American Journal of Plant Sciences
Vol.4 No.1(2013), Article ID:27649,11 pages DOI:10.4236/ajps.2013.41019
Effect of 1-MCP on Gas Exchange and Carbohydrate Concentrations of the Cotton Flower and Subtending Leaf under Water-Deficit Stress
![]()
Department of Crop, Soil, and Environmental Sciences, University of Arkansas, Fayetteville, USA.
Email: *dloka@uark.edu
Received October 6th, 2012; revised November 17th, 2012; accepted December 20th, 2012
Keywords: Cotton; 1-Methylcyclopropene; Ethylene; Water-Deficit Stress; Carbohydrates; Gas Exchange
ABSTRACT
Ethylene is an endogenous plant hormone that increases under adverse environmental conditions, resulting in leaf and fruit abscission and ultimately yield reduction. In cotton, however, the effects of water-deficit stress on ethylene production have been uncertain. In this study it was hypothesized that application of an ethylene inhibitor 1-Methylcyclopropene (1-MCP) would prevent ethylene production and result in alleviation of water-deficit stress consequences on the physiology and metabolism of the cotton flower and subtending leaf. To test this hypothesis, growth chamber experiments were conducted in 2009-2010 with treatments consisting of (C) untreated well-watered control, (C + 1MCP) well-watered plus 1-MCP, (WS) untreated water-stressed control, and (WS + 1MCP) water-stressed plus 1-MCP. The plants were subjected to two consecutive drying cycles during flowering, approximately 8 weeks after planting, and 1-MCP was foliar applied at a rate of 10 g. ai/ha at the beginning of each drying cycle. The results showed that 1-MCP application had no significant effect on gas exchange functions and did not prevent reductions from water stress in leaf photosynthesis, respiration and stomatal conductance. However, application of 1-MCP resulted in a decrease in sucrose content of water-stressed pistils compared to the control indicating that 1-MCP has the potential to interfere in carbohydrate metabolism of reproductive units.
1. Introduction
Plant growth and crop yields are greatly affected by limited water supply [1]. Approximately one third of cultivated areas around the world are subjected to inadequate supplies of water [2], and the severity of the problem is expected to increase due to the changing climatic trends [3].
Cotton (Gossypium hirsutum L.), a perennial with a complex and indeterminate growth habit that originated in hot and arid areas [4], is considered to be relatively drought tolerant. However, defense mechanisms such as leaf and root osmotic adjustment [5,6] accumulation of compatible osmolytes [7], production of heat shock proteins [8] as well as high water use efficiency [9] are associated with cotton’s indeterminate growth habit [10]. As a result, due to its domestication and cultivation as an annual crop, modern cotton cultivars suffer significant yield decreases under conditions of limited water supply [11].
Ethylene is a plant growth hormone involved in numerous physiological functions [12] and its synthesis is continually occurring at low rates under normal conditions. However, the production of ethylene significantly increases under conditions of biotic or abiotic stresses [13-15]. Water-deficit stress and ethylene interaction has been the subject of much debate since contrasting results have been reported for different crops [12,14,16-18].
In cotton, water-deficit stress has been observed to increase ethylene production ultimately resulting in leaf and fruit abscission [19-21]. However, in later studies, Morgan et al. [13] reported that ethylene rates of water-stressed intact plants were decreased, and suggested that ethylene production depends on the rate that the stress is imposed, and those findings were confirmed by Bugbee (2011) [22] and Klassen and Bugbee (2003) [18].
Ethylene biosynthesis inhibitors such as silver, silver thiosulfate (STS), and aminoethylvinylglycine (AVG) as well as blockers of ethylene receptors have provided valuable help in ethylene research. 1-Methylcyclopropene (1-MCP) is a gaseous material that acts by binding on ethylene receptors [23] and reducing plant sensitivity to ethylene due to its high affinity to the ethylene recaptors (nearly 10-fold) compared to ethylene [24]. Application of 1-MCP on climacteric fruits has been shown to decrease ethylene production [24-26], respiration [24-27] and chlorophyll degradation [24,27,28]. However, in cotton, application of 1-MCP has produced contrasting results. Kawakami et al. (2010) [29] observed that application of 1-MCP on 4-week-old water-stressed cotton plants increased stomatal resistance, water potential and activity of antioxidant enzymes while it decreased membrane leakage compared to the control [29]. On the contrary, da Costa and Cothren (2011) [30] reported that 1-MCP had no effect on gas exchange, chlorophyll content and dry matter partitioning of 16-week-old water-stressed cotton plants while the increase in the number of reproductive nodes that was observed in 1-MCP treated water-stressed plants did not result in higher yield since 1-MCP caused higher fruit abscission [30]. Nevertheless, no further explanation was given on the reason for higher abscission. Recent research in other crops [31-34] has indicated that ethylene increases due to water-deficit stress result in perturbations in the carbohydrate metabolism of reproductive units that consequently result in yield losses. Bearing in mind that the leaf subtending to the fruit is providing the majority of photosynthates to the developing boll in cotton [35], the objective of these studies was to evaluate the possible ameliorating effect of the anti-ethylene plant regulator, 1-MCP on cotton’s floral buds and subtending leaves under conditions of limited water supply during reproductive development. It was hypothesized that application of 1- MCP would prevent ethylene action and result in alleviation of water deficit stress effects on the cotton flower and consequently prevent yield loss.
