 Open Journal of Respiratory Diseases, 2012, 2, 37-42 http://dx.doi.org/10.4236/ojrd.2012.22006 Published Online May 2012 (http://www.SciRP.org/journal/ojrd) Influence of MnTE-2-PyP on Inflammation and Lipid Peroxidation in Mouse Asthma Model Lyudmil Terziev1, Veneta Shopova2, Violeta Dancheva2, Galya Stavreva3, Milena Atanasova4, Angelina Stoyanova5, Tzvetan Lukanov1, Anelia Dimitrova6 1Sector of Clinical Immunology and Allergology, Medical University, Pleven, Bulgaria 2Sector of Disaster Medicine, Medical University, Pleven, Bulgaria 3Sector of Experimental and Clinical Pharmacology, Medical University, Pleven, Bulgaria 4Sector of Biology, Medical University, Pleven, Bulgaria 5Sector of Chemistry, Medical University, Pleven, Bulgaria 6Sector of Pathophysiology, Medical University, Pleven, Bulgaria Email: luterzi@mail.bg Received January 13, 2012; revised March 6, 2012; accepted March 15, 2012 ABSTRACT Our aim was to investigate the effects of MnTE-2-PyP on some markers of inflammation and lipid peroxidation in mouse asthma model. 24 female mice were divided into four groups: group 1, controls; group 2, injected with ovalbu- min (OVA); group 3, treated with MnTE-2-PyP; and group 4, treated with ovalbumin and MnTE-2-PyP. The mice from groups 2 and 4 were injected with 10 μg OVA and 1 mg Imject Alum® in 100 μL phosphate buffered saline (PBS) on days 0 and 14. The animals from groups 1 and 3 were injected with 100 μL PBS + Imject Alum® (1:1). The animals from groups 2 and 4 were subjected to a 30 min aerosol challenge of 1% ovalbumin on days 24, 25 and 26 and those from groups 1 and 3 were subjected to aerosol challenge of PBS at the same time and duration. One hour before inhala- tion, and 12 hours later the animals from groups 3 and 4 were injected with 100 μL MnTE-2-PyP solution in PBS con- taining 5 mg/kg. The total cell number, total protein content and 8-isoprostane, IL-4 and IL-5 levels in the bronchial- veolar lavage fluid increased in group 2 as compared to the control group. Malone dialdehyde content in the lung ho- mogenate and IgE levels in the serum also increased in this group. The total cell number, total protein content, and lev- els of 8-isoprostane, IL-4, IL-5 and IgE decreased significantly in group 4 as compared to the OVA group. The parame- ters set out above in group 3 did not differ significantly from those of the control group. MnTE-2-PyP administered intraperitoneally, 48 hours after the last nebulization, reduced the inflammation and lipid peroxidation in mouse asthma model. Keywords: Asthma; Inflammation; Interleukins; 8-Isoprostane; Lipid Peroxidation; MnTE-2-PyP 1. Introduction Asthma is a lung disease characterized by airspace in- flammation and oxidative stress [1-4]. Elevated levels of reactive oxygen species (ROS), released by inflammatory cells, either directly or through the formation of products of lipid peroxidation, play a role in enhancing the in- flammatory response in these diseases. The presence of oxidative stress is important in the pathogenesis, severity and treatment of asthma [5]. Increasing evidence sug- gests that abnormalities in mitochondria are involved in several mitochondrial diseases, but also in the develop- ment of asthma [6,7]. Recently, antioxidants to prevent and to treat mitochondria in patients with mitochondrial diseases, including asthma, has received much attention, especially because antioxidant approaches seem to have few or no adverse effects [8]. Different classes of anti- oxidants are known. Among them, the group of catalytic manganese metalloporphyrins takes center stage with their