Paper Menu >>
Journal Menu >>
 Energy and Power Engineering, 2010, 2, 95-102 doi:10.4236/epe.2010.22014 Published Online May 2010 (http://www.SciRP.org/journal/epe) Copyright © 2010 SciRes. EPE 95 Co-liquefaction of Coal and Used Tire in Supercritical Water Kwanruthai Onsri1, Pattarapan Prasassarakich1,2, Somkiat Ngamprasertsith1,2* 1Fuels Research Center, Department of Chemical Technology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand 2Center for Petroleum, Petrochemicals and Advance Materials, Chulalongkorn University, Bangkok, Thailand E-mail: somkiat.n@chula.ac.th Received December 19, 2009; revised January 8, 2010; accepted March 3, 2010 Abstract The co-liquefaction of lignite coal and used tire was performed in a 250-ml batch reactor, in supercritical water under a nitrogen atmosphere to investigate the effects of temperature (380-440℃), water/feedstock ratio (4/1-10/1 (wt./wt.)) and the % used tire content in the feedstock (0-100 wt.%) on the conversion effi- ciency, liquid yield and oil composition attained. The maximum conversion and oil yield were 67 and 50%, respectively, obtained at 400℃ at 1 min, with water/feedstock ratio of 10/1 and 80% used tire content. The distillation characteristics of the oil products, analyzed by simulated distillation gas chromatography, re- vealed that the oil composition depended significantly on the reaction temperature. The co-liquefaction of coal and used tire yielded a synergistically increased level of oil production. Moreover, the total conversion level obtained with co-liquefaction alone was almost equal to those obtained in the presence of either Fe2O3 or NiMo as catalysts, under the same conditions. Therefore, supercritical water is a good medium for the dissolution of the volatile matter from a coal and used tire matrix. Keywords: Co-liquefaction, Coal, Used Tire, Supercritical Water 1. Introduction In recent years, the growth in tire consumption has con- tinued to expand concomitantly leading to the problem of the disposal of an ever increasing number of essentially non-biodegredable but flammable spent scrap tires with- out causing environmental pollution (including combus- tion). With in excess of 3.0 million tons per year of waste tires being produced in just the USA and Japan alone [1], this has become a major challenge. Indeed, currently perhaps only 60-70% of all used tires are recycled, and evens this requires the use of environmentally and eco- nomically costly processes including the use of solvents like n-hexane, toluene and tetralin [2,3]. Used tires are comprised of vulcanized natural and synthetic rubbers, zinc, sulfur and carbon black and, as such, contain poly- meric aromatic structures that are somewhat similar to those in coal. Hence, the well-developed techniques used in coal utilization should theoretically be applicable to the pyrolytic destruction of waste tires and there has been an increasing amount of attention paid to the co-utiliza- tion of coal and waste tires. Indeed, given that coal liq- uefaction is enhanced by the addition of crude oil [4] which is a source of rubber constituents in tires, the co- liquefaction of coal and used tires is of obvious interest. A number of different concepts for the degradation of spent tires in the presence of coal have hitherto contrib- uted to the background knowledge. The processing of used tire and/or coal have been subjected to thermal py- rolysis and supercritical extraction using toluene, helium, nitrogen and water. Mastral et al. [5] investigated subbi- tuminous coal-tire hydroprocessing and reported that oil formation and total solid conversion reached 45% and 70%, respectively, at a reaction temperature of 400℃, with a coal: tire ratio of 0.5 and 10 