Health
Vol. 4 No. 9 (2012) , Article ID: 23079 , 4 pages DOI:10.4236/health.2012.49104
High pregnancy and implantation rates can be obtained with preincubation of oocytes before insemination in IVF and ICSI procedures
![]()
1Laboratory of Assisted Reproduction, Alcival Hospital, Guayaquil, Ecuador; *Corresponding Author: jgarciaf@fertilab.pe
2FERTILAB Laboratory of Assisted Reproduction, Lima, Perú
Received 18 January 2012; revised 17 February 2012; accepted 1 March 2012
Keywords: Oocytes; Embryo Development; Maturation; Blastocyst; ART
ABSTRACT
Purpose: Evaluate the effect of preincubation of oocytes prior to IVF or ICSI cycles with embryo transfer at blastocyst stage. Methods: Retrospective non randomized study based on secondary analysis of data. Setting: Laboratory of Assisted Reproduction at the Alcivar Hospital. Patients: One hundred-eighteen cycles of IVF and ICSI were analyzed in the present study. The evaluated groups were formed for those patients whose oocytes, after retrieval, were inseminated at 1 - 3 h (Group I) or 4 - 6 h (Group II). Results: There was no difference in fertilization rate (83.6% and 78.1%), Day 3 cleavage rate (95.1% and 97.1%), and blastocyst formation (31.1% and 39.1%) for groups I and II respectively. Clinical pregnancy rates (PR: 53.0% vs 22.9%) and implantation rates (IR: 38.1% vs 13.0%) were significantly higher in group II versus group I, respectively (P < 0.05). Conclusions: Preincubation of oocytes before insemination is a factor which raises the PR and IR after the blastocyst transfer.
1. INTRODUCTION
The science of In Vitro Fertilization (IVF) has improved considerably in the last 25 years since the first “test-tube” baby was born. A greater understanding of the nutritional and environmental requirements of oocytes and embryos led to advances in culture conditions that have improved IVF pregnancy rates.
Oocyte quality impacts early embryonic survival, the establishment and maintenance of pregnancy, fetal development, and even adult disease. The oocytes are considered to be meiotically mature after extrusion of the first polar body, which is characteristic of metaphase II (MII). However, nuclear and cytoplasmic maturation were reached independently during oocyte maturation [1].
Quality, or developmental competence, is acquired during folliculogenesis as the oocyte grows and during the period of oocyte maturation. As an oocyte grows and matures, it acquires the abilities to resume and complete meiosis, successfully undergo the fertilization process, and initiate and sustain embryonic development [2]. In the course of acquiring these competences, cytoplasmic changes occur, which may include such cellular processes as mRNA transcription [3-5], protein translation [6], ultrastructural changes [7,8] and post-translational modification of proteins [9], all involved in meiotic progression and cell cycle control [10], and other proteins involved in cellular processes critical for developmental success before and after activation of the zygote genome [11,12].
Trounson et al. [13] postulated that preincubation of cumulus-corona-oocyte complexes (CCOC) before conventional insemination improves the outcome of IVF. They proposed that a period of culture in vitro is beneficial for the completion of oocyte maturation and to obtain high rates of fertilization and embryo development in vitro.
Several studies evaluating the effect of preincubation period of oocytes in IVF [14] and ICSI procedures [14-16] showed that the fertilization rate significantly increased with a minimal of 3 h of preincubation before insemination. Cleavage rates and embryo quality were not affected by the preincubation period. With regard to clinical pregnancy rate, only Falcone et al. [14] observed a significant increase from 2 to 5 h of preincubation with embryo transfer at 48 - 72 h post insemination [13-16].
Physiologically, the uterus provides a nutritional environment different from than in the fallopian tubes. Therefore, embryo transfer in the cleavage stage would cause homeostatic stress of the embryo and reduction in its implantatory potential [17]. Consequently, the transfer in blastocysts stage would allow a better synchronization with rhythm of uterine contractions and the embryo [18,19], and enhance the likelihood of pregnancy [20-22]. Some studies including >100 selected patients have reported high pregnancy rates after single blastocysts transfer on Day 5 [23-25].