accumulation into mitochondria. They have at least four antioxidant properties, such as removal of superox- ide (), hydrogen peroxide (H2O2), peroxynitrite (ONOO–), and lipoperoxides [9,10]. Based on this in- formation, we set the goal to investigate the effects of MnTE-2-PyP (Manganese(III) 5, 10, 15, 20-tetrakis (N- ethylpyridinium-2-yl)porphyrin), a manganese-mesopor- phyrin also known as AEOL-10113, on markers of in- flammation and lipid peroxidation in a mouse ovalbumin (OVA) sensitization model of asthma [11]. – 2 O 2. Materials and Methods 2.1. Chemicals Ovalbumin, grade V, and phosphate buffered saline (PBS), were purchased from Sigma-Aldrich Company, Nitrocellulose filters with 5 μm pores were from Milli- C opyright © 2012 SciRes. OJRD  L. TERZIEV ET AL. 38 pore Corp, IL-4 and 5 ELISA Kits were from R&D Sys- tems, 8-Isoprostane EIA Kit was from Cayman chemi- cals, Mouse IgE ELISA Sets were purchased from BD Biosciences, and the Imject Alum® was from the Pierce Chemical Company (USA). MnTE-2-PyP was kindly provided by Prof. Ines Batinić-Haberle from the Depart- ment of Radiation Oncology, Duke University Medical Center, Durham, North Carolina, USA. 2.2. Animals and Experimental Protocol The experiment was performed in accordance with Ani- mal Welfare Regulations and was approved by the Uni- versity Ethics Committee. The study was carried out on 24 female C57Bl/6 mice (weight 20 ± 2 g, 8 - 10 week old). The animals were raised at the University vivarium at a temperature of 22˚C ± 2˚C and humidity of 50% ± 10%, and were given a normal pelleted diet and water ad libitum. The mice were divided into four groups: group1, controls; group 2, in- jected with ovalbumin; group 3, treated with MnTE-2- PyP and group 4, treated with OVA and MnTE-2-PyP. Airway inflammation was induced by OVA immuniza- tion and challenge. The animals from groups 1 and 3 were injected i.p. with a 100 μL phosphate-buffed saline (PBS) + Imject AlumR (1:1) on days 0 and 14. The animals from groups 2 and 4 were injected with a 100 μL ovalbumin solution, containing 20 μg OVA on the same days. On days 24, 25 and 26, mice from groups 1 and 3 were given inhalation with PBS for 30 min, and those from groups 2 and 4 were given inhalation with 1% ovalbumin solution (OVA dissolved in PBS). For this purpose, a special plexiglass chamber was used. One hour before inhalation, and 12 hours later the animals from groups 1 and 2 were injected i.p. with 100 μL PBS, and those from groups 3 and 4 received a 100 μL MnTE-2-Pyp dissolved in PBS, containing 5 mg/kg, that is, the total daily dose was 10 mg/kg. The solution was sterilized by filtration through 0.2 μm filters. 2.3. Bronchoalveolar Lavage Fluid (BALF) To obtain BALF, the animals were sacrificed on day 28 (48 hours after the last inhalation) under thiopental anaesthesia (50 mg/kg). The chest was opened and the lungs were perfused in situ via the right heart ventricle with saline (10 mL). Triple lavage of the left lung through the trachea was performed with a total volume of 2.5 mL of saline. The right lung was ligated at the hilum, cut and then removed from the chest and used to prepare the lung homogenate. Cytological, Biochemical and Immunological Assays of BALF One aliquot of the BALF was used for the purpose of total cell number × 105/mL. The cells were then removed by centrifugation at 300 × g for 10 min. The supernatant of BALF was used to measure interleukins and 8-iso- prostane levels. The cell pellet was resuspended in 0.5 mL of saline, and differential cell count using Millipore filters by the method of Danos and Keebler, modified by