MPa initial hydrogen pressure. Moreover, the presence of rubber tire had a positive effect as an additive for coal hydropyrolysis and this was more relevant when tire feeds were coprocessed. In a similar vein, synergistic effects including increased total conversion levels and the yields of oil and asphal- tene were attained during the simultaneous hydrogenoly- sis of coal and tire were reported [2-9]. Joung et al. [3] 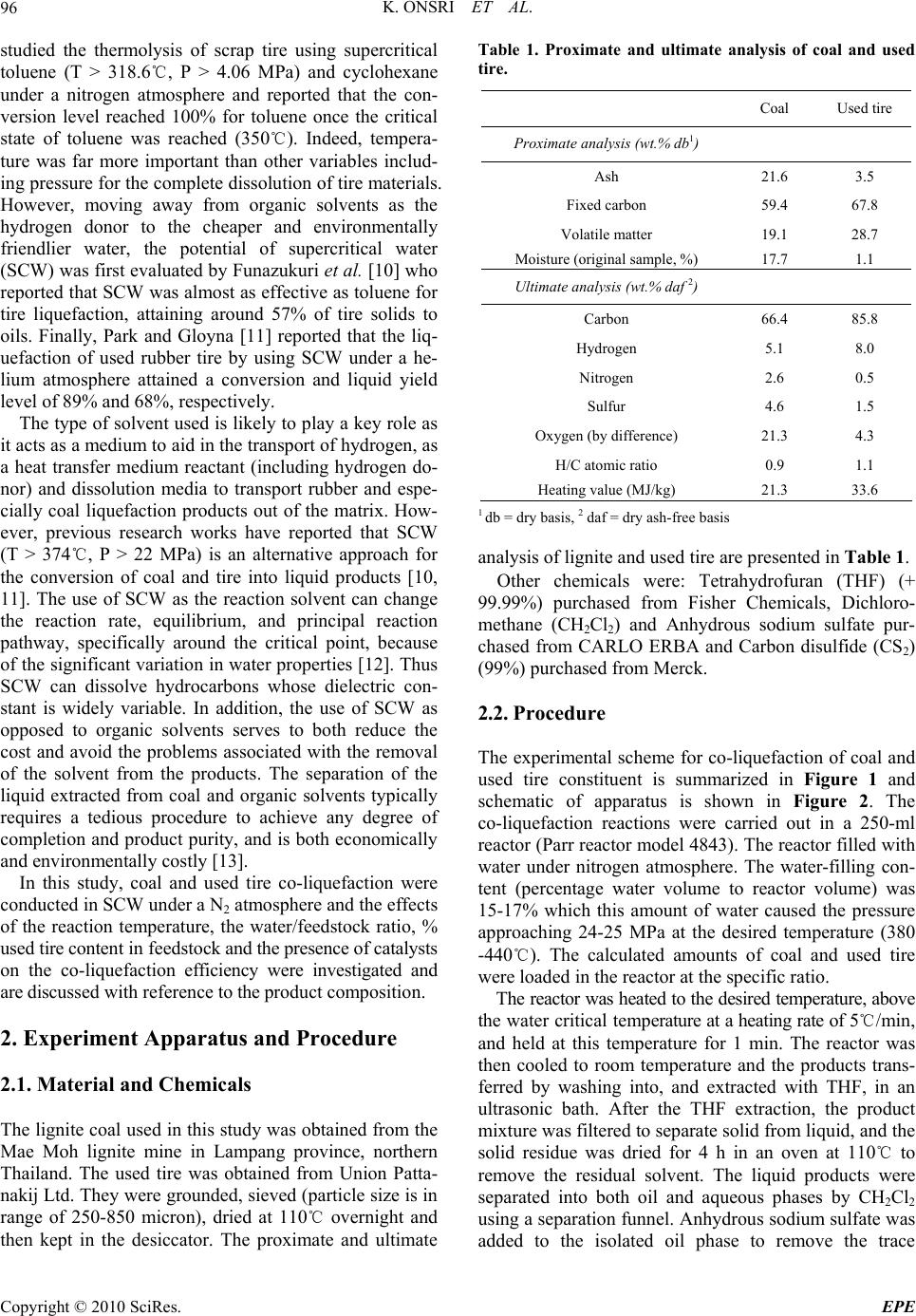 K. ONSRI ET AL. 96 studied the thermolysis of scrap tire using supercritical toluene (T > 318.6℃, P > 4.06 MPa) and cyclohexane under a nitrogen atmosphere and reported that the con- version level reached 100% for toluene once the critical state of toluene was reached (350℃). Indeed, tempera- ture was far more important than other variables includ- ing pressure for the complete dissolution of tire materials. However, moving away from organic solvents as the hydrogen donor to the cheaper and environmentally friendlier water, the potential of supercritical water (SCW) was first evaluated by Funazukuri et al. [10] who reported that SCW was almost as effective as toluene for tire liquefaction, attaining around 57% of tire solids to oils. Finally, Park and Gloyna [11] reported that the liq- uefaction of used rubber tire by using SCW under a he- lium atmosphere attained a conversion and liquid yield level of 89% and 68%, respectively. The type of solvent used is likely to play a key role as it acts as a medium to aid in the transport of hydrogen, as a heat transfer medium reactant (including hydrogen do- nor) and dissolution media to transport rubber and espe- cially coal liquefaction products out of the matrix. How- ever, previous research works have reported that SCW (T > 374℃, P > 22 MPa) is an alternative approach for the conversion of coal and tire into liquid products [10, 11]. The use of SCW as the reaction solvent can change the reaction rate, equilibrium, and principal reaction pathway, specifically around the critical point, because of the significant variation in water properties [12]. Thus SCW can dissolve hydrocarbons whose dielectric con- stant is widely variable. In addition, the use of SCW as opposed to organic solvents serves to both reduce the cost and avoid the problems associated with the removal of the solvent from the products. The separation of the liquid extracted from coal and organic solvents typically requires a tedious procedure to achieve any degree of completion and product purity, and is both economically and environmentally costly [13]. In this study, coal and used tire co-liquefaction were conducted in SCW under a N2 atmosphere and the effects of the reaction temperature, the water/feedstock ratio, % used tire content in feedstock and the presence of catalysts on the co-liquefaction efficiency were investigated and are discussed with reference to the product composition. 2. Experiment Apparatus and Procedure 2.1. Material and Chemicals The lignite coal used in this study was obtained from the Mae Moh lignite mine in Lampang province, northern Thailand. The used tire was obtained from Union Patta- nakij Ltd. They were grounded, sieved (particle size is in range of 250-850 micron), dried at 110℃ overnight and then kept in the desiccator. The proximate and ultimate Table 1. Proximate and ultimate analysis of coal and used tire. Coal Used tire Proximate analysis (wt.% db1) Ash 21.6 3.5 Fixed carbon 59.4 67.8 Volatile matter 19.1 28.7 Moisture (original sample, %) 17.7 1.1 Ultimate analysis (wt.