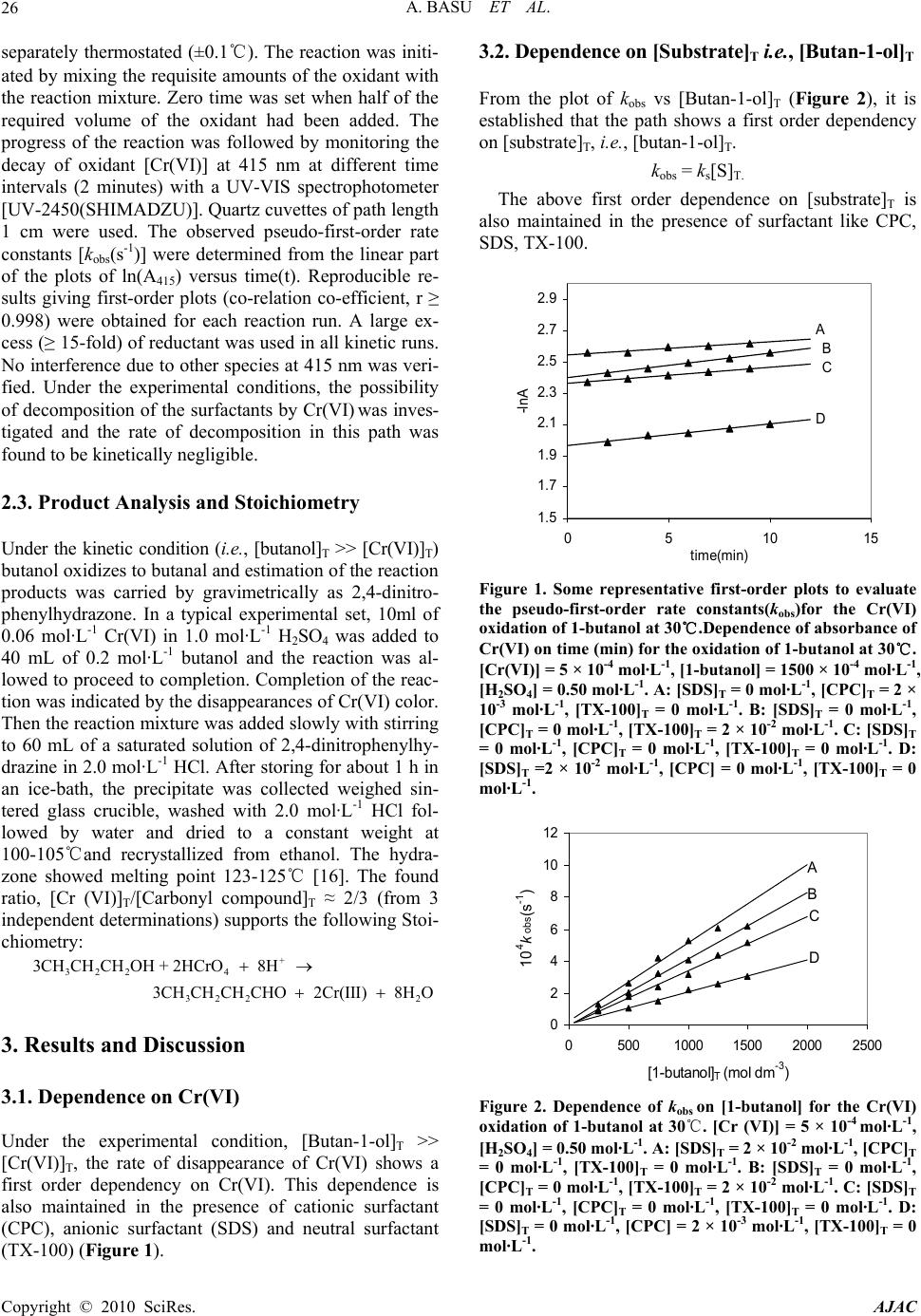 A. BASU ET AL. Copyright © 2010 SciRes. AJAC 26 separately thermostated (±0.1℃). The reaction was initi- ated by mixing the requisite amounts of the oxidant with the reaction mixture. Zero time was set when half of the required volume of the oxidant had been added. The progress of the reaction was followed by monitoring the decay of oxidant [Cr(VI)] at 415 nm at different time intervals (2 minutes) with a UV-VIS spectrophotometer [UV-2450(SHIMADZU)]. Quartz cuvettes of path length 1 cm were used. The observed pseudo-first-order rate constants [kobs(s-1)] were determined from the linear part of the plots of ln(A415) versus time(t). Reproducible re- sults giving first-order plots (co-relation co-efficient, r ≥ 0.998) were obtained for each reaction run. A large ex- cess (≥ 15-fold) of reductant was used in all kinetic runs. No interference due to other species at 415 nm was veri- fied. Under the experimental conditions, the possibility of decomposition of the surfactants by Cr(VI) was inves- tigated and the rate of decomposition in this path was found to be kinetically negligible. 2.3. Product Analysis and Stoichiometry Under the kinetic condition (i.e., [butanol]T >> [Cr(VI)]T) butanol oxidizes to butanal and estimation of the reaction products was carried by gravimetrically as 2,4-dinitro- phenylhydrazone. In a typical experimental set, 10ml of 0.06 mol·L-1 Cr(VI) in 1.0 mol·L-1 H2SO4 was added to 40 mL of 0.2 mol·L-1 butanol and the reaction was al- lowed to proceed to completion. Completion of the reac- tion was indicated by the disappearances of Cr(VI) color. Then the reaction mixture was added slowly with stirring to 60 mL of a saturated solution of 2,4-dinitrophenylhy- drazine in 2.0 mol·L-1 HCl. After storing for about 1 h in an ice-bath, the precipitate was collected weighed sin- tered glass crucible, washed with 2.0 mol·L-1 HCl fol- lowed by water and dried to a constant weight at 100-105℃and recrystallized from ethanol. The hydra- zone showed melting point 123-125℃ [16]. The found ratio, [Cr (VI)]T/[Carbonyl compound]T ≈ 2/3 (from 3 independent determinations) supports the following Stoi- chiometry: + 322 4 3CHCHCHOH + 2HCrO 8H 322 2 3CHCHCHCHO 2Cr(III) 8HO 3. Results and Discussion 3.1. Dependence on Cr(VI) Under the experimental condition, [Butan-1-ol]T >> [Cr(VI)]T, the rate of disappearance of Cr(VI) shows a first order dependency on Cr(VI). This dependence is also maintained in the presence of cationic surfactant (CPC), anionic surfactant (SDS) and neutral surfactant (TX-100) (Figure 1). 