2. Materials and Methods
Growth chamber studies were conducted and repeated at the Altheimer Laboratory, University of Arkansas, during 2009-2010. Cotton (Gossypium hirsutum L.,) cultivar ST 5288B2F was planted in 2L pots containing Sunshine potting media mix#1 (SunGro Distribution Inc., Bellevue, WA). Pots were arranged in a growth chamber (Conviron PGW36, Conviron Inc., Winnipeg, Canada) that was equipped with incandescent and fluorescent lamps and set for a 12h photoperiod with a photosynthetic flux density (PPFD) of 800 - 850 µmol/m²s and a relative humidity of 60%. Normal day/night temperatures of 32˚C/24˚C (maximum during the day and minimum during the night) were imposed throughout the duration of the experiments simulating a normal diurnal variation. All pots received half-strength Hoagland’s nutrient solution daily to maintain adequate nutrients and water until flowering, approximately eight weeks after planting and induction of water-deficit stress and 1-MCP application treatments, after which plants were watered only with deionized water. The experiments were arranged in a completely randomized design with two factors that consisted of water-deficit stress and 1-MCP application. Application of 1-MCP started at flowering (approximately eight weeks after planting) and the treatments consisted of: Untreated well-watered control (C), Control + 1-MCP (C + 1MCP), Untreated water stressed (WS), and Water Stress + 1- MCP (WS + 1MCP) with fifteen replications for each treatment (Table 1). The rate of 1-MCP was 10 g a.i./ha. Control plants received optimum quantities of water during the experiment. Optimum quantity was determined by weighing the plants the day before and after watering to saturation and allowing for excess drainage. A water-stress cycle consisted of withholding water from the pots until stomatal closure, after which the stress was relieved by re-watering with optimum quantity. This process was repeated twice. 1-MCP was applied with a CO2 backpack sprayer calibrated to deliver 187 l/ha two days after water supply had been discontinued (day 2 and day 8). The adjuvant AF-400 (Rohm Hass, Philadelphia, PA) was used for all 1-MCP applications at 0.375% v/v.
2.1. Stomatal Conductance Measurements
Stomatal conductance measurements (n = 10) were taken daily from the fourth uppermost main-stem leaf from 11:00 a.m. until 1:00 p.m. using a Decagon SC-1 Porometer (Decagon Inc., Pullman, WA). Three measurements on various areas of the leaf were taken and then averaged. The results were expressed as mmol/m²s.
2.2. Photosynthesis and Respiration Measurements
A Li-Cor Model 6200 portable photosynthesis system (LICOR Inc., Lincoln, NE) was used to determine photosynthetic and respiratory rates for the attached, fourth main-stem leaf from the terminal of the plant (n = 10). Photosynthesis measurements were taken at 1:00 p.m. one and four days after spraying. Respiratory rates were taken at 2:00 p.m. one and four days after spraying after turning off the lights in the growth chambers for 15 minutes and additionally covering the plant with a black cloth during the measurement.
2.3. Carbohydrate Content Measurements
White flowers and their subtending leaves were collected the last two days of each drying cycle at noon. Soluble carbohydrate content was measured according to a modification of the Hendrix protocol (1993) [36]. Ten white
Table 1. Rate and timing of treatments applied.
flowers and their subtending leaves were sampled from each replication-plant and they were oven dried for 3 days at 50˚C and then ground with a mortar and pestle. The ground tissue was extracted 3 times with 80% aqueous ethanol (800 ml ethanol/L) and the samples were centrifuged after each extraction at 5000 rpm and finally the fractions were pooled. Active charcoal was then added to the pooled fractions to remove substances that could interfere with the carbohydrate measurements and the samples were centrifuged again at 3500 rpm. The supernatant was immediately stored at −80˚C for later determination of sucrose and hexose (fructose and glucose) with a MultiScan Ascent Microplate Reader (Thermo Fisher Scientific Inc., Waltham, MA). A glucose HKassay kit (Sigma Chemical Company, St Louis, MO) was used. A 10µl aliquot of each extract was pipetted into a well of a microtitration plate and the plate was incubated at 50˚C for 40 min to evaporate ethanol. Ten microliters of water were then added to each well along with 100 µl of glucose assay reagent and the plate was incubated again for 15 min at 30˚C. The absorbance was measured at 340 nm using a microplate reader. Subsequently, 0.25 enzyme units of phosphoglucose isomerase was added to the extracts in each well of the plate and the absorbance was again measured at 340 nm after which, 83 enzyme units of invertase were added to the extracts and the microtitration plate was incubated at 30˚C for 60 min. The absorbance was measured at 340 nm.
2.4. Statistical Analysis
A two factor factorial statistical analysis was used to evaluate the results using JMP8 software (SAS Institute, Cary, NC). The factors consisted of experiment, water regime, and 1-MCP application. No interaction was observed between the two separate experiments, so the results were pooled and the means were taken. Analysis of variance and student’s t-test were used to analyze statistical significance. The days of the experiment were not considered a factor and a single ANOVA was done for each day to compare differences among treatment combinations.
3. Results
3.1. Stomatal Conductance
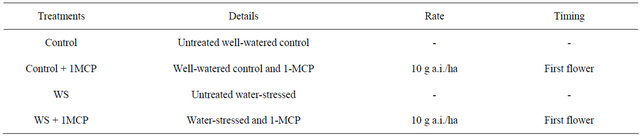
Leaf stomatal conductance was significantly lower in water-stressed plants compared to the control (Figure 1) in both watering cycles of the study. Stomatal conductance began to decrease 3 days after water supply was stopped, reaching its lowest at 48 mmol/m²s for untreated water-stressed plants and 42 mmol/m²s for 1- MCP treated water-stressed plants in 6 days. Upon rewatering, stomatal conductance measurements returned to same levels as the control. No significant interaction (P = 0.1043) was observed between 1-MCP application and water-deficit stress regime in any day of the experiment while a significant effect of the water regime (P < 0.001) was observed on days 0, 1, 3, 4, 6, 7, 8, 10 and 11 with water-deficit stress significantly lowering stomatal conductance rates compared to the control. On the other hand, stomatal conductance rates of 1-MCP treated water-stressed plants were similar to the rates of untreated water-stressed leaves and significantly lower compared to the control.
3.2. Photosynthesis and Respiration
Photosynthetic rates for the fourth main-stem leaf from the terminal were significantly decreased under conditions of water-deficit stress compared to the control (Figure 2). 1-MCP had no significant effect on preventing reductions in photosynthetic rates under waterlimited supply, since photosynthetic rates were decreased by 43% without 1-MCP application, and by 41% after 1-MCP application, compared to the control, respectively.