% daf 2) Carbon 66.4 85.8 Hydrogen 5.1 8.0 Nitrogen 2.6 0.5 Sulfur 4.6 1.5 Oxygen (by difference) 21.3 4.3 H/C atomic ratio 0.9 1.1 Heating value (MJ/kg) 21.3 33.6 1 db = dry basis, 2 daf = dry ash-free basis analysis of lignite and used tire are presented in Table 1. Other chemicals were: Tetrahydrofuran (THF) (+ 99.99%) purchased from Fisher Chemicals, Dichloro- methane (CH2Cl2) and Anhydrous sodium sulfate pur- chased from CARLO ERBA and Carbon disulfide (CS2) (99%) purchased from Merck. 2.2. Procedure The experimental scheme for co-liquefaction of coal and used tire constituent is summarized in Figure 1 and schematic of apparatus is shown in Figure 2. The co-liquefaction reactions were carried out in a 250-ml reactor (Parr reactor model 4843). The reactor filled with water under nitrogen atmosphere. The water-filling con- tent (percentage water volume to reactor volume) was 15-17% which this amount of water caused the pressure approaching 24-25 MPa at the desired temperature (380 -440℃). The calculated amounts of coal and used tire were loaded in the reactor at the specific ratio. The reactor was heated to the desired temperature, above the water critical temperature at a heating rate of 5℃/min, and held at this temperature for 1 min. The reactor was then cooled to room temperature and the products trans- ferred by washing into, and extracted with THF, in an ultrasonic bath. After the THF extraction, the product mixture was filtered to separate solid from liquid, and the solid residue was dried for 4 h in an oven at 110℃ to remove the residual solvent. The liquid products were separated into both oil and aqueous phases by CH2Cl2 using a separation funnel. Anhydrous sodium sulfate was added to the isolated oil phase to remove the trace Copyright © 2010 SciRes. EPE  K. ONSRI ET AL. Copyright © 2010 SciRes. EPE 97 amounts of residual water remaining in the oil phase and then evaporated in a vacuum rotary evaporator at 60℃ and 400 mmHg to remove the CH2Cl2 and THF solvent residues. The evaporated liquid (oil) was weighed to calculate the liquid yield. The total conversion, liquid and gas yields and solid residue levels were calculated by the following expressions: The liquid product was analyzed by Simulated Distil- lation Gas Chromatography (SIM/DIS GC, Varian Model CP-3800) according to ASTM D2887. Star Simu- lated Distillation version 5.5 software was used for data collection and processing. The liquid was dissolved in 1% (v/v) CS2 and a 15 m × 0.25 mm Cp-SIL5CB column was used for separation. The oven temperature was raised from 30 to 370℃ at a constant heating rate of 20℃/min. The distillation curve was evaluated to fractions as fol- lows: IBP-200℃, gasoline; 200-250℃, kerosene; 250- 350℃, light gas oil; 350-370℃, gas oil; and 370℃-FBP, long residue. % Conversion = 100[(Wdaf – WRT)/Wdaf] % Liquid yield = 100[Wliq/Wdaf] % Solid residue = 100[WRT/Wdaf] % Gas yield = 100 - % Liquid yield - % Solid residue where: Wdaf = wt. of dry-ash free coal and used tire mixture, WRT = wt. of dry-ash free residue remaining after THF solvent wash and dry, Wliq = wt. of liquid. All experiments were conducted in duplicate. The gaseous product was analyzed by Gas Chromato- graph with a Thermal Conductivity Detector (GC-TCD, Shimadzu GC-2014). The GC-TCD conditions were as follows: 90℃ injection temperature; He carrier gas; Po- rapak Q column and 50℃ column temperature. 2.3. Product Analysis In this study, the raw coal and used tire was analyzed for proximate analysis, total sulfur and determined the heat- ing value by ASTM D2492, D3177 and D2015, respec- tively. The carbon, hydrogen and oxygen were per- formed using a CHN analyzer (Leco CHN-2000). 3. Results and Discussion 3.1. Preliminary Study In the initial stage of study co-liquefaction of coal and used tire, the factorial design was used to analyze the significant process variables. Three variables such as temperature, water/feedstock ratio (wt./wt.) and used tire content in feedstock (wt.