Saltini [12] was performed. The total protein content in ng/mL by the method of Lowry et al. [13], the levels of IL-4 and IL-5 in pg/mL by the ELISA method, and the level of 8-isoprostane in ng/mL by the ELISA method in accordance with manufacturer’s instructions, were inves- tigated in the supernatant of BALF. 2.4. Biochemical Assays of Lung Homogenate Lung homogenate was obtained from the right lung. The tissue was homogenized with potassium chloride (KCl) in 1:10 ratio (lung mass by KCl solution volume). The homogenate was centrifuged (9000 × g, 30 min), and the supernatant was stored on ice. Malone dialdehyde (MDA) content in nmol/g was measured by the method of Oh- kawa et al. [14]. 2.5. Immunological Assay of Serum Blood was drown from the abdominal aorta by using vacuum blood collection tubes. The blood was allowed to clot for 30 minutes, and then centrifuged at 1000 × g for 10 min to achieve serum separation. The samples were kept frozen at –20˚C until serum IgE analysis in ng/mL was made by the ELISA method in accordance with manufacturer’s instructions. 2.6. Statistical Analysis Experimental data were analyzed using SPSS 14. When we tested for normality, one variable-MDA showed non- parametric distribution, and we used medians, interquar- tile range and Mann-Whitney test for comparison. For the rest of the variable we applied post-hoc ANOVA test and data were presented as mean ± standart error of mean (SEM). P < 0.05 was considered statistically significant. 3. Results The total cell number in group 2 (OVA-sensitized mice) increased more than four fold in BALF (479% as com- pared to the controls, P = 0.025). The increase of this parameter in group 4 (OVA + MnTE-2-PyP) was sig- nificantly lower (178%) than that in group 2. The eosi- nophil percentage was 28% in group 2 and 18% in group 4, versus 0.5% in the control group (Table 1). The total protein content in group 2 showed the same dynamics (Table 1). The levels of IL-4 and IL-5 increased sharply in group 2 (OVA) up to 1849% (P = 0.0006) and 350% P = 0.016) respectively, in comparison with the control ( Copyright © 2012 SciRes. OJRD 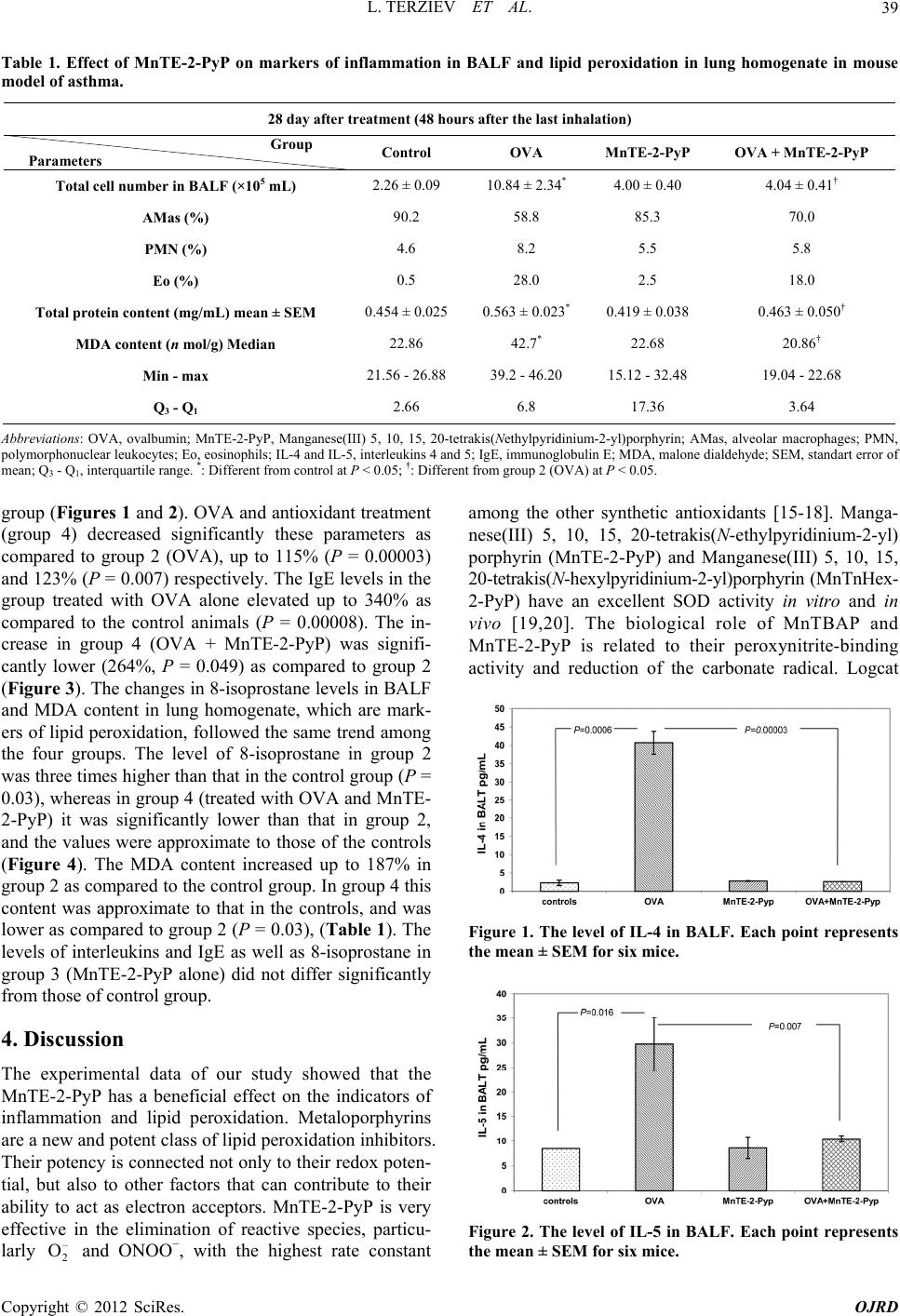 L. TERZIEV ET AL. Copyright © 2012 SciRes. OJRD 39 Table 1. Effect of MnTE-2-PyP on markers of inflammation in BALF and lipid peroxidation in lung homogenate in mouse model of asthma. 28 day after treatment (48 hours after the last inhalation) Group Parameters Control OVA MnTE-2-PyP OVA + MnTE-2-PyP Total cell number in BALF (×105 mL) 2.26 ± 0.09 10.84 ± 2.34* 4.00 ± 0.40 4.04 ± 0.41† AMas (%) 90.2 58.8 85.3 70.0 PMN (%) 4.6 8.2 5.5 5.8 Eo (%) 0.5 28.0 2.5 18.0 Total protein content (mg/mL) mean ± SEM 0.454 ± 0.025 0.563 ± 0.023* 0.419 ± 0.038 0.463 ± 0.050† MDA content (n mol/g) Median 22.86 42.7* 22.68 20.86† Min - max 21.56 - 26.88 39.2 - 46.20 15.12 - 32.48 19.04 - 22.68 Q3 - Q1 2.66 6.8 17.36 3.64 Abbreviations: OVA, ovalbumin; MnTE-2-PyP, Manganese(III) 5, 10, 15, 20-tetrakis(Nethylpyridinium-2-yl)porphyrin; AMas, alveolar macrophages; PMN, polymorphonuclear leukocytes; Eo, eosinophils; IL-4 and IL-5, interleukins 4 and 5; IgE, immunoglobulin E; MDA, malone dialdehyde; SEM, standart error of mean; Q3 - Q1, interquartile range. *: Different from control at P < 0.05; †: Different from group 2 (OVA) at P < 0.05. group (Figures 1 and 2). OVA and antioxidant treatment (group 4) decreased significantly these parameters as compared to group 2 (OVA), up to 115% (P = 0.00003) and 123% (P = 0.007) respectively. The IgE levels in the group treated with OVA alone elevated up to 340% as compared to the control animals (P = 0.00008). The