A similar trend was observed for the respiration rates of the fourth main-stem leaf from the terminal (Figure 3). Again water-limited supply caused a significant reducetion in the respiratory rates in the water-stressed leaves compared to the control. Similarly to stomatal conductance and photosynthesis, 1-MCP had no significant ameliorating effect on the respiration rates under water stress, with respiration rates decreasing 35% compared to the control without application of 1-MCP, and 42%
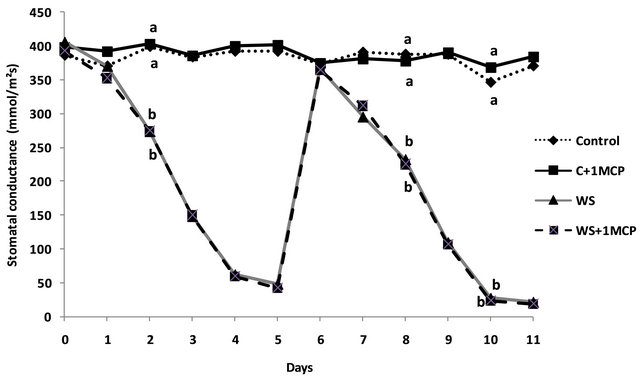
Figure 1. Effect of water-deficit stress and 1-MCP application on leaf stomatal conductance. Plants were imposed on two 5-day drying cycle. Water was withheld on day 0 and reapplied at the end of day 5. 1-MCP was applied on days 2 and 8.
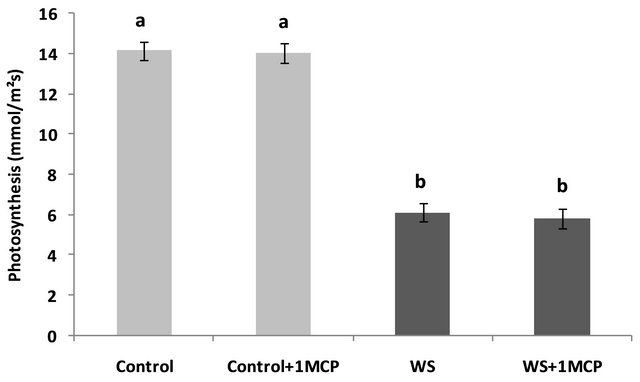
Figure 2. Effect of water-deficit stress and 1-MCP application on leaf photosynthesis 4 days after induction of stress. Bars with the same letter are not significantly different (P = 0.05). Error bars represent ± 1 standard error.
compared to the control after 1-MCP application.
3.3. Total Soluble Carbohydrate Content
Water-deficit stress caused a significant increase in sucrose concentrations of the pistil (Figure 4), however, sucrose concentrations of the subtending leaf remained unaffected (Figure 5). On the other hand, glucose levels of the pistil remained at the same levels as the control (Figure 6), whereas a significant increase was observed in the glucose concentrations of the water-stressed subtending leaves compared to the control (Figure 7). Both fructose levels of both the pistil and the subtending leaf remained unaffected under conditions of water-deficit stress and their levels were similar to the control (data not shown).
Application of 1-MCP resulted in a significant decrease in sucrose concentration of the pistils under conditions of water-deficit stress (Figure 4). No effect of 1-MCP application was observed in pistil glucose (Figure 6) and fructose concentrations (data not shown). Similarly, no significant effects of 1-MCP were observed on glucose, fructose and sucrose levels of water-stressed subtending leaves compared to the control.
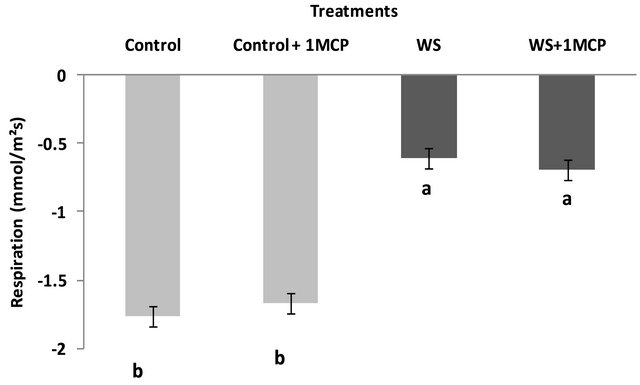
Figure 3. Effect of water-deficit stress and 1-MCP application on leaf respiration 4 days after induction of stress. Bars with the same letter are not significantly different (P = 0.05). Error bars represent ± 1 standard error.
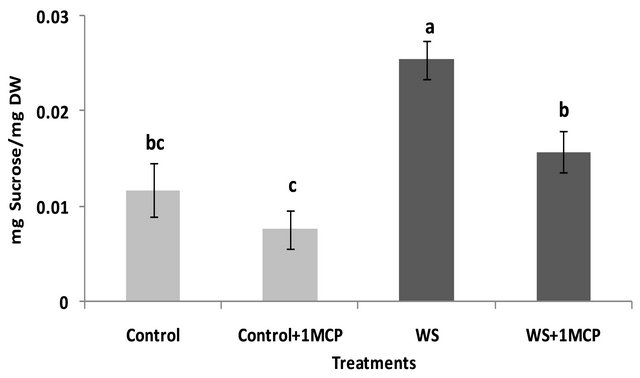
Figure 4. Effect of water-deficit stress and 1-MCP application on pistil sucrose content. Columns with the same letter are not significantly different (P = 0.05). Error bars represent ± 1 standard error.
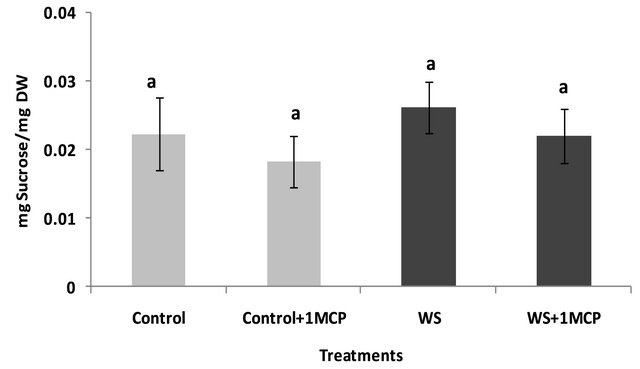
Figure 5. Effect of water-deficit stress and 1-MCP application on leaf sucrose content. Columns with the same letter are not significantly different (P = 0.05). Error bars represent ± 1 standard error.

Figure 6. Effect of water-deficit stress and 1-MCP application on pistil glucose content. Columns with the same letter are not significantly different (P = 0.05). Error bars represent ± 1 standard error.