3.2. Dependence on [Substrate]T i.e., [Butan-1-ol]T From the plot of kobs vs [Butan-1-ol]T (Figure 2), it is established that the path shows a first order dependency on [substrate]T, i.e., [butan-1-ol]T. kobs = ks[S]T. The above first order dependence on [substrate]T is also maintained in the presence of surfactant like CPC, SDS, TX-100. 1.5 1.7 1.9 2.1 2.3 2.5 2.7 2.9 0510 time(min) -l nA 15 D A C B Figure 1. Some representative first-order plots to evaluate the pseudo-first-order rate constants(kobs)for the Cr(VI) oxidation of 1-butanol at 30℃.Dependence of absorbance of Cr(VI) on time (min) for the oxidation of 1-butanol at 30℃. [Cr(VI)] = 5 × 10-4 mol·L-1, [1-butanol] = 1500 × 10-4 mol·L-1, [H2SO4] = 0.50 mol·L-1. A: [SDS]T = 0 mol·L-1, [CPC]T = 2 × 10-3 mol·L-1, [TX-100]T = 0 mol·L-1. B: [SDS]T = 0 mol·L-1, [CPC]T = 0 mol·L-1, [TX-100]T = 2 × 10-2 mol·L-1. C: [SDS]T = 0 mol·L-1, [CPC]T = 0 mol·L-1, [TX-100]T = 0 mol·L-1. D: [SDS]T =2 × 10-2 mol·L-1, [CPC] = 0 mol·L-1, [TX-100]T = 0 mol·L-1. 0 2 4 6 8 10 12 05001000 150020002500 [1-butanol] T (mol dm -3 ) 10 4 k obs (s -1 ) A B C D Figure 2. Dependence of kobs on [1-butanol] for the Cr(VI) oxidation of 1-butanol at 30℃. [Cr (VI)] = 5 × 10-4 mol·L-1, [H2SO4] = 0.50 mol·L-1. A: [SDS]T = 2 × 10-2 mol·L-1, [CPC]T = 0 mol·L-1, [TX-100]T = 0 mol·L-1. B: [SDS]T = 0 mol·L-1, [CPC]T = 0 mol·L-1, [TX-100]T = 2 × 10-2 mol·L-1. C: [SDS]T = 0 mol·L-1, [CPC]T = 0 mol·L-1, [TX-100]T = 0 mol·L-1. D: [SDS]T = 0 mol·L-1, [CPC] = 2 × 10-3 mol·L-1, [TX-100]T = 0 mol·L-1. 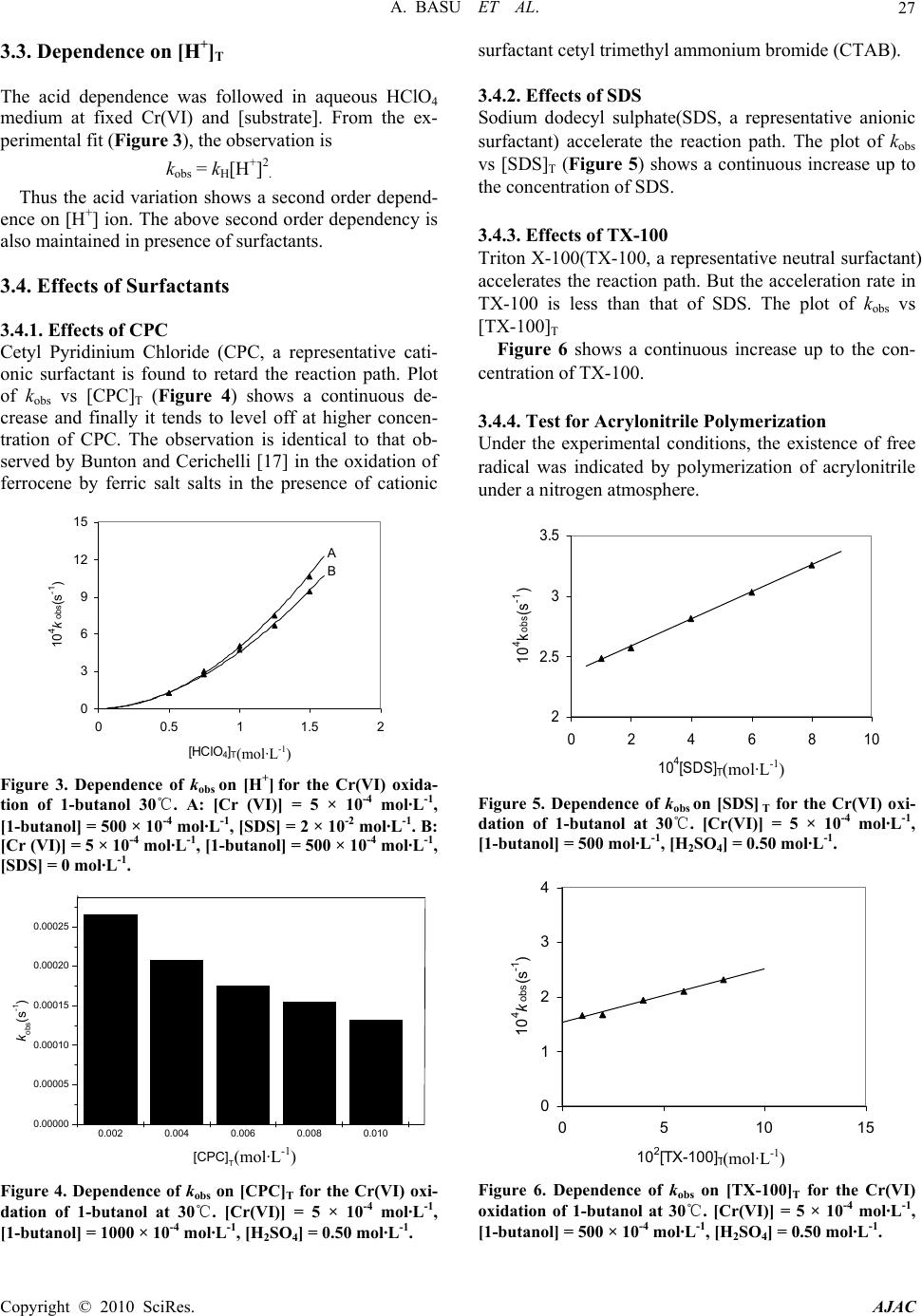 A. BASU ET AL.27 3.3. Dependence on [H+]T The acid dependence was followed in aqueous HClO4 medium at fixed Cr(VI) and [substrate]. From the ex- perimental fit (Figure 3), the observation is kobs = kH[H+]2 . Thus the acid variation shows a second order depend- ence on [H+] ion. The above second order dependency is also maintained in presence of surfactants. 3.4. Effects of Surfactants 3.4.1. Effects of CPC Cetyl Pyridinium Chloride (CPC, a representative cati- onic surfactant is found to retard the reaction path. Plot of kobs vs [CPC]T (Figure 4) shows a continuous de- crease and finally it tends to level off at higher concen- tration of CPC. The observation is identical to that ob- served by Bunton and Cerichelli [17] in the oxidation of ferrocene by ferric salt salts in the presence of cationic 0 3 6 9 12 15 00.511.5 [HClO 4 ] 2 10 4 k obs (s -1 ) T (mol dm -3 ) A B Figure 3. Dependence of kobs on [H+] for the Cr(VI) oxida- tion of 1-butanol 30℃. A: [Cr (VI)] = 5 × 10-4 mol·L-1, [1-butanol] = 500 × 10-4 mol·L-1, [SDS] = 2 × 10-2 mol·L-1. B: [Cr (VI)] = 5 × 10-4 mol·L-1, [1-butanol] = 500 × 10-4 mol·L-1, [SDS] = 0 mol·L-1. 