in- crease in group 4 (OVA + MnTE-2-PyP) was signifi- cantly lower (264%, P = 0.049) as compared to group 2 (Figure 3). The changes in 8-isoprostane levels in BALF and MDA content in lung homogenate, which are mark- ers of lipid peroxidation, followed the same trend among the four groups. The level of 8-isoprostane in group 2 was three times higher than that in the control group (P = 0.03), whereas in group 4 (treated with OVA and MnTE- 2-PyP) it was significantly lower than that in group 2, and the values were approximate to those of the controls (Figure 4). The MDA content increased up to 187% in group 2 as compared to the control group. In group 4 this content was approximate to that in the controls, and was lower as compared to group 2 (P = 0.03), (Table 1). The levels of interleukins and IgE as well as 8-isoprostane in group 3 (MnTE-2-PyP alone) did not differ significantly from those of control group. 4. Discussion The experimental data of our study showed that the MnTE-2-PyP has a beneficial effect on the indicators of inflammation and lipid peroxidation. Metaloporphyrins are a new and potent class of lipid peroxidation inhibitors. Their potency is connected not only to their redox poten- tial, but also to other factors that can contribute to their ability to act as electron acceptors. MnTE-2-PyP is very effective in the elimination of reactive species, particu- larly and ONOO−, with the highest rate constant among the other synthetic antioxidants [15-18]. Manga- nese(III) 5, 10, 15, 20-tetrakis(N-ethylpyridinium-2-yl) porphyrin (MnTE-2-PyP) and Manganese(III) 5, 10, 15, 20-tetrakis(N-hexylpyridinium-2-yl)porphyrin (MnTnHex- 2-PyP) have an excellent SOD activity in vitro and in vivo [19,20]. The biological role of MnTBAP and MnTE-2-PyP is related to their peroxynitrite-binding activity and reduction of the carbonate radical. Logcat – 2 O Figure 1. The level of IL-4 in BALF. Each point represents the mean ± SEM for six mice. Figure 2. The level of IL-5 in BALF. Each point represents the mean ± SEM for six mice.  L. TERZIEV ET AL. 40 Figure 3. The level of IgE in serum. Each point represents the mean ± SEM for six mice. Figure 4. The level of 8-isoprostane in BALF. Each point represents the mean ± SEM for six mice. () of MnTBAP is about 3.16, which is about 5 - 6 times less-than the SOD activity of the powerful SOD mimetic MnTE-2-PyP and CuZn SOD. Positively charged MnTE-2-PyP and related analogues are very suitable for SOD mimetics and ONOO–/ cleaners. MnTE-2- PyP has a potent catalytic antioxidant-like effect of ex- tracellular superoxide dismutase. The strong porphyrin- based compounds are based on a “structure- activity” rela- tionship, such as Mn(III) meso-tetreakis (N-ethylpyridi- nium-2-yl)porphyrin (MnTE-2-PyP) and its hexyl ana- logue (MnTnHex-2-PyP) [21]. – 2 O – 3 CO We applied antioxidants in a dose of 10 mg/kg/daily, divided into doses over 12 hours, starting with the asser- tion that good tolerance in mice is observed at two doses of 15 mg/kg/day and the plasma half-life varies consid- erably. This is the most commonly used (as single or multiple) therapeutic dose of this porphyrin [22]. MnTE- 2-PyP administered i.p. or orally reaches maximum con- centration in plasma in 0.33 hours [16,17]. It accumulates at high levels in liver, kidney, and spleen, at moderate levels in lungs and heart, and at low levels in brain. The plasma half-life for a single i.p. dose of 10 mg/kg in mice is about 1 hour and the half-life in the body is signifi- cantly longer ranging from 60 - 135 hours [22-24]. Stud- ies reveal that MnTBAP and MnTM-4-PyP