Paper Menu >>
Journal Menu >>
 Engineering, 2010, 2, 387-390 doi:10.4236/eng.2010.25050 Published Online May 2010 (http://www.SciRP.org/journal/eng) Copyright © 2010 SciRes. ENG 387 Microwave-Assisted Synthesis of Silver Nanoparticles in Alkalic Carboxymethyl Chitosan Solution Binghui Wang1, Xupin Zhuang1, Wenjian Deng1, Bowen Cheng2* 1Department of Nonwovens, School of Textile, Tianjin Polytechnic University, Tianjin, China 2Tianjin Key Laboratory of Fiber modification and functional fiber, Tianjin Polytechnic University, Tianjin, China *E-mail: bowen15@tjpu.edu.cn Received December 16, 2009; revised February 18, 2010; accepted February 26, 2010 Abstract Silver nanoparticles were prepared by microwave irradiation of silver nitrate solution with carboxymethyl chitosan as reducing agent and a stabilizer. The optical properties, morphology and structure were character- ized using UV–Visible spectrophotometer, transmission electron microscope (TEM) and X-ray diffraction (XRD). Appearance of surface plasmon band at 413 nm indicated the formation of silver nanoparticles within 5 s microwave irradiation. TEM images show most silver nanoparticles are between 2 nm and 20 nm. XRD results identified the nanoparticles as face-centered cubic phase. Keywords: Silver Nanoparticles, Carboxymethyl Chitosan, Microwave Irradiation, Chemical Reduction 1. Introduction Metal nanoparticles have attracted significant attention because of their unusual size-dependent optical and elec- tronic properties. Among these metals, silver nanoparti- cles (AgNPs) show potential applications in various fields such as the environment, biomedicinal, catalysis, optics and electronics [1]. AgNPs can be synthesized using various methods such as chemical reduction [2], electrochemical [3], γ-radiation [4], laser ablation [5], photochemical [6]. Among these methods, the most popular method is chemical reduction of silver salt in the presence of stabilizer. Most commonly used stabilizing agents are polymers [7] and surfactants [8]. Utilization of nontoxic chemicals and renewable materials has attracted considerable attention due to their advantage in reducing the environmental risk. Polysaccharide, as a polyhy- droxylated macromolecule, has been reported used as ‘green’ capping agent in the synthesis of silver nanopar- ticles. Chitosan, a natural polysaccharide widely found in the shells and exoskeletons of some crustacean, has been used to prepare AgNPs and act as both reducing agent and stabilizer in the process. The alkalic environment is benefit for the formation while the solubility of chitosan in neutral and alkalic solution is poor. Carboxymethyl chitosan (CMCT), which is a common derivative of in- troduction carboxymethyl group onto the hydroxyl and amine groups, possess solubility in wide pH range even alkalic solutions [9]. CMCT has a higher sorption ability of metal ions than chitosan which is taken for its in- creased chain flexibility [10] and higher concentrations of chelating groups [11]. Therefore, it is of interest to study the formation of AgNPs with CMCT as matrix material. On the other hand, for the preparation of metal nano- particles, microwave heating has been reported recently