0.002 0.004 0.006 0.008 0.010 0.00000 0.00005 0.00010 0.00015 0.00020 0.00025 Fig 5 kobs(s-1) [CPC]T(mol dm-3) Figure 4. Dependence of kobs on [CPC]T for the Cr(VI) oxi- dation of 1-butanol at 30℃. [Cr(VI)] = 5 × 10-4 mol·L-1, [1-butanol] = 1000 × 10-4 mol·L-1, [H2SO4] = 0.50 mol·L-1. surfactant cetyl trimethyl ammonium bromide (CTAB). 3.4.2. Effects of SDS Sodium dodecyl sulphate(SDS, a representative anionic surfactant) accelerate the reaction path. The plot of kobs vs [SDS]T (Figure 5) shows a continuous increase up to the concentration of SDS. 3.4.3. Effects of TX-100 Triton X-100(TX-100, a representative neutral surfactant) accelerates the reaction path. But the acceleration rate in TX-100 is less than that of SDS. The plot of kobs vs [TX-100]T Figure 6 shows a continuous increase up to the con- centration of TX-100. 3.4.4. Test for Acrylonitrile Polymerization Under the experimental conditions, the existence of free radical was indicated by polymerization of acrylonitrile under a nitrogen atmosphere. 2 2.5 3 3.5 02468 10 4 [SDS] 10 10 4 k obs (s -1 ) T (mol dm -3 ) (mol·L-1) (mol·L-1) Figure 5. Dependence of kobs on [SDS] T for the Cr(VI) oxi- dation of 1-butanol at 30℃. [Cr(VI)] = 5 × 10-4 mol·L-1, [1-butanol] = 500 mol·L-1, [H2SO4] = 0.50 mol·L-1. 0 1 2 3 4 0510 10 2 [TX-100] T (mol dm -3 ) 10 4 k obs (s -1 ) 15 (mol·L-1) (mol·L-1) Figure 6. Dependence of kobs on [TX-100]T for the Cr(VI) oxidation of 1-butanol at 30℃. [Cr(VI)] = 5 × 10-4 mol·L-1, [1-butanol] = 500 × 10-4 mol·L-1, [H2SO4] = 0.50 mol·L-1. Copyright © 2010 SciRes. AJAC 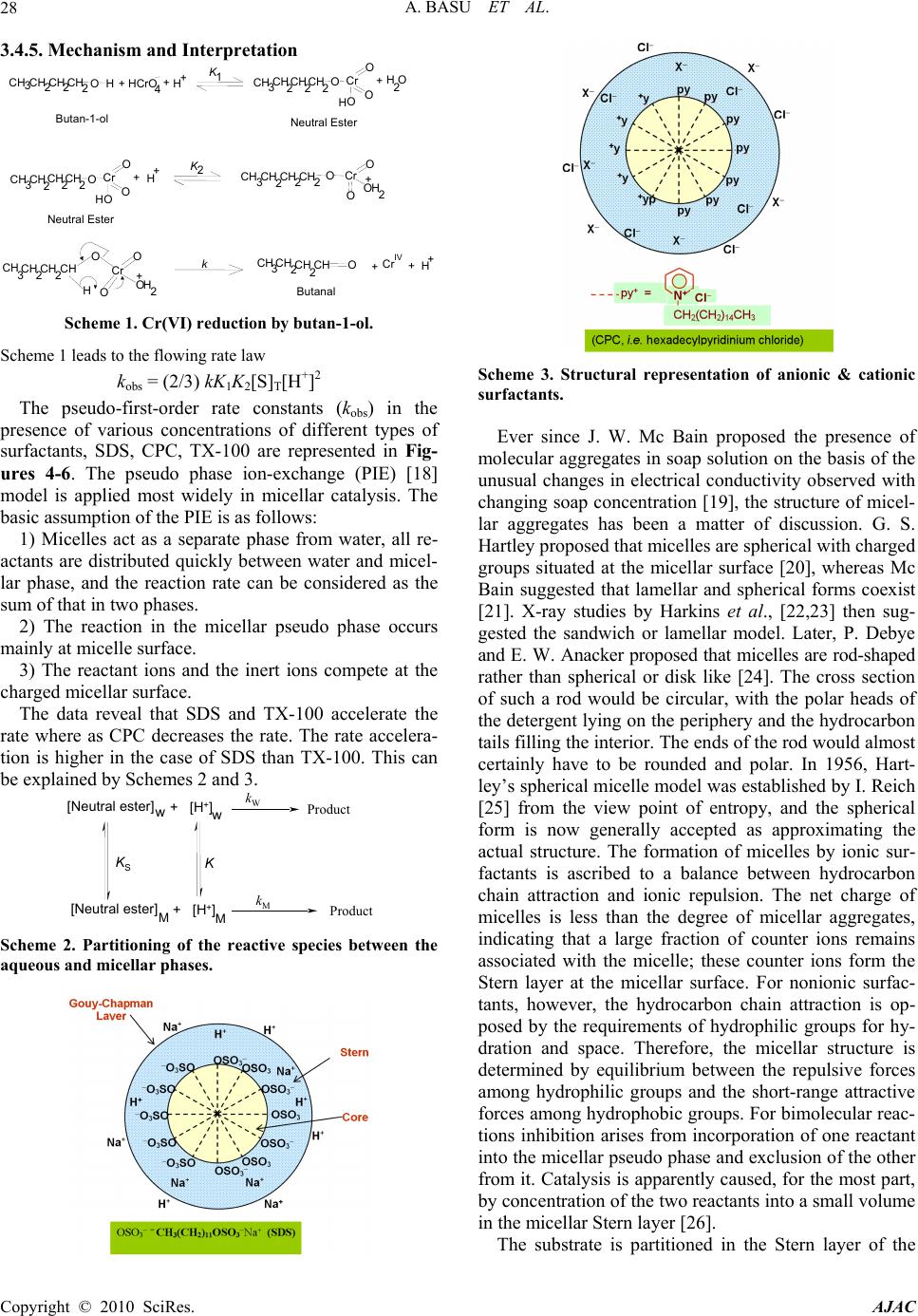 A. BASU ET AL. Copyright © 2010 SciRes. AJAC 28 3.4.5. Mechanism and Interpretation 1CH 3222 Cr O O O H H +2 Neutral Ester Butan-1-ol H O 2 + K CH3 CH 2 CH 2 CH 2OH+HCrO 4+H+K CH CH CH O CH 3222Cr O O H Neutral Ester + CH CH CH O O CH CHCH CH 3222 OCr O OOH2 + CH3 CH CH CH 22 O Cr O O OH 2 + H CH CH CH CH 322O Butanal + CrIV kH + + Scheme 1. Cr(VI) reduction by butan-1-ol. Scheme 1 leads to the flowing rate law kobs = (2/3) kK1K2[S]T[H+]2 The pseudo-first-order rate constants (kobs) in the presence of various concentrations of different types of surfactants, SDS, CPC, TX-100 are represented in Fig- ures 4-6. The pseudo phase ion-exchange (PIE) [18] model is applied most widely in micellar catalysis. The basic assumption of the PIE is as follows: 1) Micelles act as a separate phase from water, all re- actants are distributed quickly between water and micel- lar phase, and the reaction rate can be considered as the sum of that in two phases. 