%) were chosen. Each of the variables was coded at two levels: -1 and +1 as shown in Table 2 and the average 23 (= 8) experimental results including % conversion and % liquid yield of 2 replica- tions were showed in Table 3. Liquefaction Coal and used tire Water Gas THF extraction Insoluble Residue Soluble CH 2 Cl 2 Oil Water phase SIM/DIS GC G C TCD N 2 Init. Pres. : 1 atm 4/1 10/1 380-440℃ 1-30 min The analysis using the 2-level factorial design method was based on an evaluation of variance ratios. Such comparison helped to determine whether or not signifi- cant difference existed among the means of several groups of observation. It was assumed that each group followed a normal distribution and its trend of the re- sponse between ranges of studied variables is linear. The F-test (95% confidence limits) method was used. The calculated results were showed in Table 4. These results showed that only temperature had effect on % conver- sion, while all three variables (temperature, Water/feed- stock (wt./wt.) ratio and % used tire in feedstock) had effect on % liquid yield. Figure 1. Experimental scheme. However, the effect of reaction time on the product distribution of the co-liquefaction was not studied, be- Table 2. Factorial design of experiments. Factor Level -1 +1 Temperature (℃), A 380 440 Water/feedstock ratio (wt./wt.), B 4/1 10/1 % Used tire content in feedstock (wt.%), C 20 80 Figure 2. Schematic diagram of the apparatus. (1) N2 gas cylinder. Parr reactor model 4843 consisted of; (2) Heater; (3) 250-ml high temperature and pressure reactor; (4) Temperature and pressure controller. (1) (2) (3) (4)  K. ONSRI ET AL. 98 Table 3. Experimental results obtained from factorial design for co-liquefaction experiments. Factorial Factor % Conversion % Liquid Design* Temperature Water/feedstock % Used tire content yield (℃), A Ratio (wt./wt.), B in feedstock (wt.%), C (1) 380 4/1 20 53.9 15.0 a 440 4/1 20 62.6 24.9 b 380 10/1 20 56.3 25.5 ab 440 10/1 20 63.4 28.6 c 380 4/1 80 53.2 26.6 ac 440 4/1 80 62.4 36.0 bc 380 10/1 80 60.0 40.2 abc 440 10/1 80 68.2 41.6 * Reaction conditions are denoted according to statistical nomenclature; e.g. ab represents high levels of factors A and B and low levels of C and D (see Table 2) Table 4. Analysis of variance for co-liquefaction experi- ments. (a) for % conversion; (b) for % liquid yield. Source of Sum of Degrees of Mean F0 Variation Square Freedom Square A 137.74 1 137.74 37.21* B 30.69 1 30.69 8.29 AB** 0.90 1 0.90 0.24 C 7.29 1 7.29 1.97 Error 11.10 3 3.70 Total 187.73 7 (a) Source of Sum of Degrees of Mean F0 Variation Square Freedom Square A 70.92 1 70.92 9.13* B 138.83 1 138.83 17.87* C 315.63 1 315.63 40.63* Error 31.07 4 7.77 Total 556.45 7 (b) * Significant in F-test with 95% confidence limits, F0.05,1,3 = 10.13 (F0 > F0.05,1,3) * Significant in F-test with 95% confidence limits, F0.05,1,4 = 7.71 (F0 > F0.05,1,4) ** Two-factor interaction effects of factors A and B (see Table 2) cause the results from initial experiments of liquefaction of the used tire, as shown in Figure 3, showed that the reaction time (elapsed time after the temperature reached the desired temperature) did not affect the product dis- tribution. Moreover, the previous studies [14-16] re- ported that the coal and some polymers including rubber 67.4 67.5 67 .6 67.6 54.1 53.653.1 53.0 32.6 32.5 32.4 32. 4 14.5 14.5 14. 0 13.3 0 20 40 60 80 100 01020 time, (min) % wt (daf) 30 % conversion% liquid% solid % gas Time Figure 3. The effect of temperature on the distribution of products for used tire liquefaction. (Temperature 420℃; water/feedstock ratio of 10/1). and plastics can be decomposed and give the highest oil yield within 1 min. Thus, we used the reaction time of 1 min for all of co-liquefaction experiments. 