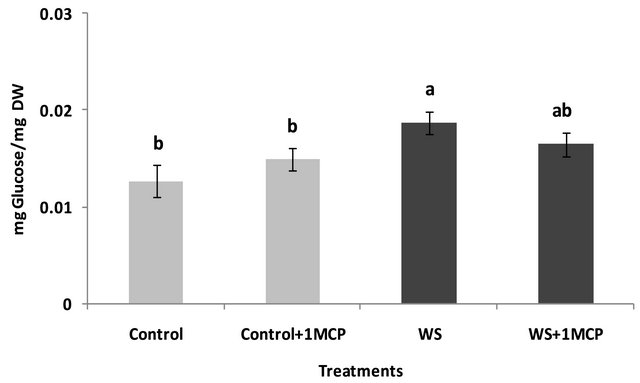
Figure 7. Effect of water-deficit stress and 1-MCP application on leaf glucose content. Columns with the same letter are not significantly different (P = 0.05). Error bars represent ± 1 standard error.
4. Discussion
Stomatal closure and consequently decreased stomatal conductance is a well-documented plant response to limited water conditions [37] in order to prevent excess water loss and in our study significant reductions of leaf stomatal conductance rates were observed in waterstressed plants compared to the control. Similar reductions in leaf stomatal conductance rates under waterdeficit stress have been reported in cotton by numerous researchers [24,25,33,34] with the exception of Ackerson et al. (1977) [35] who observed that leaf stomatal conductance of field-grown cotton was only slightly affected. However, we speculate that this differential response was due to the differences in light intensity as well as the more gradual imposition of water-deficit stress in the field compared to our potted experiments.
Despite past reports that ethylene production of cotton increases under conditions of limited water supply [13,20,21] inducing stomatal closure and lowering stomatal conductance [38,39], no such responses were observed in our study since application of 1-MCP had no significant effect on stomatal conductance of waterstressed plants. In accordance with our results, Klassen and Bugbee (2003) [18], Bugbee (2011) [22], and da Costa and Cothren (2011) [30] reported that 1-MCP application on potted cotton plants subjected to water deficit during their reproductive stage had no significant effect on leaf stomatal conductance rates and concluded that water-deficit stress had no effect on ethylene evolution rates. The opposite results were reported by Kawakami et al. (2010) [29] where it was observed that 1-MCP treated water-stressed plants exhibited higher stomatal resistance compared to untreated water-stressed plants and the authors concluded that ethylene production increases under conditions of limited water supply. However, in their experiment plants were subjected in water stress during their vegetative stage and not during their reproductive stage leading us to speculate that the differential to our study results are due to the differential ethylene production depending on the growth stage [40].
As expected, photosynthetic and respiratory rates of water-stressed plants were significantly compromised compared to well-watered control. Regarding photosynthesis, similar decreases in leaf photosynthesis under conditions of limited water supply have been observed in cotton [25,41,42] as well as several other species [43,44]. Unlike photosynthesis, diverse opinions have been expressed on the effect of water-deficit stress on leaf respiration with several researchers reporting significant decreases [45-47] while Ghashgaie et al. (2001) [48] noticed the opposite, and Lawlor and Fock (1977) [49] reported no change. In cotton, contrary to our results, Pallas et al. (1967) [50] reported an increase in respiretion under water stress, however we speculate that this is due to the different growth stages the stress was imposed in their studies.While exogenous application of ethylene has been reported to result in decreases in photosynthesis and respiration rates in soybean (Glycine max L.) [51], peanut (Arachis hypogea L.) [35] wheat (Triticum aestivum L.), maize (Zea mays L.), pea (Pisum sativum L.), beans (Phaseolus vulgaris L.), cotton [53] and lettuce (Lactuca sativa L.) [53], application of 1-MCP failed to prevent reduction of both photosynthetic and respiratory rates. In accordance with our results Bugbee (2011) [22] and da Costa and Cothren (2011) [30] reported that 1-MCP application had no effect on water-stressed cotton leaf gas exchange functions, leading us to accept their conclusion that water-deficit stress has no effect on leaf ethylene production rates and leaf gas exchange functions in cotton.
Similarly to leaf gas exchanger functions, leaf carbohydrate metabolism was significantly affected from water-deficit stress, while 1-MCP application had no significant effect. In our study, limited water-supply resulted in increases in leaf glucose concentrations whereas, fructose and sucrose concentrations remained similar to the control. Increases in leaf hexose concentrations under conditions of water-deficit stress have also been reported in other crops such as soybean [54], and barley (Hordeum vulgare, L.) [55], while in cotton Eaton and Ergle (1948) [56], Timpa et al. (1986) [57] and Parida et al. (2007) [58] observed that water-stressed leaves contained higher glucose concentrations and similar sucrose levels compared to the control indicating an impairment in photosynthate translocation mechanism. Observations in Arabidopsis have suggested that ethylene is implicated in leaf carbohydrate metabolism through modulating translocation of photosynthates [59], and also through regulation of enzymes such as invertase and sucrose synthase [60] and carbohydrate concentrations [61,62]. However, in our study leaf glucose, fructose and sucrose concentrations of 1-MCP treated plants were similar to those of untreated plants under both water-stressed and well-watered conditions leading us to assume that ethylene is not implicated in leaf carbohydrate metabolism of cotton.
Pistil carbohydrate metabolism was expected to be similar to leaf carbohydrate metabolism due to the subtending leaf being the main provider of photosynthate products to the pistil [63]. However, pistil carbohydrate metabolism was significantly affected by both waterdeficit stress and 1-MCP application. Water-deficit resulted in significant increases in pistil sucrose concentrations, while pistil glucose and fructose concentrations remained unaffected. The differential responses between leaf and pistil carbohydrate concentrations were attributed to tissue specific regulation of sucrose cleaving enzymes, with invertase being up-regulated in the leaves and down-regulated in the fruiting forms under conditions of water-deficit stress [27,62]. In support of our speculation increases in sucrose accumulation of reproductive units under limited water supply have been previously observed in soybean, wheat, maize and rice (Oryza sativa L.) [34,54,64-66] and have been associated with cessation of ovary and anther growth due to inhibittion of sucrose cleaving enzymes function. In cotton, Guinn (1976) [21] reported that no significant differences were observed in carbohydrate accumulation of 4-day old bolls that had been subjected to limited water supply. We speculate that the contrasting to our study results are due to differences in water-deficit stress imposition and age of tissue. Nevertheless, Guinn (1976) [21] reported that ethylene levels of water-stressed bolls were significantly higher compared to control.