2) The reaction in the micellar pseudo phase occurs mainly at micelle surface. 3) The reactant ions and the inert ions compete at the charged micellar surface. The data reveal that SDS and TX-100 accelerate the rate where as CPC decreases the rate. The rate accelera- tion is higher in the case of SDS than TX-100. This can be explained by Schemes 2 and 3. kWProduct kMProduct [Neutral ester]+ [H+] [Neutral ester]+ [H+] K KS ww M M Scheme 2. Partitioning of the reactive species between the aqueous and micellar phases. Scheme 3. Structural representation of anionic & cationic surfactants. Ever since J. W. Mc Bain proposed the presence of molecular aggregates in soap solution on the basis of the unusual changes in electrical conductivity observed with changing soap concentration [19], the structure of micel- lar aggregates has been a matter of discussion. G. S. Hartley proposed that micelles are spherical with charged groups situated at the micellar surface [20], whereas Mc Bain suggested that lamellar and spherical forms coexist [21]. X-ray studies by Harkins et al., [22,23] then sug- gested the sandwich or lamellar model. Later, P. Debye and E. W. Anacker proposed that micelles are rod-shaped rather than spherical or disk like [24]. The cross section of such a rod would be circular, with the polar heads of the detergent lying on the periphery and the hydrocarbon tails filling the interior. The ends of the rod would almost certainly have to be rounded and polar. In 1956, Hart- ley’s spherical micelle model was established by I. Reich [25] from the view point of entropy, and the spherical form is now generally accepted as approximating the actual structure. The formation of micelles by ionic sur- factants is ascribed to a balance between hydrocarbon chain attraction and ionic repulsion. The net charge of micelles is less than the degree of micellar aggregates, indicating that a large fraction of counter ions remains associated with the micelle; these counter ions form the Stern layer at the micellar surface. For nonionic surfac- tants, however, the hydrocarbon chain attraction is op- posed by the requirements of hydrophilic groups for hy- dration and space. Therefore, the micellar structure is determined by equilibrium between the repulsive forces among hydrophilic groups and the short-range attractive forces among hydrophobic