are effective in animal models of oxidative stress by forming super- oxide [25-27], hydrogen peroxide [28,29] and peroxini- ganese porphyrins have a molecular weight above 800, quickly pass through cell membranes and are distributed in mitochondria [23]. MnTE-2-PyP in the liver cells showed slightly higher accumulation in the mitochondria, as compared with the cytosol (Spasojevich I., unpub- lished data). This compo trite [30,31]. Many water-soluble meso substituted man- und not only decreases the primary insult of 5. Acknowledgements the financial support of REFERENCES [1] P. Kirkham ane Stress in Asthma and reactive species to biological molecules, but also in- hibits the activation of transcription factors which in turn leads to suppression of expression of those cytokines and enzymes that perpetuate secondary oxidative stress [20,32]. According to Gauter-Fleckenstein et al. [33] the inhabita- tion of excessive cellular activity takes place through the suppression of the transcriptional activity, particularly suppressing HIF-1α (hypoxia inducible factor 1α) active- tion in a long-lasting effect. Therefore reducing the total number of cells, the amount of total protein observed after treatment with mangan porphyrin can be explained by the ability of the antioxidants to inhibit the expression of VCAM-1 (vascular cell adhesion molecule-1) res- ponsible for the accumulation of inflammatory cells, and thus to decrease airway hyperreactivity [34]. After intra- tracheal introduction, the antioxidant dramatically re- duces the severity of airway inflammation in the airways in OVA-induced murine asthma. The reduction in the number of eosinophils, neutrophils and lymphocytes in BALF is more than 80% [34-36]. VCAM-1 and ICAM-1 (intra-cellular adhesion molecule) participate in the mi- gration of eosinophils and neutrophils and contribute to eosinophilic inflammation in animal models. MnTE-2- PyP has been shown to alter cell signaling and reduce inflammation by reducing NF-kB activity [37]. Piganelli et al. showed that MnTE-2-PyP inhibits T cell prolifera- tion, while lipopolysaccha-ride-stimulated macrophages treated with the compound, inhibit TNF-α (tumor necrosis factor-α) and NADPH release of superoxide [38]. The beneficial effects of Mn alkylpyri-dylporphyrins have been observed in various diseases, associated with oxida- tive stress, such as radiation injury, Alzheimer’s disease, cancer, diabetes, central nervous system injuries, ische- mia/reperfusion conditions, pulmonary emphysema, and other diseases [19,33,38-43]. This study was carried out with Medical University-Pleven through the University Grants Commission (Project N 17/2009). d I. Rahman, “Oxidativ COPD: Antioxidants as a Therapeutic Strategy,” Phar- macology and Therapeutics, Vol. 111, No. 2, 2006, pp. 476-494. doi:10.1016/j.pharmthera.2005.10.015 Copyright © 2012 SciRes. OJRD  L. TERZIEV ET AL. 41 [2] J. Bousquet, P. K. Jeffery, W. W. Busse, M. Johnso ess in Asthma,” Thorax, Vol. 55, n and A. M. Vignola, “Asthma. From Bronchoconstriction to Air- ways Inflammation and Remodeling,” American Journal of Respiratory and Critical Care Medicine, Vol. 161, No. 5, 2000, pp. 1720-1745. [3] R. Dworski, “Oxidant Str No. 2, 2000, pp. S51-S53. doi:10.1136/thorax.55.suppl_2.S51 [4] P. K. Jeffery, “Comparison of the Structural and Inflam- matory Features of Chronic Obstructive Pulmonary Dis- ease and Asthma Giles F. Filley Lecture,” Chest, Vol. 117.5, No. 1, 2000, pp. 251S-260S. doi:10.1378/chest.117.5_suppl_1.251S [5] J. Ciencewicki, S. Trivedi and S. R. Kleenberger, “Oxi- dants and the Pathogenesis of Lung