3.2. The Effect of Temperature Thermal cracking is the principal reaction in SCW and the effect of temperature on thermal cracking is impor- tant. Thermogravimetric analysis curves revealed the weight loss of used tire when pyrolyzed at a constant heating rate of 10℃/min within a temperature range of 25-900℃ under a nitrogen atmosphere (Figure 4). The decomposition of used tire begins in earnest near 380℃ and is essentially completely decomposed at 480℃. Therefore, the chosen temperature range for all subse- quent co-liquefaction experiments was 380-440℃. Fig- ure 5 summarizes the variation in product yield with reaction temperature at a reaction time of 1 min, a wa- ter/feed stock ratio of 10/1 and a substrate composition C opyright © 2010 SciRes. EPE 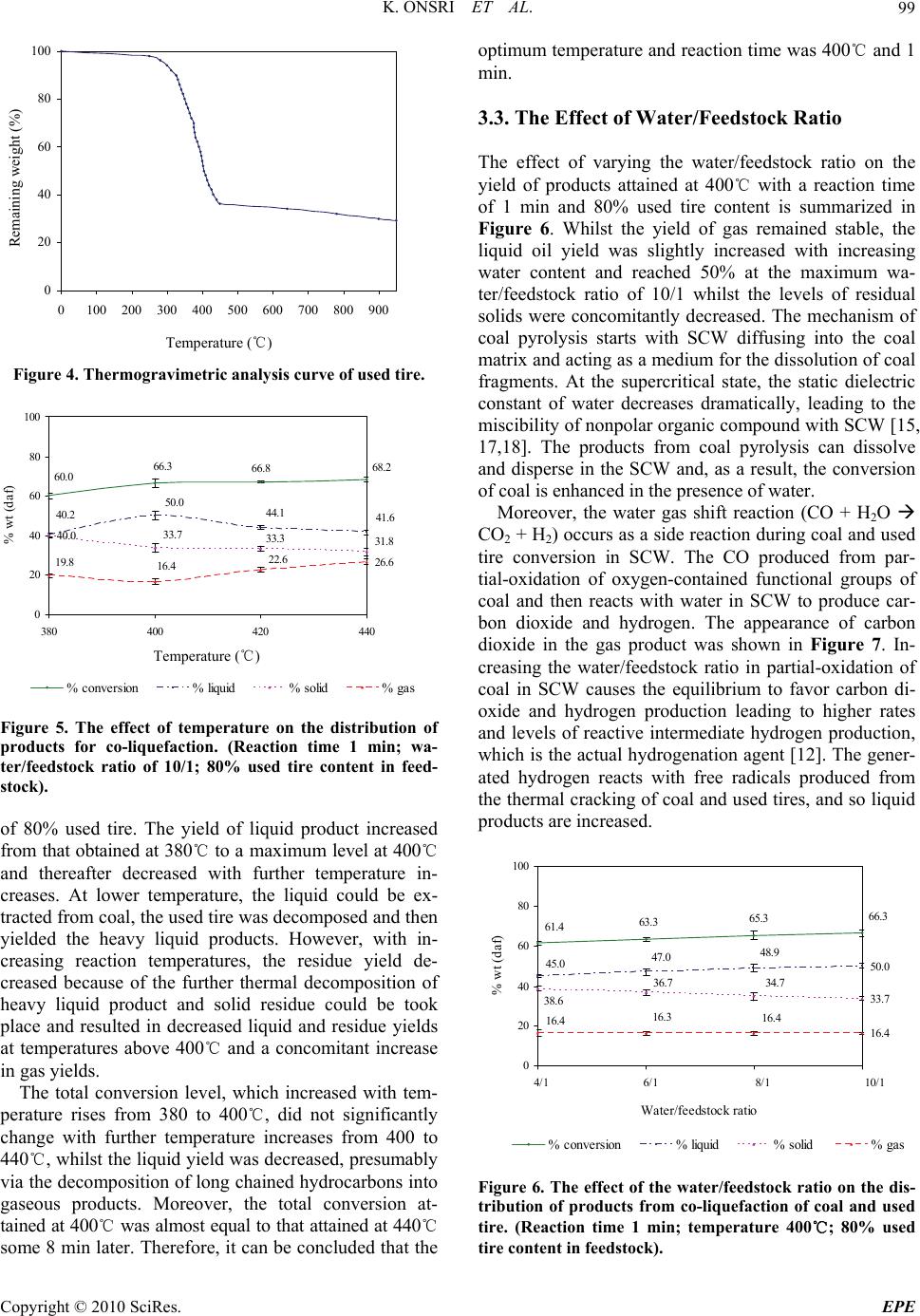 K. ONSRI ET AL.99 0 20 40 60 80 100 0100 200 300 400 500 600 700 800 900 Temperature (℃) Figure 4. Thermogravimetric analysis curve of used tire. 40.0 26.6 68.2 66.8 66.3 60.0 41.6 44.1 50.0 40.2 33.7 33.3 31.8 22.6 16.4 19.8 0 20 40 60 80 100 380 400420 440 temperature, (oC) % wt (daf) % conversion% liquid% solid% gas Figure 5. The effect of temperature on the distribution of products for co-liquefaction. (Reaction time 1 min; wa- ter/feedstock ratio of 10/1; 80% used tire content in feed- stock). of 80% used tire. The yield of liquid product increased from that obtained at 380℃ to a maximum level at 400℃ and thereafter decreased with further temperature in- creases. At lower temperature, the liquid could be ex- tracted from coal, the used tire was decomposed and then yielded the heavy liquid products. However, with in- creasing reaction temperatures, the residue yield de- creased because of the further thermal decomposition of heavy liquid product and solid residue could be took place and resulted in decreased liquid and residue yields at temperatures above 400℃ and a concomitant increase in gas yields. The total conversion level, which increased with tem- perature rises from 380 to 400℃, did not significantly change with further temperature increases from 400 to 440℃, whilst the liquid yield was decreased, presumably via the decomposition of long chained hydrocarbons into gaseous products. Moreover, the total conversion at- tained at 400℃ was almost equal to that attained at 440℃ some 8 min later. Therefore, it can be concluded that the optimum temperature and reaction time was 400℃ and 1 min. 3.3. The Effect of Water/Feedstock Ratio Remaining weigh t (%) The effect of varying the water/feedstock ratio on the yield of products attained at 400℃ with a reaction time of 1 min and 80% used tire content is summarized in Figure 6. Whilst the yield of gas remained stable, the liquid oil yield was slightly increased with increasing water content and reached 50% at the maximum wa- ter/feedstock ratio of 10/1 whilst the levels of residual solids were concomitantly decreased. The mechanism of coal pyrolysis starts with SCW diffusing into the coal matrix and acting as a medium for the dissolution of coal fragments. At the supercritical state, the static dielectric constant of water decreases dramatically, leading to the miscibility of nonpolar organic compound with SCW [15, 17,18]. The products from coal pyrolysis can dissolve and disperse in the SCW and, as a result, the conversion of coal is enhanced in the presence of water. Moreover, the water gas shift reaction (CO + H2O CO2 + H2) occurs as a side reaction during coal and used tire conversion in SCW. The CO produced from par- tial-oxidation of oxygen-contained functional groups of coal and then reacts with water in SCW to produce car- bon dioxide and hydrogen. The