groups. For bimolecular reac- tions inhibition arises from incorporation of one reactant into the micellar pseudo phase and exclusion of the other from it. Catalysis is apparently caused, for the most part, by concentration of the two reactants into a small volume in the micellar Stern layer [26]. The substrate is partitioned in the Stern layer of the 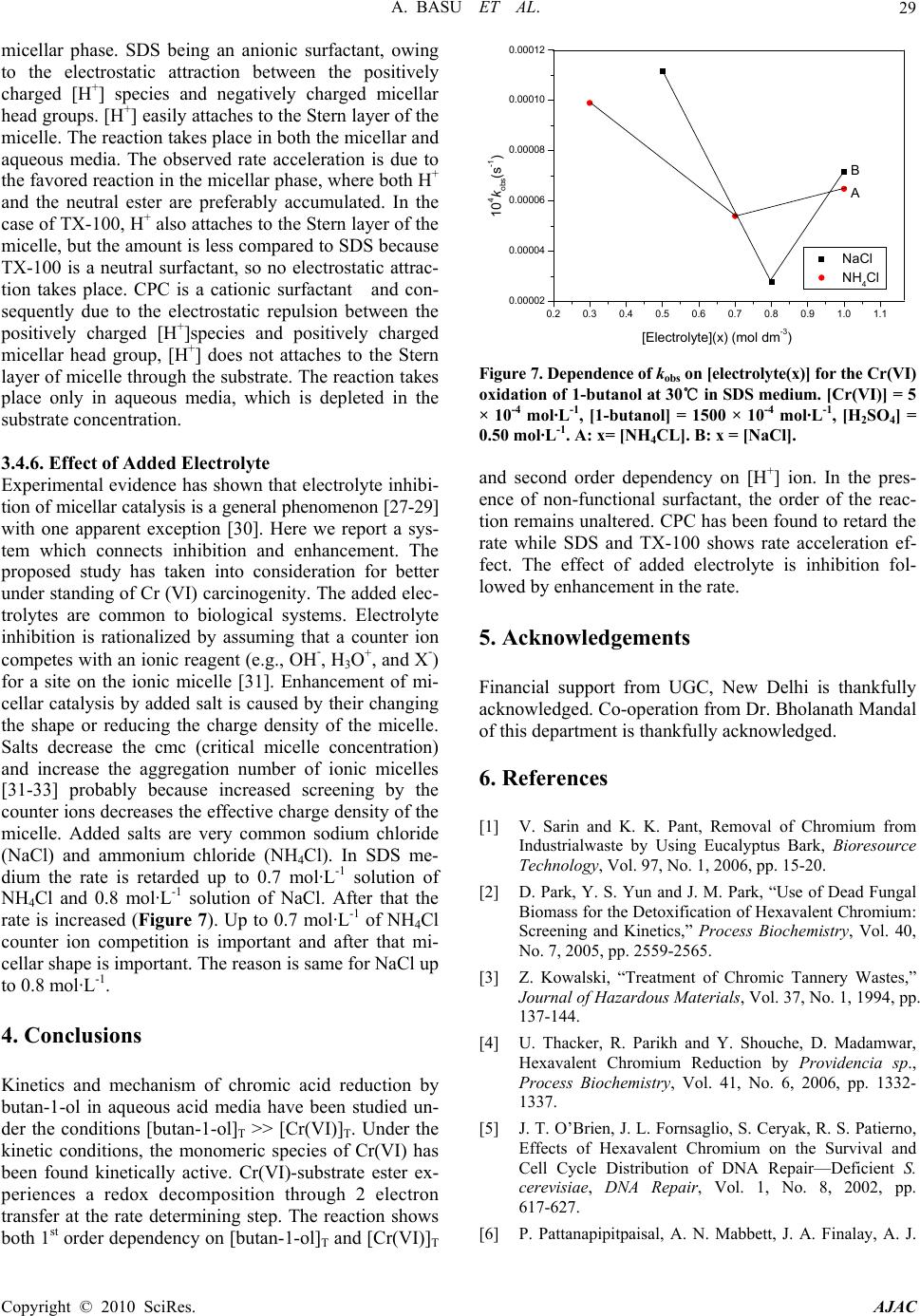 A. BASU ET AL.29 micellar phase. SDS being an anionic surfactant, owing to the electrostatic attraction between the positively charged [H+] species and negatively charged micellar head groups. [H+] easily attaches to the Stern layer of the micelle. The reaction takes place in both the micellar and aqueous media. The observed rate acceleration is due to the favored reaction in the micellar phase, where both H+ and the neutral ester are preferably accumulated. In the case of TX-100, H+ also attaches to the Stern layer of the micelle, but the amount is less compared to SDS because TX-100 is a neutral surfactant, so no electrostatic attrac- tion takes place. CPC is a cationic surfactant and con- sequently due to the electrostatic repulsion between the positively charged [H+]species and positively charged micellar head group, [H+] does not attaches to the Stern layer of micelle through the substrate. The reaction takes place only in aqueous media, which is depleted in the substrate concentration. 3.4.6. Effect of Added Electrolyte Experimental evidence has shown that electrolyte inhibi- tion of micellar catalysis is a general phenomenon [27-29] with one apparent exception [30]. Here we report a sys- tem which connects inhibition and enhancement. The proposed study has taken into consideration for better under standing of Cr (VI) carcinogenity. The added elec- trolytes are common to biological systems. Electrolyte inhibition is rationalized by assuming that a counter ion competes with an ionic reagent (e.g., OH-, H3O+, and X-) for a site on the ionic micelle [31]. Enhancement of mi- cellar catalysis by added salt is caused by their changing the shape or reducing the charge density of the micelle. Salts decrease the cmc (critical micelle concentration) and increase the aggregation number of ionic micelles [31-33] probably because increased screening by the counter ions decreases the effective charge density of the micelle. Added salts are very common sodium chloride (NaCl) and ammonium chloride (NH4Cl). In SDS me- dium the rate is retarded up to 0.7 mol·L-1 solution of NH4Cl and 0.8 mol·L-1 solution of NaCl. After that the rate is increased (Figure 7). Up to 0.7 mol·L-1 of NH4Cl counter ion competition is important and after that mi- cellar shape is important. The reason is same for NaCl up to 0.8 mol·L-1. 