to have better promise over thermal heating. In the mi- crowave frequency range 300 MHz to 300 GHz, polar molecules such as H2O try to orient with the electric field. When the dipolar molecules try to re-orient with respect to an alternating electric field, they lose energy in the form of heat by molecular friction [12]. This study described the formation of AgNPs in CMCT solutions with microwave assisted, and the char- acteristics of AgNPs were studied using UV–visible (UV–vis), transmission electron microscopy (TEM), zeta potential, polarizing optical microscopy (POM) and X-ray diffraction (XRD). 2. Experimental Carboxymethyl chitosan was synthesized according to our previously reported method using a chitoasn with Mw 35 kDa as start material and the degree of substitu- tion was 0.79 determined by pH titration. CMCT was used in the form of sodium salt. For the synthesis of sil-  B. H. WANG ET AL. 388 ver nanoparticles, a Midea brand microwave oven (model: AU23B-AQ) was used. In a typical procedure, 10 ml of 0.1 M AgNO3 (99.5%, analytical grade) aque- ous solution was mixed with 25 ml of 0.1% wt CMCT aqueous solution. The mixture was adjusted to 100 ml by adding of purified water. All the aqueous solutions were prepared using ultrahigh purity water purified by a mill-Q system. Then the solution was taken in a closed round flask, and placed in a microwave oven that was operated at a power of 800 W and frequency 2450 MHz for different time. The solution turned colloidal and yel- low in color which suggested the formation of silver nanoparticles. The silver nanoparticles solutions with different reac- tion time were characterized with UV-vis spectrum (SHIMADZU UV-2401P). Transmission electron mi- croscope (TEM) was performed on a HITACHI H-7650 machine to observe the morphology of the nanoparticles. The samples for TEM were prepared by dropping a drop of dilute aqueous solution on carboncoated copper grids and air-dried. The diameters of nanoparticles were cal- culated with the aid of DigitalMicrograph™ software (Gatan, Inc). Delsa™Nano submicron particle size and zeta potential analyzer was also applied to examine the size of AgNPs-CMCT composite. The solution with reaction time of 15 s was casted film and dried at vacuum oven. Then the film was examined with polarizing optical microscopy (POM, OLYMPUS BX51 equipped with 5050 ZOOM digital camera) and wide angle X-ray diffraction (XRD, Bruker Axs D8 Discover with Gadds, Cu Kα). 3. Results and Discussions It was found that the colourless solution turned yellow even within 5 s of microwave irradiation, which sug- gested that silver nanoparticles were successfully synthe- sized with CMCT and AgNO3 promoted by microwave. Figure 1 shows the UV–vis spectra of the samples at different reaction time during the reactive process. An absorption peak named surface plasmon absorption band (SPB) is observed at about 413 nm, and the intensity became stronger with the passage of reactive time. The results indicate more AgNPs crystal nucleus amount formation and the size of nanoparticles negligible in- crease. Also, there is no obvious absorption in the range of 450-800 nm indicates the neglectable aggregation oc- curs