appearance of carbon dioxide in the gas product was shown in Figure 7. In- creasing the water/feedstock ratio in partial-oxidation of coal in SCW causes the equilibrium to favor carbon di- oxide and hydrogen production leading to higher rates and levels of reactive intermediate hydrogen production, which is the actual hydrogenation agent [12]. The gener- ated hydrogen reacts with free radicals produced from the thermal cracking of coal and used tires, and so liquid products are increased. Temperature (℃) 50.0 33.7 16.4 66.3 65.3 63.3 61.4 45.0 47.0 48.9 34.736.7 38.6 16.4 16.3 16.4 0 20 40 60 80 100 1234 Water/feedstock ratio % wt (daf) % conversion% liquid% solid % gas 4/1 6/1 8/1 10/1 Figure 6. The effect of the water/feedstock ratio on the dis- tribution of products from co-liquefaction of coal and used tire. (Reaction time 1 min; temperature 400℃; 80% used tire content in feedstock). Copyright © 2010 SciRes. EPE 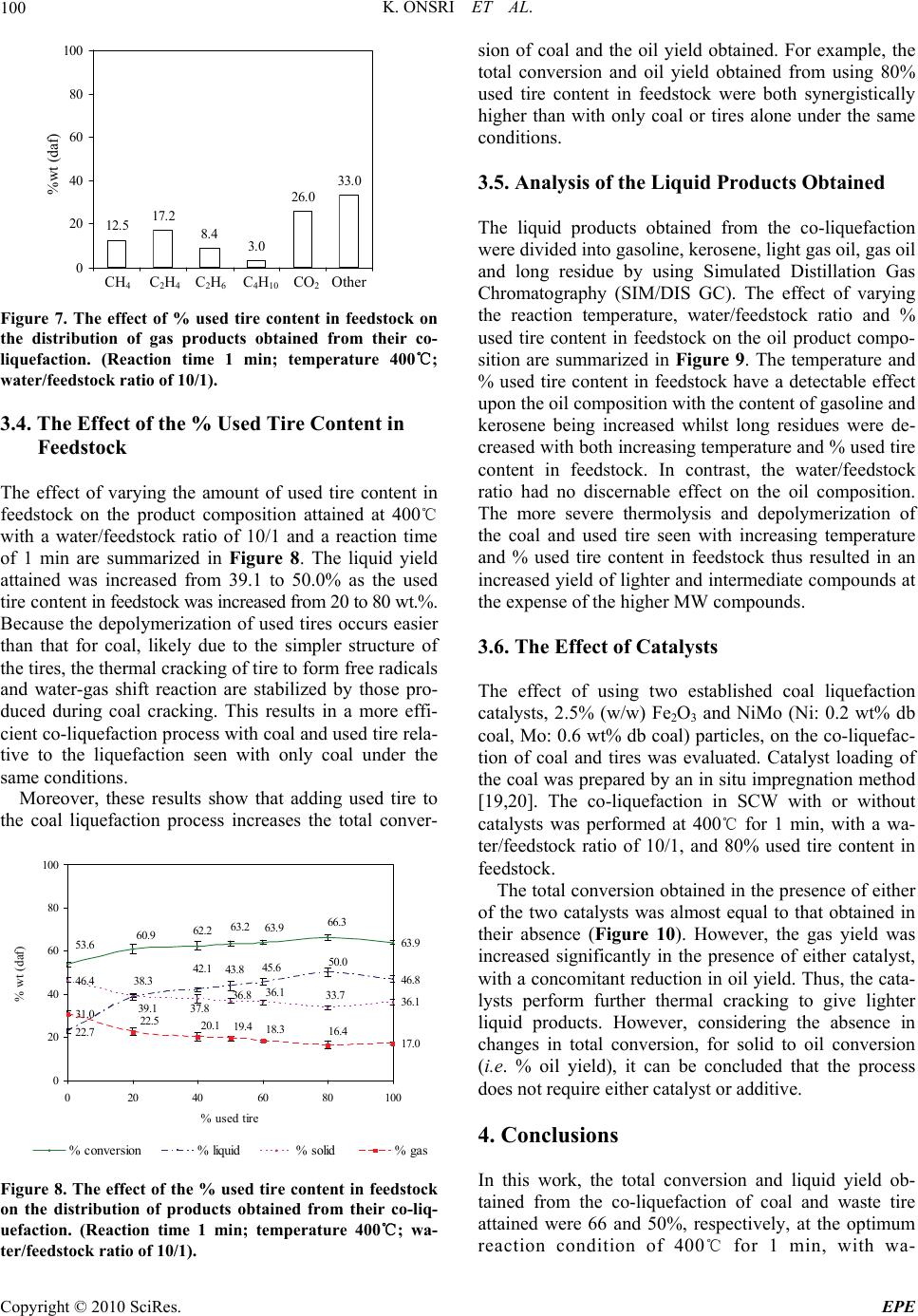 K. ONSRI ET AL. Copyright © 2010 SciRes. EPE 100 12.5 17.2 8.4 3.0 26. 0 33.0 0 20 40 60 80 100 CH4C2H4 C3H6C4H10 CO2Other sion of coal and the oil yield obtained. For example, the total conversion and oil yield obtained from using 80% used tire content in feedstock were both synergistically higher than with only coal or tires alone under the same conditions. %wt (daf) 3.5. Analysis of the Liquid Products Obtained The liquid products obtained from the co-liquefaction were divided into gasoline, kerosene, light gas oil, gas oil and long residue by using Simulated Distillation Gas Chromatography (SIM/DIS GC). The effect of varying the reaction temperature, water/feedstock ratio and % used tire content in feedstock on the oil product compo- sition are summarized in Figure 9. The temperature and % used tire content in feedstock have a detectable effect upon the oil composition with the content of gasoline and kerosene being increased whilst long residues were de- creased with both increasing temperature and % used tire content in feedstock. In contrast, the water/feedstock ratio had no discernable effect on the oil composition. The more severe thermolysis and depolymerization of the coal and used tire seen with increasing temperature and % used tire content in feedstock thus resulted in an increased yield of lighter and intermediate compounds at the expense of the higher MW compounds. CH4 C2H4 C 2H6 C 4H10 CO 2 Other Figure 7. The effect of % used tire content in feedstock on the distribution of gas products obtained from their co- liquefaction. (Reaction time 1 min; temperature 400℃; water/feedstock ratio of 10/1). 3.4. The Effect of the % Used Tire Content in Feedstock The effect of varying the amount of used tire content in feedstock on the product composition attained at 400℃ with a water/feedstock ratio of 10/1 and a reaction time of 1 min are summarized in Figure 8. The liquid yield attained was increased from 39.1 to 50.0% as the used tire content in feedstock was increased from 20 to 80 wt.%. Because the depolymerization of used tires occurs easier than that for coal, likely due to the simpler structure of the tires, the thermal cracking of tire to form free radicals and water-gas shift reaction are stabilized by those pro- duced during coal cracking. This results in a more effi- cient co-liquefaction process with coal and used tire rela- tive to the liquefaction seen with only coal under the same conditions. 3.6. The Effect of Catalysts Moreover, these results show that adding used tire to the coal liquefaction process increases the total conver- 63.9 22.7 46.8 36.1 31.0 17.0 66.3 63.9 63.2 62.2 60.9 53.6 38.3 42.1 43.8 45.6 50.0 33.7 36.1 36.8 37.8 46.4 39.1 22.5 20.1 19.4 18.3 16. 