Diseases,” The Jour- nal of Allergy and Clinical Immunology, Vol. 122, No. 3, 2008, pp. 456-470. doi:10.1016/j.jaci.2008.08.004 [6] B. A. Raby, B. Klanderman, A. Murphy, S. Mazza, C. A. Camargo Jr., E. K. Silverman and S. T. Weiss, “A Com- mon Mitochondrial Haplogroup Is Associated with Ele- vated Total Serum IgE Levels,” The Journal of Allergy and Clinical Immunology, Vol. 120, No. 2, 2007, pp. 351- 358. doi:10.1016/j.jaci.2007.05.029 [7] U. Mabalirajan, A. K. Dinda, S. Kumar, R. Roshan, P ion and Oxidativ . Gupta, S. K. Sharma and B. Ghosh, “Mitochondrial Structural Changes and Dysfunction Are Associated with Experimental Allergic Asthma,” Journal of Immunology, Vol. 181, No. 5, 2008, pp. 3540-3548. [8] P. H. Reddy, “Mitochondrial Dysfuncte Stress in Asthma: Implications for Mitochondria-Targeted Antioxidant Therapeutics,” Pharmaceuticals, Vol. 4, No. 3, 2011, pp. 429-456. doi:10.3390/ph4030429 [9] M. Patel and B. J. Day, “Metalloporphyrin Class of Therapeutic Catalytic Antioxidants,” Trends in Pharma- cological Sciences, Vol. 2, No. 9, 1999, pp. 359-364. doi:10.1016/S0165-6147(99)01336-X [10] B. J. Day, “Catalytic Antioxidants: A Radical Approach to New Therapeutics,” Drug Discovery Today, Vol. 9, No. 13, 2004, pp. 557-566. doi:10.1016/S1359-6446(04)03139-3 [11] A. T. Nials and S. Uddin, “Mouse Models of Allergic Asthma: Acute and Chromic Allergen Challenge,” Dis Models and Mechanisms, Vol. 1, No. 4-5, 2008, pp. 213- 220. doi:10.1242/dmm.000323 [12] C. Saltini, A. J. Hance, V. J. Ferrance, F. Basset, N. J. Rosenhbrough, A. L. Farr and R. J. d K. Yagi, “Assay for Lipid P. B, Bitterman and R. G. Crystal, “Acurate Quantification of Cells Received by Bronchoalveolar Lavage,” The Ameri- can Review of Respiratory Disease, Vol. 130, No. 4, 1984, pp. 650-658. [13] O. H. Lowry, Randall, “Protein Measurement with the Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, No. 1, 1951, pp. 265-275. [14] H. Ohkawa, N. Ohishi an Peroxides in Animal Tissues by Thiobarbituric Acid Re- action,” Analytical Biochemistry, Vol. 95, No. 2, 1979, pp. 351-358. doi:10.1016/0003-2697(79)90738-3 [15] G. DeFreitas-Silva, J. S. Rebouçças, I. Spasojević, L. Benov, Y. M. Idemori and I. Batinić-Haberle, “SOD-Like Activity of Mn(II)beta-octabromo-meso-tetrakis(N-methyl- pyridinium-3-yl)porphyrin Equals That of the Enzyme It- self,” Archives of Biochemistry and Biophysics, Vol. 477, No. 1, 2008, pp. 105-112. doi:10.1016/j.abb.2008.04.032 [16] I. Batinić-Haberle, I. Spasojevich, R. D. Stevens, P. Ham- ić, D. H. Tjahjono, A. Richaud, . brigh and I. Fridovich, “Manganese(III)meso -tetra kis(ortho- N-alkylpyridyl)porphyrins. Synthesis, Characterization and Catalysis of O2– Dismutation,” Journal of the Chemical Society Dalton Transactions, 2002, No. 13, pp. 2689-2696. [17] I. Batinić-Haberle, I. Spasojevich, R. D. Stevens, P. Hambright, P. Neta, A. Okado-Matsumoto and I. Fridovich, “New Class of Potent Catalysts of O2– Dismutation. Mn(III)ortho-methoxyethylpyridyl and di-ortho-meth- oxyethylimidazolylporphyrins,” Dalton Transactions, Vol. 11, 2004, pp. 1696-1702. [18] J. S. Rebouças, I. Spasojev F. Méndez, L. Benov and I. Batinić-Haberle, “Redox Modulation of Oxidative Stress by Mn Porphyrin-Based Therapeutics: The Effect of Charge Distribution,” Dalton Transactions, Vol. 9, 2008, pp. 1233-1242. [19] H. Saba, I. Batinić-Haberle, S. Munusamy, T. Mitchell, C Lichti, J. Megyesi and L. A. MacMillan-Crow, “Manga- nese Porphyrin Reduces Renal Injury and Mitochondrial Damage during Ischemia/Reperfusion,” Free Radical Bi- ology and Medicine, Vol. 42, No. 10, 2007, pp. 1571- 1578. doi:10.1016/j.freeradbiomed.2007.02.016 [20] Y. Zhao, L. Chaiswing, J. M. Velez, I. Batinić-Haberle, N. H. Colburn, T. D. Oberley and D. K. St. Clair, “p53 Translocation to Mitochondria Precedes Its Nuclear Translocation and Targets Mitochondrial Oxidative De- fense Protein-Manganese Superoxide Dismutase,” Cancer Research, Vol. 65, No. 9, 2005, pp. 3745-3750. doi:10.1158/0008-5472.CAN-04-3835 [21] I. Batinić-Haberle, S. Cuzzocrea, J. Rebouças, G. Ferrer- .2008.09.042 Sueta; E. Mazzon, R. Di Paola, R. Radi, I. Spasojević, L. Benov and D. Salvemini, “Pure MnTBAP Selectively Scavenges Peroxynitrite over Superoxide: Comparison of Pure and Commercial MnTBAP Samples to MnTE-2-PyP in Two Models of Oxidative Stress Injury, an SOD-Spe- cific Escherichia coli Model and Carrageenan-Induced Pleurisy,” Free Radical Biology and Medicine, Vol. 46, No. 2, 2009, pp. 192-201. doi:10.1016/j.freeradbiomed pasojević, “Su- [22] I. Batinić-Haberle, J. S. Rebouças and I. S peroxide Dismutase Mimics: Chemistry, Pharmacology, and Therapeutic Potencial,” Antioxidants and Redox Sig- naling, Vol. 13, No. 6, 2010, pp. 877-918. doi:10.1089/ars.2009.2876 [23] I. Spasojevic, C. Yumin, T. Noel, Y. Yu, M. P. Cole, L. Zhang, Y. Zhao, D. K. St Clair and I. Batinić-Haberle, “Mn Porphyrin-Based SOD Mimic, MnTE-2-PyP5+ Tar- gets Mouse Heart Mitochondria,” Free Radical Biology and Medicine, Vol. 42, No. 8, 2007, pp. 1193-1200. doi:10.1016/j.freeradbiomed.2007.01.019 [24] I. Spasojevic, Y. Chen, T. J. Noel, P. Fan, L. Zhang, J. S. Rebouças, D. K. St Clair and I. Batinić-Haberle, “Phar- macokinetics of the Potent Redox-Modulating Manganese Porphyrin, MnTE-2-PyP5+, in Plasma and Major Organs of B6C3F1 Mice,” Free Radical Biology and Medicine, Copyright © 2012 SciRes. OJRD  L. TERZIEV ET AL. Copyright © 2012 SciRes. OJRD 42 .015 Vol. 45, No. 7, 2008, pp. 943-949. doi:10.1016/j.freeradbiomed.2008.05 . D. Crapo, “A and J. O. [25] B. J. Day, S. Shawen, S. I. Liochev and J Metalloporphyrin Superoxide Dismutase Mimetic Protects against Paraquat-Induced Endothelial Cell Injury, in Vitro,” The Journal of Pharmacology and Experimental Thera- peutics, Vol. 275, No. 3, 1995, pp. 1227-1232. [26] M. Patel, B. J. Day, J. D. Crapo, I. Fridovich McNamara, “Requirement for Superoxide in Excitotoxic Cell Death,” Neuron, Vol. 16, No. 2, 1996, pp. 345-355. doi:10.1016/S0896-6273(00)80052-5 [27] B. J. Day and J. D. Crapo, “A Metalloporphyrin Super- oxide Dismutase Mimetic Protect against Paraquat-Induced Lung Injury in Vivo,” Toxicology and Applied Pharma- cology, Vol. 140, No. 1, 1996, pp. 94-100. doi:10.1006/taap.1996.0201 [28] B. J. Day, I. Fridovich and J. D. Crapo, “Manganic Por- phyrins Possess Catalase Activity and Protect Endothelial Cells against Hydrogen Peroxide-Mediated Injury,” Ar- chives Biochemistry Biophysics, Vol. 347, No. 2, 1997, pp. 256-262. doi:10.1006/abbi.1997.0341 [29] J. Milano and B. J. Day, “A Catalytic Antioxidant Metal- loporphyrin Blocks Hydrogen Peroxide-Induced Mito- chondrial DNA Damage,” Nucleic Acids Research, Vol. 28, No. 4, 2000, pp. 968-973. doi:10.1093/nar/28.4.968 [30] C. Szabó, B. J. Day and A. L. Salzman, “Evaluation of the Relative Contribution of Nitric Oxide and Peroxyni- trite to the Suppression of Mitochondrial Respiration in Immunostimulated Macrophages Using a Manganese Meso- porphyrin Superoxide Dismutase Mimetic and Peroxyni- trite Scavenger,” FEBS Letters, Vol. 381, No. 1-2, 1996, pp. 82-86. doi:10.1016/0014-5793(96)00087-7 [31] G. Ferrer-Sueta, L. Ruiz-Ramirez and R. Radi, “Ternary Copper Complexes and Manganese(III) Tetrakis(4-benzoic acid)porphyrin Catalyze Peroxynitrite-Dependent