in this reactive system and the nanoparticles are well dispersed. CMCT is an oxygen-rich natural polysaccharide con- sisting of anhydroglucose units joined by an oxygen linkage as chitosan. When AgNO3 was mixed with CMCT solution, Ag+ ions could be bound to CMCT macromolecules chains probably via electrostatic (i.e., ion–dipole) interactions, because the electron-rich oxy- 300 400 500 60 0 0.0 0.2 0.4 0.6 0.8 Absorbance Wavelength(nm) 18s 15s 12s 10s 8s 5s Figure 1. UV–vis spectra of the silver colloidal solution at different microwave irradiation time. gen atoms of polar hydroxyl and CMCT are expected to interact with electropositive Ag+ ions and form CMCT- Ag+ complex [13]. With microwave heating, continuing reduction of Ag+ to Ag causes the aggregation of silver clusters into nanoparticles. The morphology of the monodispersed AgNPs was observed with the aid of TEM (c.a. Figure 2). The AgNPs are observed as spherical particles and uniformly distributed which illustrates the synthesis of AgNPs through reduction of Ag+ inside the polymer templates. The histogram of the particle size distribution shows that most particles are between 2 nm and 20 nm and have similar distributions at 5 s and 15 s. The results demon- strate that use of microwave irradiation in the synthesis is a promise method not only due to faster heating but it also gives internal uniform heating resulting into uni- formly distributed monodispersed particles. The particles size of the AgNPs was further tested us- ing the laser diameter measurement method as shown in Figure 3. From the results, the size distribution is be- tween 55.7 nm and 111.4 nm which is higher than the value tested by TEM. Considering that the energy distri- bution of laser of dispersed and diffracted is used to evaluate the size of particles in this method, the test con- firms the structure of AgNPs-CMCT composites in which AgNPs are stabilized by CMCT macromolecules. Figure 4 shows the POM image of the casted film from AgNPs-CMCT composites solution. The branch configuration is observed in which the bright points rep- resent the silver nanoparticles bind to the CMCT chains. The image further confirmed the structure of AgNPs- CMCT composites. The XRD pattern of AgNPs-CMCT powder prepared from the colloidal solution is given in Figure 5. The fig- ure shows the bragg diffraction peaks at 38.1°, 44.0°, 64.0° and 77.7° which belong to (111), (200), (220) and (311) planes respectively. This is in good agreement Copyright © 2010 SciRes. ENG 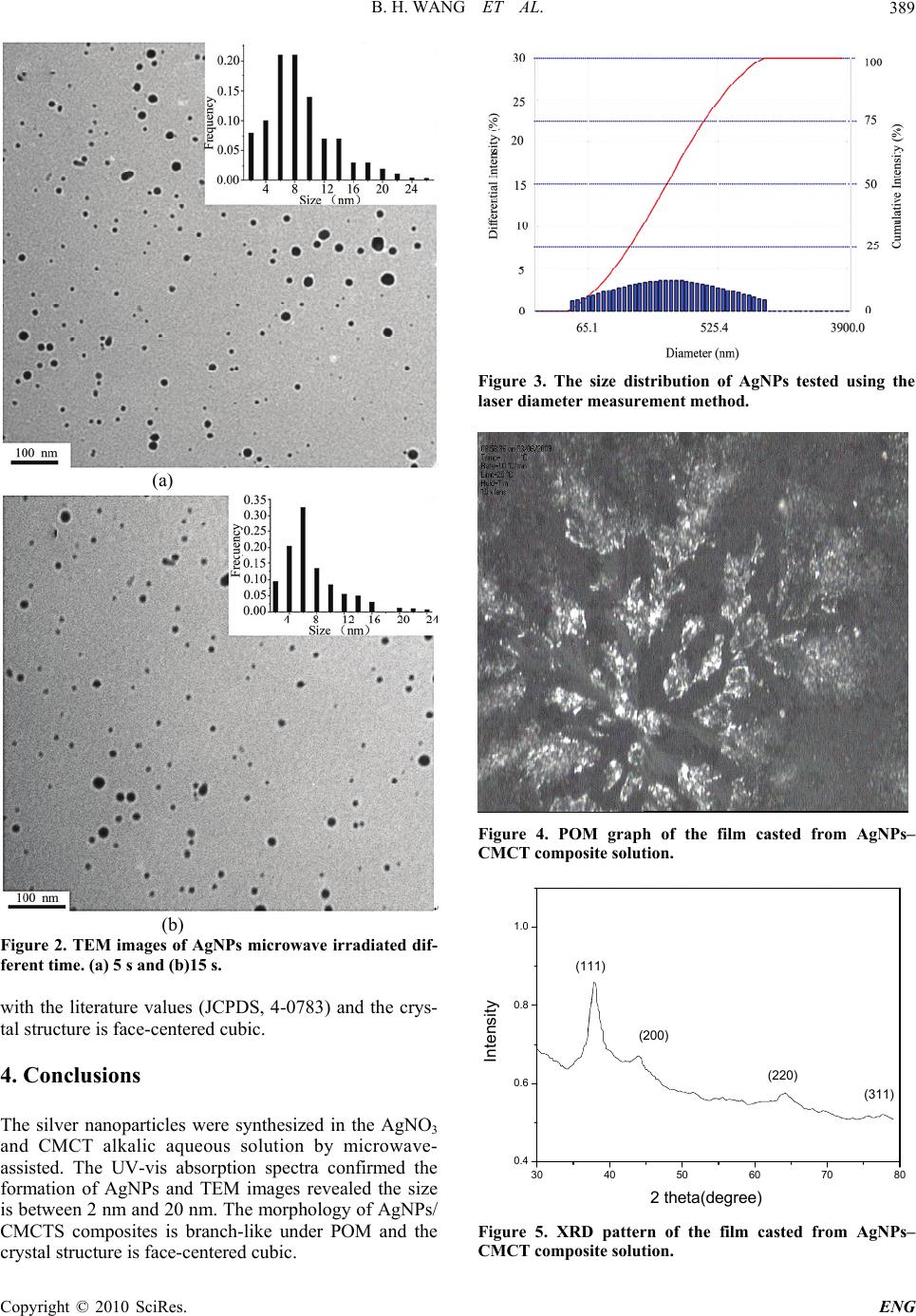 B. H. WANG ET AL.389 (a) (b) Figure 2. TEM images of AgNPs microwave irradiated dif- ferent time. (a) 5 s and (b)15 s. with the literature values (JCPDS, 4-0783) and the crys- tal structure is face-centered cubic. 4. Conclusions The silver nanoparticles were synthesized in the AgNO3 and CMCT alkalic aqueous solution by microwave- assisted. The UV-vis absorption spectra confirmed the formation of AgNPs and TEM images revealed the size is between 2 nm and 20 nm. The morphology of AgNPs/ CMCTS composites is branch-like under POM and the crystal structure is face-centered cubic. Figure 3. The size distribution of AgNPs tested using the laser diameter measurement method. Figure 4. POM graph of the film casted from AgNPs– CMCT composite solution. 