Ethylene has been observed to modulate sucrose concentrations of reproductive units in sugar beet (Beta vulgaris L.) [67], grape berry (Vitis vinifera L.) [68] and rice [69]. In support of these observations, reductions in grain filling rate and weight due to increases in ethylene under conditions of water stress have been reported in wheat [70] and rice [34], while Mohapatra et al. (2000) [71] and Naik and Mohapatra (2000) [72] observed that application of ethylene inhibitors on rice improved grain filling and enhanced sucrose synthase activity under conditions of water stress. In accordance to the above reports, in our study application of 1-MCP had a pronounced effect on pistil sucrose concentration, significantly inhibiting sucrose accumulation under water-stress compared to the untreated water-stressed plants. However, reductions in sucrose concentrations, even though not significant, were observed between the treated wellwatered and the untreated well-watered leading us to assume 1-MCP decreased plant sensitivity to ethylene that is produced naturally during cotton flowering [13, 73]. Nevertheless, Morgan et al. (1990) [13] reported that flowering cotton plants subjected to water stress showed a decrease in ethylene production rates, while no changes in ethylene production rates were reported by Bugbee (2011) [22] and da Costa and Cothren (2011) [30]. Since ethylene rates were not determined in our study we cannot comment if 1-MCP affected ethylene production rates, however we can conclude that ethylene affects carbohydrate metabolism of cotton reproductive units. We speculate that the differential response of 1-MCP observed in cotton leaves and pistils is attributed either to the lower number of receptors existing in the leaves compared to the reproductive units [74] or to the differential regulation of sucrose cleaving enzymes due to tissue specific response of ethylene under conditions of water-deficit stress [40].
In summary, the results of our study indicated that water-deficit stress during reproductive development resulted in significant decreases in cotton leaf stomatal conductance, photosynthesis and respiration. Application of 1-MCP failed to ameliorate the negative consequences of water-deficit stress on cotton gas exchange functions indicating that either ethylene evolution from the leaves is minimal under conditions of water stress or ethylene evolution is uncoupled from cotton leaf’s gas exchange functions. However, the significant decrease in pistil sucrose concentrations indicates that ethylene plays a rather important role in the regulation of carbohydrate metabolism in reproductive tissues. Further research is needed to elucidate the exact site of modulation and factors controlling the interaction between ethylene production under water-deficit stress and cotton carbohydrate accumulation.
REFERENCES
- P. J. Kramer, “Water Deficits and Plant Growth,” In: P. J. Kramer, Ed., Water Relations of Plants, Academic Press, New York, 1983, pp. 342-389. doi:10.1016/B978-0-12-425040-6.50015-1
- A. Massaci, S. M. Nabiev, L. Petrosanti, S. K. Nematov, T. N. Chernikova, K. Thor and J. Leipner, “Response of the Photosynthetic Apparatus of Cotton (Gossypium hirsutum L.) to the Onset of Drought Stress Under Field Conditions Studied by Gas-Exchange Analysis and Chlorophyll Fluorescence Imaging,” Plant Physiology and Biochemistry, Vol. 46, No. 2, 2008, pp. 189-195. doi:10.1016/j.plaphy.2007.10.006
- H. N. L. Houerou, “Climate Changes, Drought and Desertification,” Journal of Arid Environment, Vol. 34, No. 2, 1996, pp. 133-185. doi:10.1006/jare.1996.0099
- J. A. Lee, “Cotton as a World Crop,” In: R. J. Kohel and C. F. Lewis, Eds., Cotton Agronomy Monogram 24. American Statistical Association, CSSA, SSSA, Madison, 1984, pp. 1-25.
- D. M. Oosterhuis and S. D. Wullschleger, “Osmotic Adjustment in Cotton (Gossypium Hisrsutum L.) Leaves and Roots in Response to Water Stress,” Plant Physiology, Vol. 84, No. 4, 1987, pp. 1154-1157. doi:10.1104/pp.84.4.1154
- A. L. Nepomuceno, D. M. Oosterhuis and J. M. Stewart, “Physiological Responses of Cotton Leaves and Roots to Water Deficit Induced by Polythelene Glycol,” Environmental and Experimental Botany, Vol. 40, No. 1, 1998, pp. 29-41. doi:10.1016/S0098-8472(98)00018-5
- V. V. Kuznetsov, V. Rakitin and V. N. Zholkevich, “Effects of Preliminary Heat-Shock Treatment on Accumulation of Osmolytes and Drought Resistance in Cotton Plants during Water Deficiency,” Physiologia Plantarum, Vol. 107, No. 4, 1999, pp. 399-406. doi:10.1034/j.1399-3054.1999.100405.x
- J. J. Burke, J. L. Hatfield, R. R. Klein and J. E. Mullet, “Accumulation of Heat Shock Proteins in Field Grown Soybean,” Plant Physiology, Vol. 78, No. 2, 1985, pp. 394- 398. doi:10.1104/pp.78.2.394
- R. C. Ackerson, D. R. Krieg, T. D. Miller and R. E. Zartman, “Water Relations of Field Grown Cotton and Sorghum: Temporal and Diurnal Changes in Leaf Water, Osmotic, and Turgor Potentials,” Crop Science, Vol. 17, No. 1, 1977, pp. 76-78. doi:10.2135/cropsci1977.0011183X001700010022x
- J. E. Quisenberry and B. Roark, “Influence of Indeterminate Growth Habit on Yield and Irrigation Water-Use Efficiency in Upland Cotton,” Crop Science, Vol. 16, No. 1976, pp. 762-765. doi:10.2135/cropsci1976.0011183X001600060005x
- H. Basal, C. W. Smith, P. S. Thaxton and J. K. Hemphill, “Seedling Drought Tolerance in Upland Cotton,” Crop Science, Vol. 45, No. 2, 2005, pp. 766-771. doi:10.2135/cropsci2005.0766
- F. B. Abeles, “Regulation of Ethylene Production by Internal, Environmental and Stress Factors. IV. Abiotic Stress,” In: F. B. Abeles, P. W. Morgan and M. E. Saltveit, Eds., Ethylene in Plant Biology, Academic Press Inc., San Diego, 1973, pp. 83-101.