We hypothesize that a preincubation period of oocytes after their retrieval from IVF and ICSI procedures will affect embryo quality and clinical outcomes when the embryo transfers are at blastocysts stage. Thus, the objective of this study was to report clinical outcomes obtained with two preincubation periods of oocytes (≤3 h or ≥4 h) before the insemination procedure with blastocyst stage transfer.
2. MATERIALS AND METHODS
2.1. Patients
This is a retrospective non randomized study based on secondary analysis of data obtained from 118 cycles of IVF and ICSI at the Laboratory of Assisted Reproduction of Alcivar Hospital (Guayaquil, Ecuador) during the period January 2009 to December 2010. This study was approved by the Institutional Review Board (IRB) and the corresponding Ethics Committee at the Alcival Hospital (Guayaquil, Ecuador).
The evaluated groups were formed for those patients whose oocytes after retrieval were inseminated at 1 - 3 h (Group I) or 4 - 6 h (Group II). The age range of the patients was 26 - 41 year-old in both groups. All embryo transfers were made at blastocyst stage.
2.2. Controlled Ovarian Stimulation and Oocyte Collection
The menstrual cycles of patients were stimulated using recombinant FSH (rFSH) (Puregon®, Organ-on Laboratories, Ecuador), HMG (Merional®, IBSA Laboratories, Ecuador) and GnRH antagonist (Orgalutran® Organon, Laboratories, Ecuador) according to the stimulation protocols previously established and starting on Day 2 of the menstrual cycle until at the least three follicles reached ~18 mm in diameter. The oocyte retrieval was performed by vaginal ultrasound 36 h after i.m. application of hCG (Pregnyl® 10,000 UI, Organon Laboratories, Ecuador). For the procedure, the patient was under general anesthesia with 200 mg of Propofol iv (Diprivan® 1% P/V; AstraZeneca laboratories, UK).
During follicular aspiration procedure the oocytes were recovered in Global®-HEPES-buffered medium (IVFonline, Canada) supplemented with 10% vol/vol Serum Substitute Supplement (SSS; Irvine Scientific, USA). After retrieval with the help of two sterile needles, cumulus-oocyte complexes were trimmed of excess cumulus cells to prevent accumulation of debris and red blood cells and then maintained in ~200 µl drops of Global®-Fertilization medium (IVFonline, Canada) plus 10% SSS under oil at 37˚C and an atmosphere containing 5.8% CO2 in air until the recovered oocytes were inseminated or injected. None of the oocytes were damaged by cleaning procedure.
2.3. Preparation of Spermatozoa
The semen samples were collected by masturbation from the recipients’ partners. Motile spermatozoa were separated from the seminal plasma by centrifugation through 1.0 ml 95% and 45% Isolate gradients (Irvine Scientific, USA). For oligospermic samples the sperm were washed and resuspended in varying amounts of sperm washing medium depending on initial concentration and motility and then placed into 10 µl drops of HEPES-buffered Global medium + 10% SSS for ICSI.
2.4. Insemination, Fertilization and Embryo Culture
All oocytes were inseminated or injected, depending about seminal characteristics, with sperma-tozoa from recipient’s husband. After insemination procedure (day 0), all oocytes were cultures at 37˚C in an atmosphere of 5.8% CO2 in air.
In both groups, the fertilization was evaluated 16 - 18 h after injection by presence of two pronuclei (day 1). The zygotes were individually cultured under mineral oil, in 10 µl droplets of Global® medium (IVFonline, Canada) supplemented with 10% vol/vol SSS from day 1 to day 3. On day 3, the embryos were moved to fresh 10 µl droplets of Global® medium + 10% SSS.
2.5. Embryo Quality
On day 3 the embryos were evaluated for cell number, fragmentation, and multinucleation. Good quality day 3 embryos were defined as those with 6 - 8 cell and ≤10% of fragmentation. Good quality blastocysts were defined as having an inner cell mass (ICM) and trophoectoderm type A or B [26]. The ICM score was evaluated as follow: type A = compact area, many cells present; type B = cells are loosely grouped. The trophoectoderm was scored as follows: type A = many cells forming a tight epithelial network of cells; type B = few cells forming a loose network of cells.