30 40 50 60 70 80 0.4 0.6 0.8 1.0 Intensity 2 theta(degree) (111) (200) (220) (311) Figure 5. XRD pattern of the film casted from AgNPs– CMCT composite solution. Copyright © 2010 SciRes. ENG  B. H. WANG ET AL. Copyright © 2010 SciRes. ENG 390 5. Acknowledgements The authors are grateful for the support of Tianjin Col- lege Science and Technology Development Fund under Grant 20060911. 6 . References [1] T. J. Norman, C. D. Grant, D. Magana, et al., “Near In- frared Optical Absorption of Gold Nanoparticle Aggre- gates,” The Journal of Physical Chemistry B, Vol. 106, No. 28, 2002, pp. 7005-7012. [2] A. Pal, S. Shah and S. Devi, “Synthesis of Au, Ag and Au-Ag Alloy Nanoparticles in Aqueous Polymer Solu- tion,” Colloids Surface A: Physicochemical and Engi- neering Aspects, Vol. 302, No. 1-3, 2007, pp. 51-57. [3] M. T. Reetz and W. Helbig, “Size-Selective Synthesis of Nanostructured Transition Metal Clusters,” Journal of the American Chemical Society, Vol. 116, No. 16, 1994, pp. 7401-7402. [4] S. H. Choi, Y. P. Zhang, A. Gopalan, K. P. Lee and H. D. Kang, “Preparation of Catalytically Efficient Precious Metallic Colloids by γ-irradiation and Characterization,” Colloids and Surfaces A: Physicochemical and Engi- neering Aspects, Vol. 256, No. 2-3, 2005, pp. 165-170. [5] H. Willwohl, J. Wolfrum, V. Zumbach, P. Albers and K. Seibold, “Production and Characterization of Highly Dispersed Catalytic Active Platinum and Palladium Pow- ers by Excimer Laser Photolysis,” Journal of Physical Chemistry, Vol. 98, 1994, pp. 2242-2247. [6] Z. Li, Y. Li, X. F. Qian, J. Yin and Z. K. Zhu, “A Simple Method for Selective Immobilization of Silver Nanopar- ticles,” Applied Surface Science, Vol. 250, No. 1-4, 2005, pp. 109-116. [7] A. Murugadoss and A. Chattopadhyay, “A ‘Green’ Chi- tosan-Silver Nanoparticle Composite as a Heterogeneous as well as Micro-Heterogeneous Catalyst,” Nanotechnol- ogy, Vol. 19, No.1, 2008, pp. 1-9. [8] A. Pal, S. Shah and S. Devi, “Preparation of Silver, Gold and Silver-Gold Bimetallic Nanoparticles in w/o Mi- croemulsion Containing TritonX-100,” Colloids and Sur- faces A: Physicochemical and Engineering Aspects, Vol. 302, No. 1-3, 2007, pp. 483-487. [9] X. P. Zhuang, X. F. Liu, Z. Li, Y. L. Guan and K. D. Yao, Chinese Journal of Polymer Science, Vol. 22, 2004, p. 521 [10] E. Guibal, “Interactions of Metal Ions with Chito- san-Based Sorbents: A Review,” Purification Technology, Vol. 38, No.1, 2004, pp. 43-74. [11] W. S. W. Ngah and K. H. Liang, “Adsorption of Gold(III) Irons onto Chitosan and N-Carboxymethyl Chitosan: Equilibrium Studies,” Industrial and Engineering Chem- istry Research, Vol. 38, No. 4, 1999, pp. 1411-1414. [12] L. Huang, M. L. Zhai, D. W. Long, J. Peng, et al., “UV-Induced Synthesis, Characterization and Formation Mechanism of Silver Nanoparticles in Alkalic Carboxy- methylated Chitosan Solution,” Journal of Nanoparticle Research, Vol. 10, No. 7, 2008, pp. 1193-1202. [13] H. Huang, Q. Yuan, and X. Yang, “Preparation and Characterization of Metal-Chitosan Nanocomposites,” Colloids and Surfaces B: Biointerfaces, Vol. 39, No. 1-2, 2004, pp. 31-37. |