- P. W. Morgan, C. J. He, J. A. De Greef and M. P. De Proft, “Does Water Deficit Stress Promote Ethylene Synthesis by Intact Plants?” Plant Physiology, Vol. 94, No. 4, 1990, pp. 1616-1624. doi:10.1104/pp.94.4.1616
- P. W. Morgan and M. C. Drew, “Ethylene and Plant Responses to Stress,” Physiologia Plantarum, Vol. 100, No. 3, 1997, pp. 620-630. doi:10.1111/j.1399-3054.1997.tb03068.x
- I. Narayana, S. Lalonde and H. S. Saini, “WaterStress-Induced Ethylene Production in Wheat,” Plant Physiology, Vol. 96, 1991, pp. 406-410. doi:10.1104/pp.96.2.406
- N. J. Stumpff and J. D. Johnson, “Ethylene Production by Loblolly Pine Seedlings Associated with Water Stress,” Physiologia Plantarum, Vol. 69, No. 1, 1987, pp. 167- 172. doi:10.1111/j.1399-3054.1987.tb01962.x
- J. J. Irigoyen, D. W. Emerich and M. Sanchez-Diaz, “Alfalfa Leaf Senescence Induced by Drought Stress: Photosynthesis, Hydrogen Peroxide Lipid Metabolism, Lipid Peroxidation and Ethylene Evolution,” Physiologia Plantarum, Vol. 84, No. 1, 1992, pp. 67-72. doi:10.1111/j.1399-3054.1992.tb08766.x
- S. P. Klassen and B. Bugbee, “Ethylene Synthesis and Sensitivity in Crop Plants,” Horticulture Science, Vol. 39, No. 7, 2003, pp. 1546-1552.
- B. L. McMichael, W. R. Jordan and R. D. Powell, “An Effect of Water Stress on Ethylene Production by Intact Cotton Petioles,” Plant Physiology, Vol. 49, 1972, pp. 658-660. doi:10.1104/pp.49.4.658
- B. L. McMichael, W. R. Jordan and R. D. Powell, “Abscission Processes in Cotton: Induction by Plant Water Deficit,” Agronomy Journal, Vol. 65, No. 2, 1973, pp. 202-204. doi:10.2134/agronj1973.00021962006500020005x
- G. Guinn, “Water Deficit and Ethylene Evolution by Young Cotton Bolls,” Plant Physiology, Vol. 57, No. 3, 1976, pp. 403-405. doi:10.1104/pp.57.3.403
- B. Bugbee, “Effect of Environment on Ethylene Synthesis and Cotton,” In: D. M. Oosterhuis, Ed., Stress Physiology in Cotton, The Cotton Foundation, Cordova, 2011, pp. 85-96.
- E. C. Sisler and M. Serek, “Inhibitors of Ethylene Responses in Plants at the Receptor Level: Recent Developments,” Physiologia Plantarum, Vol. 100, No. 3, 1997, pp. 577-582. doi:10.1111/j.1399-3054.1997.tb03063.x
- S. M. Blankenship and J. M. Dole, “1-Methylcyclopropene: A Review,” Postharvest Biology and Technology, Vol. 28, No. 1, 2003, pp. 1-25. doi:10.1016/S0925-5214(02)00246-6
- L. Dong, H. Zhou and S. Lurie, “Ripening of Red Rosa Plums: Effect of Ethylene and 1-Methylcyclopropene,” Australian Journal of Plant Physiology, Vol. 28, No. 10, 2001, pp. 1039-1045.
- J. D. Jeong, D. J. , Huber and S. A. Sargent, “Influence of 1-Methylcyclopropene (1-MCP) on Ripening and Cell Wall Matrix Polysaccharides of Avocado (Persea americana) Fruit,” Postharvest Biology and Technology, Vol. 25, No. 3, 2002, pp. 241-256. doi:10.1016/S0925-5214(01)00184-3
- X. T. Fan and J. P. Mattheis, “Yellowing of Broccoli in Storage Is Reduced by 1-Methylcyclopropene” Horticulture Science, Vol. 35, No. 5, 2000, pp. 885-887.
- W. B. Jiang, Q. Sheng, X. J. Zhou, M. J. Zhang and X. J. Liu, “Regulation of Detached Coriander Leaf Senescence by 1-Methylcyclopropene and Ethylene,” Postharvest Biology and Technology, Vol. 26, No. 3, 2002, pp. 339-345. doi:10.1016/S0925-5214(02)00068-6
- E. M. Kawakami, D. M. Oosterhuis and J. L. Snider, “Physiological Effects of 1-Methylcyclopropene on WellWatered and Water-Stressed Cotton Plants,” Journal of Plant Growth Regulation, Vol. 29, No. 3, 2010, pp. 280- 288. doi:10.1007/s00344-009-9134-3
- V. A. Da Costa and J. T. Cothren, “Drought Effects on Gas Exchange, Chlorophyll, and Plant Growth of 1-Methylcyclopropene Treated Cotton,” Agronomy Journal, Vol. 103, No. 4, 2011, pp. 1230-1241. doi:10.2134/agronj2010.0479
- M. N. Andersen, F. Asch, F. Wu, C. R. Jensen, H. Naested, V. O. Mogensen and K. E. Koch, “Soluble Invertase Expression Is an Early Target of Drought Stress during the Critical, Abortion-Sensitive Phase of Young Ovary Development in Maize,” Plant Physiology, Vol. 130, No. 2, 2002, pp. 591-604. doi:10.1104/pp.005637
- J. Yang, J. Zhang, Y. Ye, Z. Wang, Q. Zhu and L. Liu, “Involvement of Abscisic Acid and Ethylene in the Responses of Rice Grains to Water Stress during Grain Filling,” Plant Cell and Environment, Vol. 27, No. 8, 2004, pp. 1055-1064. doi:10.1111/j.1365-3040.2004.01210.x
- H. Bielorai and P. A. M. Hopmans, “Recovery of Leaf Water Potential, Transpiration, and Photosynthesis of Cotton during Irrigation Cycles,” Agronomy Journal, Vol. 67, No. 5, 1975, pp. 629-632. doi:10.2134/agronj1975.00021962006700050011x