2.6. Blastocyst Transfer
Blastocysts were transferred on day 5 in all recipients. There were sixty nine and one hundred seventy one blastocyst transfers in groups I and II respectively. In group I, the rate of transfer was 1.97 ± 0.29 blastocyst/patient (Mean ± SD) and; in group II, 2.06 ± 0.29 blastocyst/patient (Mean ± SD). The blastocysts that were not transferred were cryopreserved or discarded according to their morphology. The blastocyst transfer was performed with a Labotech Embryo Transfer Catheter Set® (Labotect, Germany) that had been previously washed with culture medium. The catheter was completely filled with culture medium and the blastocysts filled in the last 10 µl of the catheter. All transfers were performed according to the methods previously described by Mansour [27].
2.7. Pregnancy Assessment
The biochemical pregnancy was assessed 12 days after the blastocyst transfer by measuring the Human Chorionic Gonadotropin beta subunit (hCG-b) in blood. The clinical pregnancy was determined by transvaginal ultrasonography to detect gestational sacs and fetal heartbeats at approximately 21 and 28 days after transfer, respectively.
2.8. Statistical Analysis
Data were statistically analyzed using the χ2 test and Student’s t-test as appropriate and differences were considered to be significant at P < 0.05. All statistical analysis was carried out using the statistic package Stata 10 (StataCorp, College Station, TX).
The normal fertilization was calculated from the number of zygotes with two pronuclei divided by the number of mature oocytes inseminated times 100. Clinical pregnancy rate per transfer was calculated from the number of patients with blastocyst transfer with at least one gestational sac divided by the total blastocyst transfers times 100. Implantation rate was calculated by dividing the number of gestational sacs (observed by ultrasound at the 21st day post transfer) by the total number of blastocysts transferred times 100. Miscarriages were defined as the number of pregnancies with total loss of the gestational sacs before the 20 weeks of gestation over the number of pregnancies times 100.
3. RESULTS
The ages of patients were similar in both evaluated groups (34.31 ± 3.59 vs 33.39 ± 3.78 years). Laboratory results obtained from group I (1 - 3 h) and control group (4 - 6 h) are shown in Table 1. A total of 314 and 950 oocytes were collected from patients of group I and group II respectively. Two hundred seventy nine oocytes from group I and six hundred ninety six oocytes from group II were inseminated. There was no difference in the number of inseminated (IVF) or injected (ICSI) oocytes in both group evaluated (data no shown). The normal fertilization (2PN) was similar in both evaluated groups (Group I: 83.6% vs Group II: 78.1%; P:NS). In group I, the cleavage rate by embryo at Day 3 was 95.1%, whereas in group II it was 97.1% (P:NS). Embryos from group I had mean cell numbers at Day 3 significantly higher compared to those embryos from group II (P < 0.05). Embryo quality derived from oocytes that were inseminated 1 - 3 h was similar to those that were inseminated at 4 - 6 h (76.0% vs 80.1%). Blastocyst development rates were similar in both study groups (31.1% vs 39.1%). In addition, embryos reaching the blastocyst stage were morphologically similar in both groups.
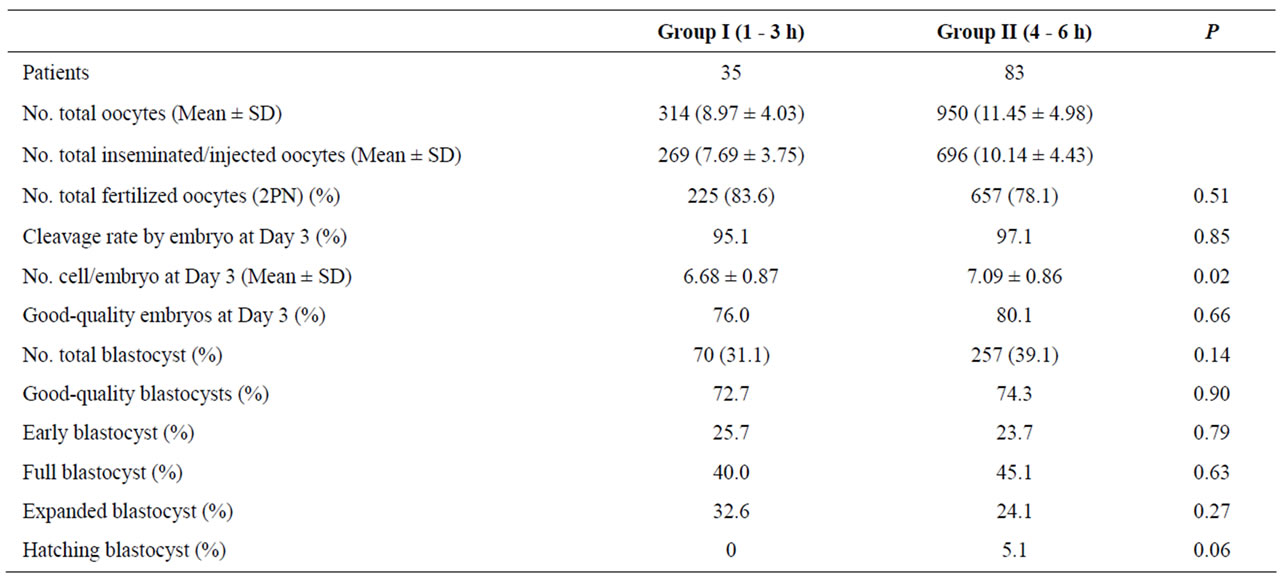
Table 1. Comparison of laboratory results between both evaluated groups.