4. Conclusions Kinetics and mechanism of chromic acid reduction by butan-1-ol in aqueous acid media have been studied un- der the conditions [butan-1-ol]T >> [Cr(VI)]T. Under the kinetic conditions, the monomeric species of Cr(VI) has been found kinetically active. Cr(VI)-substrate ester ex- periences a redox decomposition through 2 electron transfer at the rate determining step. The reaction shows both 1st order dependency on [butan-1-ol]T and [Cr(VI)]T 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 0.00002 0.00004 0.00006 0.00008 0.00010 0.00012 Fig 7 B A NaCl NH4Cl 104kobs(s-1) [Electrolyte](x) (mol dm-3) Figure 7. Dependence of kobs on [electrolyte(x)] for the Cr(VI) oxidation of 1-butanol at 30℃ in SDS medium. [Cr(VI)] = 5 × 10-4 mol·L-1, [1-butanol] = 1500 × 10-4 mol·L-1, [H2SO4] = 0.50 mol·L-1. A: x= [NH4CL]. B: x = [NaCl]. and second order dependency on [H+] ion. In the pres- ence of non-functional surfactant, the order of the reac- tion remains unaltered. CPC has been found to retard the rate while SDS and TX-100 shows rate acceleration ef- fect. The effect of added electrolyte is inhibition fol- lowed by enhancement in the rate. 5. Acknowledgements Financial support from UGC, New Delhi is thankfully acknowledged. Co-operation from Dr. Bholanath Mandal of this department is thankfully acknowledged. 6. References [1] V. Sarin and K. K. Pant, Removal of Chromium from Industrialwaste by Using Eucalyptus Bark, Bioresource Technology, Vol. 97, No. 1, 2006, pp. 15-20. [2] D. Park, Y. S. Yun and J. M. Park, “Use of Dead Fungal Biomass for the Detoxification of Hexavalent Chromium: Screening and Kinetics,” Process Biochemistry, Vol. 40, No. 7, 2005, pp. 2559-2565. [3] Z. Kowalski, “Treatment of Chromic Tannery Wastes,” Journal of Hazardous Materials, Vol. 37, No. 1, 1994, pp. 137-144. [4] U. Thacker, R. Parikh and Y. Shouche, D. Madamwar, Hexavalent Chromium Reduction by Providencia sp., Process Biochemistry, Vol. 41, No. 6, 2006, pp. 1332- 1337. [5] J. T. O’Brien, J. L. Fornsaglio, S. Ceryak, R. S. Patierno, Effects of Hexavalent Chromium on the Survival and Cell Cycle Distribution of DNA Repair—Deficient S. cerevisiae, DNA Repair, Vol. 1, No. 8, 2002, pp. 617-627. [6] P. Pattanapipitpaisal, A. N. Mabbett, J. A. Finalay, A. J. Copyright © 2010 SciRes. AJAC  A. BASU ET AL. Copyright © 2010 SciRes. AJAC 30 nmental Techno nology