- J. E. Ephrath, A. Marani and B. A. Bravdo, “Effects of Moisture Stress on Stomatal Resistance and Leaf Water Potential in Cotton (Gosssypium Hirsutum): I. Controlled Levels of Stress” Field Crops Research, Vol. 23, No. 2, 1990, pp. 117-131. doi:10.1016/0378-4290(90)90107-M
- R. C. Ackerson, D. R. Krieg, C. L. Haring and N. Chang, “Effects of Plant Water Status on Stomatal Activity, Photosynthesis, and Nitrate Reductase Activity of Field Grown Cotton,” Crop Science, Vol. 17, No. 1, 1977, pp. 81-84. doi:10.2135/cropsci1977.0011183X001700010023x
- D. L. Hendrix, “Rapid Extraction and Analysis of Nonstructural Carbohydrates in Plant Tissues,” Crop Science, Vol. 33, No. 6, 1993, pp. 1306-1311. doi:10.2135/cropsci1993.0011183X003300060037x
- T. C. Hsiao, “Plant Responses to Water Stress,” Annual Reviews of Plant Physiology, Vol. 24, 1973, pp. 519-570. doi:10.1146/annurev.pp.24.060173.002511
- J. E. Pallas and S. J. Kays, “Inhibition of Photosynthesis by Ethylene—A Stomatal Effect,” Plant Physiology, Vol. 70, No. 2, 1982, pp. 598-601. doi:10.1104/pp.70.2.598
- C. Vitogliano and G. V. Hoad, “Leaf Stomatal Resistance, Ethylene Evolution and ABA-Levels as Influenced by 2-Chloroethyl-Phosphonic Acid,” Scientia Horticulturae, Vol. 8, No. 2, 1978, pp. 101-106. doi:10.1016/0304-4238(78)90013-4
- W. Verelst, A. Skirycz and D. Inze, “Abscisic Acid, Ethylene and Gibberellic Acid Act at Different Developmental Stages to Instruct the Adaptation of Young Leaves to Stress,” Plant Signaling and Behavior, Vol. 5, No. 4, 2010, pp. 473-475. doi:10.4161/psb.5.4.11421
- M. J. Lacape, J. Wery and D. J. M. Annerose, “Relationships between Plant and Soil Water Status in Five FieldGrown Cotton Cultivars,” Field Crops Research, Vol. 57, No. 1, 1998, pp. 29-43. doi:10.1016/S0378-4290(97)00111-1
- W. T. Pettigrew, “Physiological Consequences of Moisture Deficit Stress in Cotton,” Crop Science, Vol. 44, No. 4, 2004, pp. 1265-1272. doi:10.2135/cropsci2004.1265
- J. Flexas, J. Bota, J. Galmes, H. Medrano and M. RibasCarbo, “Keeping A Positive Carbon Balance under Adverse Conditions: Responses of Photosynthesis and Respiration to Water Stress,” Physiologia Plantarum, Vol. 127, No. 3, 2006, pp. 343-352. doi:10.1111/j.1399-3054.2006.00621.x
- D. W. Lawlor and W. Tezara, “Causes of Decreased Photosynthetic Rate and Metabolic Capacity in WaterDeficient Leaf Cells: A Critical Evaluation of Mechanisms and Integration of Processes,” Annals of Botany, Vol. 103, No. 4, 2009, pp. 561-579. doi:10.1093/aob/mcn244
- F. W. T. De Vries, J. M. Witlage and D. Kremer, “Rates of Respiration and of Increase in Structural Dry Matter in Young Wheat, Ryegrass and Maize Plants in Relation to Temperature, to Water Stress and to Their Sugar Content,” Annals of Botany, Vol. 44, No. 5, 1979, pp. 595- 609.
- K. J. McCree, C. E. Kallsen and S. G. Richardson, “Carbon Balance of Sorghum Plants during Osmotic Adjustment to Water Stress,” Plant Physiology, Vol. 76, No. 4, 1984, pp. 898-902. doi:10.1104/pp.76.4.898
- M. Ribas-Carbo, N. L. Taylor, L. Giles, S. Busquets, P. M. Finnegan and D. A. Day, “Effects of Water Stress on Respiration in Soybean Leaves,” Plant Physiology, Vol. 139, No. 1, 2005, pp. 466-473. doi:10.1104/pp.105.065565
- J. Ghashgaie, M. Duranceau, F. W. Badeck, G. Cornic, M. T. Adeline and E. Deleens,” Δ C13 of CO2 Respired in the Dark in Relation to Δ C13 of Leaf Metabolites: Comparison between Nicotiana sylvestris and Helianthus annuus under Drought,” Plant Cell and Environment, Vol. 24, No. 5, 2001, pp. 505-515. doi:10.1046/j.1365-3040.2001.00699.x
- D. W. Lawlor and H. Fock, “Water Stress Induced Changes in the Amounts of Some Photosynthetic Assimilation Products and Respiratory Metabolites of Sunflower Leaves” Journal of Experimental Botany, Vol. 28, No. 2, 1977, pp. 329-337. doi:10.1093/jxb/28.2.329
- J. E. Pallas, B. E. Michel and D. G. Harris, “Photosynthesis, Transpiration, Leaf Temperature, and Stomatal Activity of Cotton Plants under Varying Water Potentials,” Plant Physiology, Vol. 42, No. 1, 1967, pp. 76-88. doi:10.1104/pp.42.1.76
- C. A. Gunderson and G. E. Taylor, “Ethylene Directly Inhibits Foliar Gas Exchange in Glycine max,” Plant Physiology, Vol. 95, No. 1, 1991, pp. 337-339. doi:10.1104/pp.95.1.337
- G. E. Taylor and C. A. Gunderson, “The Response of Foliar Gas Exchange to Exogenously Applied Ethylene,” Plant Physiology, Vol. 82, No. 3, 1986, pp. 653-657. doi:10.1104/pp.82.3.653
- C. He and F. T. Davies, “Ethylene Reduces Plant Gas Exchange and Growth of Lettuce Grown from Seed to Harvest under Hypobaric and Ambient Total Pressure,” Journal of Plant Physiology, Vol. 169, No. 4, 2012, pp. 369-378. doi:10.1016/j.jplph.2011.11.002