Clinical outcomes are shown in Table 2. The mean value for blastocysts was significantly lower for the patients of group I than those for the patients of group II (2.41 ± 1.78 vs 3.37 ± 2.03; P < 0.05). In group I, a total of sixty nine blastocysts were transferred to 35 patients with a mean value of 1.97 blastocysts. In group II, a total of one hundred seventy one blastocysts were transferred to 35 patients with a mean value of 2.06 blastocysts. The mean quality values of blastocysts transferred was similar in both analyzed groups (P:NS).
The patients of group I compared with group II had significantly higher clinical PR (22.9% vs 53.0%; P < 0.05) and IR (13% vs 38.1%; P < 0.05) respectively. Miscarriage and biochemical pregnancy rates were similar in both groups (P:NS). For group I, one and two gestational sacs were observed in seven (87.5%) and one (12.5%) patients respectively. For group II, one and two gestational sacs were observed in twenty eight (63.6%) and sixteen (36.4%) patients respectively. These percentages were similar in both evaluated groups (P:NS).
4. DISCUSSION
Complete nuclear and cytoplasmic maturation of oocytes is essential for the activation of oocytes at fertilization and the development of embryos [28]. An oocyte is considered to reach nuclear maturity when its meiosis is arrested again at MII with the presence of an extruded first polar body. However, nuclear and cytoplasmic maturation are acquired independently during oocyte maturation [1,29].
Cytoplasmic maturation encompasses a wide array of metabolic and structural modifications, including events that ensure the occurrence of normal fertilization, meiotic to mitotic cell cycle progression, and activation of pathways required for genetic and epigenetic programmes of preimplantation embryonic development [1,30- 33]. On the other hand, deficient cytoplasmic maturity may be reflected by certain cytoplasmic abnormalities such as cytoplasmic inclusions, vacuoles, smooth endoplasmic reticulum (sER) clustering visible at light microscope level [34]. In previous studies, the incidence of vacuoles in MII oocytes varied between 5.7% [35] and 12.4% [36]. De Sutter et al. [35] showed a severely reduced fertilization rate in vacuolized oocytes (40%) compared with gametes without vacuolization (69.9%). Several studies evaluating the presence of sER in human oocytes have shown no differences on fertilization and cell division rates [37]; however, negative effects on the blastocyst formation rate, clinical pregnancy rate [37-39] and implantation rate were evident [40].