4 0 20 40 60 80 100 0 20406080100 % used tire % wt (daf) % conversion% liquid% solid % gas The effect of using two established coal liquefaction catalysts, 2.5% (w/w) Fe2O3 and NiMo (Ni: 0.2 wt% db coal, Mo: 0.6 wt% db coal) particles, on the co-liquefac- tion of coal and tires was evaluated. Catalyst loading of the coal was prepared by an in situ impregnation method [19,20]. The co-liquefaction in SCW with or without catalysts was performed at 400℃ for 1 min, with a wa- ter/feedstock ratio of 10/1, and 80% used tire content in feedstock. The total conversion obtained in the presence of either of the two catalysts was almost equal to that obtained in their absence (Figure 10). However, the gas yield was increased significantly in the presence of either catalyst, with a concomitant reduction in oil yield. Thus, the cata- lysts perform further thermal cracking to give lighter liquid products. However, considering the absence in changes in total conversion, for solid to oil conversion (i.e. % oil yield), it can be concluded that the process does not require either catalyst or additive. 4. Conclusions In this work, the total conversion and liquid yield ob- tained from the co-liquefaction of coal and waste tire attained were 66 and 50%, respectively, at the optimum reaction condition of 400℃ for 1 min, with wa- Figure 8. The effect of the % used tire content in feedstock on the distribution of products obtained from their co-liq- uefaction. (Reaction time 1 min; temperature 400℃; wa- ter/feedstock ratio of 10/1). 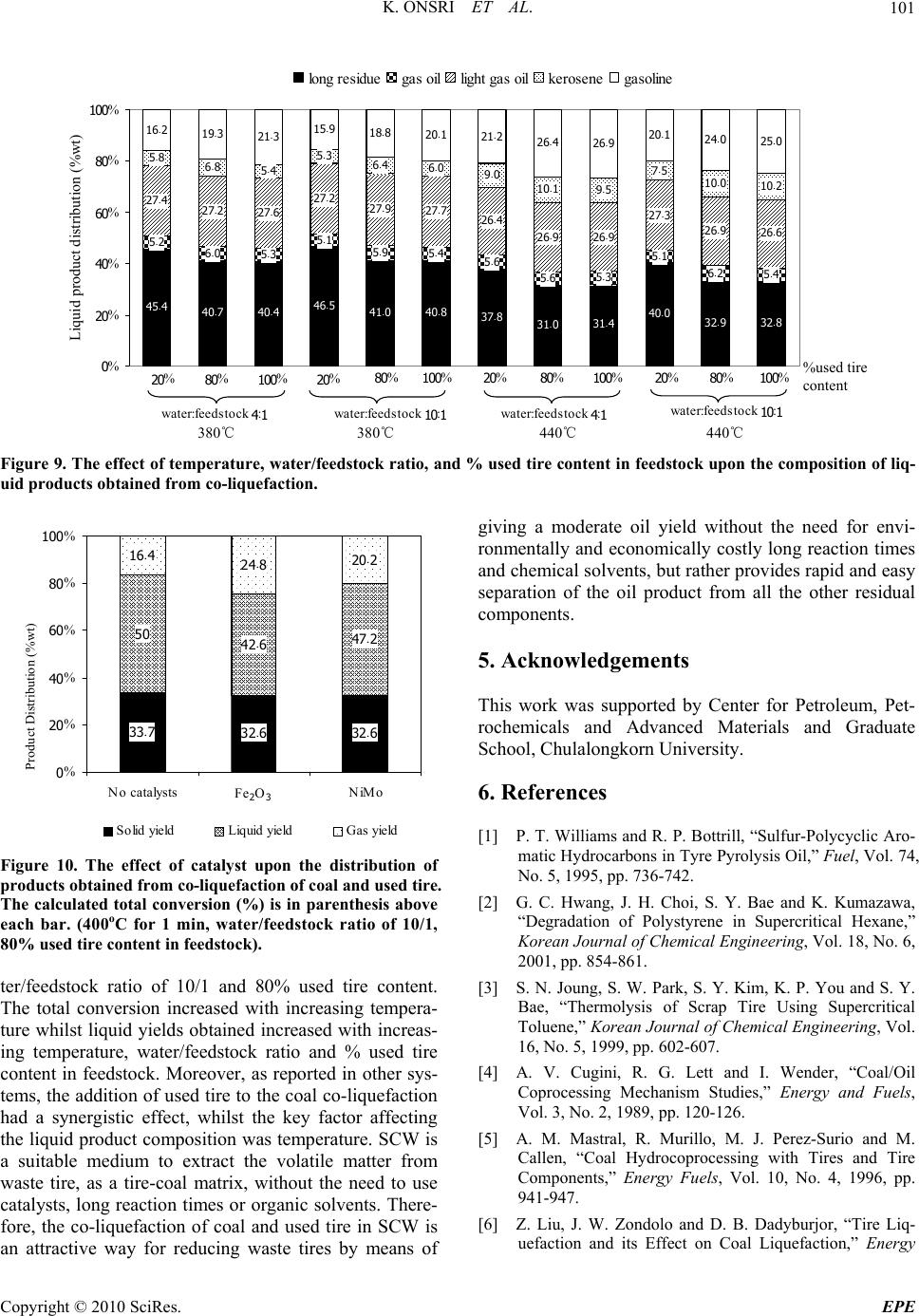 K. ONSRI ET AL. 101 45.440.740 .446.541.040 .837.831.031.4 40.0 32.932 .8 5.2 6.05.3 5.1 5.95.45.6 5.65.3 5.1 6.25.4 27.4 27.227.6 27.2 27.927.7 26.4 26.926.9 27.3 26.926.6 5.8 5.4 5.3 6.46.09.0 10.19.5 7.5 10.010.2 16.219.321.315.918.820.121.226.426.920.124.025.0 6.8 0% 20% 40% 60% 80% 100% long residuegas oillight gas oilkerosene gasoline water:feedstock 4:1water:feedstock 10:1water:feedstock 4:1water:feedstock 10:1 20%80%20%80% 100%100%20%80%20%80% 100%100% Liquid product distribution (%wt) %used tire content 380℃ 380℃ 440℃ 440℃ Figure 9. The effect of temperature, water/feedstock ratio, and % used tire content in feedstock upon the composition of liq- uid products obtained from co-liquefaction. 33.732.632.6 50 42.647.2 16.424.820.2 0% 20 % 40 % 60 % 80 % 100% No catalystsFe2O3NiMo Product Distribution (%w t Solid yieldLiquid yieldGas yield Fe2O3 Product Distribution (%wt) Figure 10. The effect of catalyst upon the distribution of products obtained from co-liquefaction of coal and used tire. The calculated total conversion (%) is in parenthesis above each bar. (400oC for 1 min, water/feedstock ratio of 10/1, 80% used tire content in feedstock). ter/feedstock ratio of 10/1 and 80% used tire content. The total conversion increased with increasing tempera- ture whilst liquid yields obtained increased with increas- ing temperature, water/feedstock ratio and % used tire content in feedstock. Moreover, as reported in other sys- tems, the addition of used tire to the coal co-liquefaction had a synergistic effect, whilst the key factor affecting the liquid product composition was temperature. SCW is a suitable medium to extract the volatile matter from waste tire, as a tire-coal matrix, without the need to use catalysts, long reaction times or organic solvents. There- fore, the co-liquefaction of coal and used tire in SCW is an attractive way for reducing waste tires by means of giving a moderate oil yield without the need for envi- ronmentally and economically costly long reaction times and chemical solvents, but rather provides rapid and easy separation of the oil product from all the other residual components. 