Letters, Vol. 23, No. 9, 2001, pp oremediation Journal, Vol. 6, No. 3, 2002, pp otechnol- ce Technology, Vol. 98, No. 12, Water Research, Vol. 27, No. 6, 1993, pp. - lysis A: Chemical, Vol. 266 emical Kinetics, (manuscript emical Kinetics, Vol. 12, No. 8, 1980, pp. Chemical Society, Vol. 89, No. 8, 1967, ac- tions of the Fara- l and Ap- rnal of Chemi- Journal of Chemical Physics, Vol. 16, 1948, pp. f Physical Chemistry, ical Chemistry, Vol. 60, Science and Engineering, ey, Chemical Physical Organic Chemistry, Vol. 8, l Chemistry, Vol. 75, No. 17, 1971, pp. mistry Reviews, Vol. 248, No. 1-2, l of Colloid Science, Vol. Physical Chemistry, Vol. 59, No. 5, 1955, pp. 432-435. Beswick, M. Paterson-Beedle and A. Essa, “Reduction of Cr(VI) and Bioaccumulation of Chromium by Gram Positive and Gram Negative Microorganisms not Previ- ously Exposed to CR-Stress, Enviro logy, pp. 4698-4703. [19] J. W. Mc Bain, “Colloids and their Viscosity,” Trans Vol. 23, No. 7, 2002, pp. 731-745. [7] A. N. Mabbett and L. E. Macaskie, “A Novel Isolate of Desulfovibrio sp. with Enhanced Ability to Reduce Cr(VI),” Biotech . da 683-687. [8] W. A. Smith, W. A. Apel and J. N. Petersen, “Effect of Carbon and Energy Source on Bacterial Chromate Re- duction,” Bi . pl 205-215. [9] J. V. Bhinde, P. K. Dhakephalkar and K. M. Paknikar, “Microbiological Process for the Removal of Cr(VI) from Chromate Bearing Cooling Tower Effluent,” Bi ogy Letters, Vol. 18, No. 6, 1996, pp. 667-672. [10] S. S. Ahluwalia and D. Goyal, “Microbial and Plant De- rived Biomass for Removal of Heavy Metals from Wastewater,” Bioresou 2007, pp. 2243-2257. [11] X. Zhou, T. Korenaga, T. Takahashi, T. Moriwake and S. Shinoda, “A Process Monitoring/Controlling System for the Treatment of Wastewater Containing Chro- mium(VI),” 1049-1054. [12] M. Islam, B. Saha and A. K. Das, “Kinetics and Mechanism of 2,2’-Bipyridyl and 1,10-Phenanthroline- Catalysed Chromium(VI) Oxidation of D-Fructose in Aqueous Micellar Media,” Journal of Molecular Cataly sis A: Chemical, Vol. 236, No. 1-2, 2005, pp. 260-266. [13] M. Islam, B. Saha and A. K. Das, “Kinetics and Mechanism of Picolinic Acid Promoted Chromic Acid Oxidation of Maleic Acid in Aqueous Micellar Media, ” Journal of Molecular Cata, Re No. 1-2, 2007, pp. 21-30. [14] S. Ghosh, A. Basu, K. K. Paul and B. Saha, “Micelle Catalyzed Oxidation of Propan-2-ol to Acetone by Penta-Valent Vanadium in Aqueous Acid Media, ” 197 Molecular Physics, Vol. 107, No. 7, 2009, pp. 615-619. [15] A. Basu, T. Ghosh and B. Saha, “Effect of some Non Functional Surfactants