- F. Liu, C. R. Jensen and M. N. Andersen, “Drought Stress Effect on Carbohydrate Concentration in Soybean Leaves and Pods during Early Reproductive Development: Its Implication in Altering Pod Set,” Field Crops Research, Vol. 86, No. 1, 2004, pp. 1-13. doi:10.1016/S0378-4290(03)00165-5
- B. Teulat, C. Borries and D. This, “New QTLs Identified for Plant Water Status, Water-Soluble Carbohydrate and Osmotic Adjustment in a Barley Population Grown in a Growth-Chamber under Two Water Regimes,” Theoretical and Applied Genetics, Vol. 103, No. 1, 2001, pp. 161- 170. doi:10.1007/s001220000503
- F. M. Eaton and D. R. Ergle, “Carbohydrate Accumulation in the Cotton Plant at Low Moisture Levels,” Plant Physiology, Vol. 23, No. 2, 1948, pp. 169-187. doi:10.1104/pp.23.2.169
- J. D. Timpa, J. J. Burke, J. E Quisenberry and C. W. Wendt, “Effects of Water Stress on the Organic Acid and Carbohydrate Composition of Cotton Plants,” Plant Physiology, Vol. 82, No. 3, 1986, pp. 724-728. doi:10.1104/pp.82.3.724
- A. K. Parida, V. S. Dagaonkar, M. S. Phalak, G. V. Umalkar and L. P. Aurangabadkar, “Alterations in Photosynthetic Pigments, Protein and Osmotic Components in Cotton Genotypes Subjected to Short-Term Drought Stress Followed by Recovery,” Plant Biotechnology Reports, Vol. 1, No. 1, 2007, pp. 37-48. doi:10.1007/s11816-006-0004-1
- L. Zhou, J. C. Jang, T. L. Jones and J. Sheen, “Glucose and Ethylene Signal Transduction Cross-Talk Revealed by an Arabidopsis Glucose-Insensitive Mutant,” Plant Biology, Vol. 95, No. 17, 1998, pp. 10294-10299.
- [61] F. Rolland, E. Baena-Gonzalez and J. Sheen, “Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms,” Annual Reviews of Plant Biology, Vol. 57, 2006, pp. 675-709. doi:10.1146/annurev.arplant.57.032905.105441
- [62] P. Leon and J. Sheen, “Sugar and Hormone Connections,” Trends in Plant Science, Vol. 8, No. 3, 2003, pp. 110-116. doi:10.1016/S1360-1385(03)00011-6
- [63] K. E. Koch, “Sucrose Metabolism:Regulatory Mechanisms and Pivotal Roles in Sugar Sensing and Plant Development,” Current Opinion in Plant Biology, Vol. 7, No. 3, 2004, pp. 235-246. doi:10.1016/j.pbi.2004.03.014
- [64] D. A. Ashley, “14C-Labelled Photosynthate Translocation and Utilization in Cotton Plants,” Crop Science, Vol. 12, No. 1, 1972, pp. 69-72. doi:10.2135/cropsci1972.0011183X001200010023x
- [65] J. R. Schussler and M. E. Westgate, “Assimilate Flux Determines Kernel Set at Low Water Potential in Maize,” Crop Science, Vol. 35, No. 4, 1995, pp. 1074-1080. doi:10.2135/cropsci1995.0011183X003500040026x
- [66] C. Zinselmeier, B. R. Jeong and J. S. Boyer, “Starch and the Control of Kernel Number in Maize at Low Water Potentials,” Plant Physiology, Vol. 121, No. 1, 1999, pp. 25-35. doi:10.1104/pp.121.1.25
- [67] H. S. Saini and M. E. Westgate, “Reproductive Development in Grain Crops during Drought,” Advances in Agronomy, Vol. 68, 1999, pp. 59-96. doi:10.1016/S0065-2113(08)60843-3
- [68] R. A. Saftner, “Effect of Ethylene on Sucrose Uptake in Root Discs of Sugar Beet,” Plant Cell Physiology, Vol. 27, No. 5, 1986, pp. 853-860.
- [69] C. Chervin, N. Terrier, A. Ageorges, F. Ribes and T. Kuapunyakoon, “Influence of Ethylene on Sucrose Accumulation in Grape bErry,” American Journal of Enology and Viticulture, Vol. 57, No. 4, 2006, pp. 511-513.
- [70] K. Ishizawa and Y. Esashi, “Action Mechanism of Ethylene in the Control of Sugar Translocation in Relation to Rice Coleoptiles Growth. I. Sucrose Metabolism,” Plant Cell Physiology, Vol. 29, No. 1, 1988, pp. 131-141.
- [71] J. Xu, G. H. Pemberton, E. C. Almira, D. R. McCarty and K. E. Koch, “The Ivr1 Gene for Invertase in Maize,” Plant Physiology, Vol. 108, No. 3, 1995, pp. 1293-1294. doi:10.1104/pp.108.3.1293
- [72] P. K. Mohapatra, P. K. Naik and R. Patel, “Ethylene Inhibitors Improve Dry Matter Partitioning and Development of Late Flowering Spikelets on Rice Panicles,” Australian Journal of Plant Physiology, Vol. 27, No. 4, 2000, pp. 311-323. doi:10.1071/PP99057
- [73] P. K. Naik and P. K. Mohapatra, “Ethylene Inhibitors Enhanced Sucrose Synthase Activity and Promoted Grain Filling of Basal Rice Kernels,” Australian Journal of Plant Physiology, Vol. 27, No. 11, 2000, pp. 997-1008.
- [74] J. A. Lipe and P. W. Morgan, “Ethylene, a Regulator of Young Fruit Abscission,” Plant Physiology, Vol. 51, No. 5, 1973, pp. 949-953. doi:10.1104/pp.51.5.949
- [75] J. K. Burns, “1-Methylcyclopropene Applications in Preharvest Systems: Focus on Citrus,” HortScience, Vol. 43, No. 1, 2008, pp. 112-114.
NOTES
*Corresponding author.