Nitra- tion of Aromatics,” Chemical Research in Toxicology, Vol. 10, No. 12, 1997, pp. 1338-1344. doi:10.1021/tx970116h [32] I. L. Jackson, L. Chen, I. Batinić-Haberle and Z. Vujaskovic, “Superoxide Dismutase Mimetic Reduces Hypoxia In- duced-O2–, TGF-β and VEGF Production by Macrophages,” Free Radical Research, Vol. 41, No. 1, 2007, pp. 8-14. doi:10.1080/10715760600913150 [33] B. Gauter-Fleckenstein, K. Fleckenstein, K. Owzar, C. 0.01.020 Jian, J. S. Rebouças, I. Batinić-Haberle and Z. Vujaskovic, “Early and Late Administration of MnTE-2-PyP5+ in Mitigation and Treatment of Radiation-Induced Lung Damage,” Free Radical Biology and Medicine, Vol. 48, No. 8, 2010, pp. 1034-1043. doi:10.1016/j.freeradbiomed.201 of Airway In-[34] L. Y. Chang and J. D. Crapo, “Inhibition flammation and Hyperreactivity by a Catalytic Antioxi- dant,” Chest, Vol. 12, No. 3, 2003, p. 446S. [35] L. Y. Chang and J. D. Crapo, “Inhibition of Airway In- flammation and Hyperreactivity by an Antioxidant Mi- metic,” Free Radical Biology and Medicine, Vol. 33, No. 3, 2002, pp. 379-386. doi:10.1016/S0891-5849(02)00919-X [36] L.-Y. Chang, M. Subramaniam, B. A. Yoder, B. J. Day, M. C. Ellison, M. E. Sunday and J. D. Crapo, “A Cata- lytic Antioxidant Attenuates Alveolar Structural Remod- eling in Bronchopulmonary Dysplasia,” American Jour- nal of Respiratory and Critical Care Medicine, Vol. 167, No. 1, 2003, pp. 57-64. doi:10.1164/rccm.200203-232OC [37] H. M. Tse, M. J. Milton and J. D. Piganelli, “Mechanistic Analysis of the Immunomodulatory Effects of a Catalytic Antioxidant on Antigen-Presenting Cells: Implication for Their Use in Targeting Oxidation-Reduction Reactions in Innate Immunity,” Free Radical Biology and Medicine, Vol. 36, No. 2, 2004, pp. 233-247. doi:10.1016/j.freeradbiomed.2003.10.029 [38] J. D. Piganelli, S. C. Flores, C. Cruz, J. Koepp, I. Batinić-Haberle, J. Crapo, B. Day, R. Kachadourian, R. Young, B. Bradley and K. Haskins, “A Metalloporphyrin- Based Superoxide Dismutase Mimic Inhibits Adoptive Transfer of Autoimmune Diabetes by a Diabetogenic T- Cell Clone,” Diabetes, Vol. 51, No. 2, 2002, pp. 347- 355. doi:10.2337/diabetes.51.2.347 [39] A. Y. Makinde, A. Rizvi, J. D. Crapo, R. D. Pearlstein, J. M. Slater and D. S. Gridley, “A Metalloporphyrin Anti- oxidant Alters Cytokine Responses after Irradiation in a Prostate Tumor Model,” Radiation Research, Vol. 173, No. 4, 2010, pp. 441-452. doi:10.1667/RR1765.1 [40] X. W. Mao, J. D. Crapo, T. Mekonnen, N. Lindsey, P. Martinez, D. S. Gridley and J. M. Slater, “Radioprotective Effect of a Metalloporphyrin Compound in Rat Eye Model,” Current Eye Research, Vol. 34, No. 1, 2009, pp. 62-72. doi:10.1080/02713680802546948 [41] T.-J. Wu, N. H. Khoo, F. Zhou, B. J. Day and D. A. Parks, “Decreased Hepatic Ischemia-Reperfusion Injury by Man- ganese-Porphyrin Complexes,” Free Radical Research, Vol. 41, No. 2, 2007, pp. 127-134. doi:10.1080/10715760600801298 [42] G. B. Mackensen, M. Patel, H. Sheng, C. L. Calvi, I. Batinić-Haberle, B. J. Day, L. P. Liang, I. Fridovich, J. D. Crapo, R. D. Pearlstein and D. S. Warner, “Neuroprotec- tion from Delayed Postischemic Administration of a Met- alloporphyrin Catalytic Antioxidant,” Journal of Neuro- science, Vol. 21, No. 13, 2001, pp. 4582-4592. [43] H. Yao, G. Arunachalam, J.-W. Hwang, S. Chung, I. K. Sundar, V. L. Kinnula, J. D. Crapo and I. Rahman, “Ex- tracellular Superoxide Dismutase Protects against Pul- monary Emphysema by Attenuating Oxidative Fragmen- tation of ECM,” Proceedings of the National Academy of Sciences of USA, Vol. 107, No. 35, 2010, pp. 15571- 15576. doi:10.1073/pnas.1007625107
|