and Electrolytes on the Chro- mium(VI) Reduction by Glycerol: A Mechanistic Study,” International Journal of Ch has sent for consideration). [16] V. R. Bhalerao and F. A. Kummerow, “Paper Chromatog- raphy of 2,4-Dinitrophenylhydrazones of Saturated Ali- 20 phatic Aldehydes,” Vol. 36, No. 10, 1959, pp. 461-463. [17] C. A. Bunton and G. Cerichelli, “Micellar Effects upon Electron Transfer from Ferrocenes,” International Jour- nal of Ch 519-533. [18] F. M. Menger and C. E. Portnoy, “Chemistry of Reac- tions Proceeding inside Molecular Aggregates,” Journal of the American tions of the Faraday Society, Vol. 9, 1913, pp. 34-46. [20] G. S. Hartle, “The application of the Debye-Hückel The- ory to Colloidal Electrolytes,” Transac y Society, Vol. 31, 1935, pp. 31-50. [21] J. W. Mc Bain, “Colloid Chemistry, Theoretica ied, In: J. Alexander, Ed., New York, 1944. [22] R. H. Mattan, R. S. Stearns and W. D. Harkins, “Struc- ture for Soap Micelles as Indicated by a Previously Un- recognized X-Ray Diffraction Band,” Jou cal Physics, Vol. 15, 1947, pp. 209-210. [23] W. D. Harkins, “A Cylindrical Model for the Small Soap Micelle,” 156-157. [24] P. Debye and E. W. Anacker, “Micelle Shape from Dis- symmetry Measurement,” Journal o Vol. 55, No. 5, 1951, pp. 644-655. [25] I. Reich, “Factors Responsible for the Stability of Deter- gent Micelles,” Journal of Phys No. 3, 1956, pp. 257-262. [26] C. A. Bunton, “Reaction Kinetics in Aqueous Surfactant Solutions,” Catalysis Reviews, Vol. 20, No. 1, 1979, pp. 1-56. [27] H. Morawetz, “Catalysis and Inhibition in Solutions of Synthetic Polymers and in Micellar Solutions,” Advances in Catalysis & Related Subject, Vol. 20, In: D. D. El Ed., Academic Press, New York, 1969, pp. 341-371. [28] E. M. Cordes and R. B. Dunlop, “Kinetics of Organic Reactions in Micellar Systems,” Accounts of search, Vol. 2, No. 11, 1969, pp. 329-337. [29] E. J. Fendler and J. H. Fendler, “Micellar Catalysis in Organic Reactions: Kinetic and Mechanistic Implica- tions,” Advances in 0, pp. 271-406. [30] C. A. Bunton, M. Minch and L. Sepulveda, “Enhance- ment of Micellar Catalysis by Added Electrolyte,” Jour- nal of Physica 2707-2709. [31] A. K. Das, “Micellar Effect on the Kinetics and Mecha- nism of Chromium(VI) Oxidation of Organic Substrates,” Coordination Che 04, pp. 81-99. [32] K. J. Mysels and L. H. Princen, “Light Scattering by Ideal Colloidal Electrolyte,” Journa 12, No. 6, 1957, pp. 594-605. [33] K. Shinoda, “The Critical Micellar Concentrations in Aqueous Solutions of Potassium Alkyl Malonates,” Journal of |