5. Acknowledgements This work was supported by Center for Petroleum, Pet- rochemicals and Advanced Materials and Graduate School, Chulalongkorn University. 6 . References [1] P. T. Williams and R. P. Bottrill, “Sulfur-Polycyclic Aro- matic Hydrocarbons in Tyre Pyrolysis Oil,” Fuel, Vol. 74, No. 5, 1995, pp. 736-742. [2] G. C. Hwang, J. H. Choi, S. Y. Bae and K. Kumazawa, “Degradation of Polystyrene in Supercritical Hexane,” Korean Journal of Chemical Engineering, Vol. 18, No. 6, 2001, pp. 854-861. [3] S. N. Joung, S. W. Park, S. Y. Kim, K. P. You and S. Y. Bae, “Thermolysis of Scrap Tire Using Supercritical Toluene,” Korean Journal of Chemical Engineering, Vol. 16, No. 5, 1999, pp. 602-607. [4] A. V. Cugini, R. G. Lett and I. Wender, “Coal/Oil Coprocessing Mechanism Studies,” Energy and Fuels, Vol. 3, No. 2, 1989, pp. 120-126. [5] A. M. Mastral, R. Murillo, M. J. Perez-Surio and M. Callen, “Coal Hydrocoprocessing with Tires and Tire Components,” Energy Fuels, Vol. 10, No. 4, 1996, pp. 941-947. [6] Z. Liu, J. W. Zondolo and D. B. Dadyburjor, “Tire Liq- uefaction and its Effect on Coal Liquefaction,” Energy C opyright © 2010 SciRes. EPE  K. ONSRI ET AL. 102 Fuels, Vol. 8, No. 3, 1994, pp. 607-612. [7] A. M. Mastral, R. Murillo, M. S. Callen and T. Garcia, “Evidence of Coal and Tire Interactions in Coal-Tire Coprocessing for Short Residence Times,” Fuel Process- ing Technology, Vol. 69, 2000, pp. 127-140. [8] M. Sugano, D. Onda and K. Mashimo, “Additive Effect of Waste Tire on the Hydrogenolysis Reaction of Coal Liquefaction Residue,” Energy Fuels, Vol. 20, No. 6, 2006, pp. 2713-2716. [9] Y. Tang and C. W. Curtis, “Thermal and Catalytic Coprocessing of Waste Tires with Coal,” Fuel Processing Technology, Vol. 46, No. 3, 1995, pp. 195-215. [10] T. Funazukuri, T. Takanashi and N. Wakoa, “Supercriti- cal Extraction of Used Automotive Tire with Water,” Journal of Chemical Engineering of Japan, Vol. 20, 1987, pp. 23-27. [11] S. Park and E. F. Gloyna, “Statistical Study of the Lique- faction of Used Rubber Tyre in Supercritical Water,” Fuel, Vol. 76, No. 11, 1997, pp. 999-1003. [12] N. Akiya and P. E. Savage, “Roles of Water for Chemical Reactions in High-Temperature Water,” Chemical Re- views, Vol. 102, No. 8, 2002, pp. 2725-2750. [13] S. Sangon, S. Ratanavaraha, S. Ngamprasertsith and P. Prasassarakich, “Coal Liquefaction Using Supercritical Toluene-Tetralin Mixture in a Semi-Continuous Reac- tor,” Fuel Processing Technology, Vol. 87, No. 3, 2006, pp. 201-207. [14] X. Su, Y. Zhao, R. Zhang and J. Bi, “Investigation on Degradation of Polyethylene to Oils in Supercritical Wa- ter,” Fuel Processing Technology, Vol. 85, No. 8-10, 2004, pp. 1249-1258. [15] L. Cheng, R. Zhang and J. Bi, “Pyrolysis of a Low-Rank Coal in Sub- and Supercritical Water,” Fuel Processing Technology, Vol. 85, No. 8-10, 2004, pp. 921-932. [16] S. Sunphorka, P. Prasassarakich and S. Ngamprasertsith, “Co-liquefaction of Coal and Plastic Mixture in Super- critical Water,” Journal of Scientific Research Chu- lalongkorn University, Vol. 32, No. 2, 2007, pp. 101-109. [17] H. Hu, S. Guo and K. Hedden, “Xtraction of Lignin with Water in Sub- and Supercritical States,” Fuel Processing Technology, Vol. 53, 1997, pp. 267-277. [18] M. Watanabe, H. Hirakoso, S. Sawamoto, T. Adschiri and K. Arai, “Poly Ethylene Conversion in Supercritical Wa- ter,” Journal of Supercritical Fluids, Vol. 13, No. 1-3, 1998, pp. 247-252. [19] Y. Artanto, W. R. Jackson, P. J. Redlich and M. Marshall, “Liquefaction Studies of Some Indonesian Low Rank Coals,” Fuel, Vol. 79, No. 11, 2000, pp. 1333-1340. [20] Z. Liu, J. Yang, J. W. Zondlo, A. H. Stiller and D. B. Dadybujor, “In situ Impregnated Iron-Based Catalysts for Direct Coal Liquefaction,” Fuel, Vol. 75, No. 1, 1996, pp. 51-57. Copyright © 2010 SciRes. EPE |