Our data suggests that overall fertilization rate is not influenced by preincubation time of oocytes before insemination. However, we observed a significantly greater cell number in embryos at Day 3 achieved from group II oocytes, which had a longer culture time (4 - 6 h) compared to group I (1 - 3 h), indicating a better preimplantation embryonic development under the same culture conditions, and similar results were obtained for Falcone et al. [14]. The present results confirm that the quality of embryo cleavage depends on the period of oocyte preincubation before insemination. Additionally, cleavage rate, cleavage stage embryo morphology, cytoplasmic fragmentation and multinucleation have been shown to be important markers of embryo quality and viability that may be observed over time during in vitro culture. After the advent of extended embryo culturing, higher IRs have been reported because of better embryo selection compared with earlier developmental stages and because
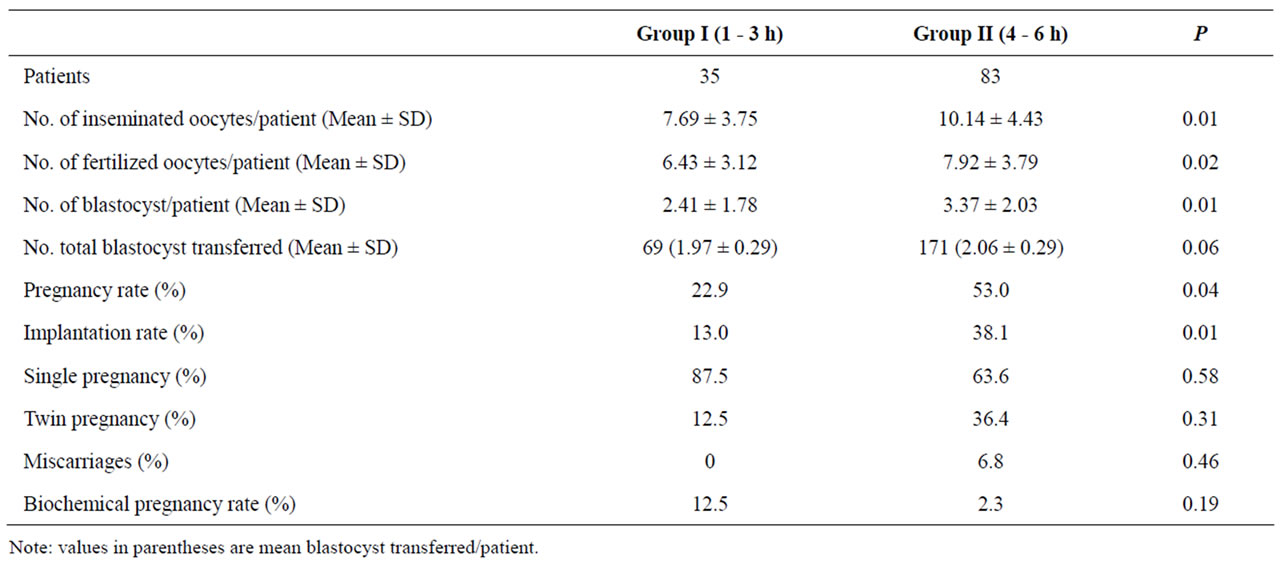
Table 2. Clinical outcomes in those patients whose oocytes were inseminated at ≤3 h or ≥4 h after oocyte retrieval.
of better synchronization between the embryo developmental stage and uterine environment.
Previous studies have shown that during the growth phase, arrested oocytes accumulate a large store of messenger RNA (mRNA) and proteins that function after fertilization to support and regulate preimplantation embryonic development [41,42], and whose oocytes with deficient mRNA or protein accumulation can not complete the cytoplasmic maturation process [43-45]. On the other hand, preimplantation mammalian embryos display an impressive capacity to adapt to the pressures that suboptimal culture environments place upon them. The embryo can compensate, at least partially, for missing components or offset the presence of deleterious components by adjusting its developmental program [46-48].
As human embryo gene expression is switched on at around the 8-cell stage before compaction [49], only embryos that undergo the transition from maternal to embryonic genome might reach the blastocyst stage. Furthermore, there are both cytoplasmic and nuclear reasons why a large proportion of embryos stop developing before implantation. For example, blastocyst formation in the mouse is dependent on the relocation of cytoplasmic organelles, alterations to membrane transport system and transcripts in the oocyte generated before fertilization during cytoplasmic maturation process [32,45,50]. Gross abnormalities in nuclear and cytoplasmic maturation process generally interrupt the meiotic cycle or block fertilization, but more subtle imperfections during oocyte cytoplasmic maturation may be manifested during the late cleavage or blastocyst stage.
Many reports have underlined the difficulties of correctly selecting the best embryo on Day 2 [51] or on Day 3 [52,53]. The aim of extending embryo culture to blastocyst stage is to select an embryo with increased probability of implantation rather than to improve embryo quality. In our study all transfers were made at blastocyst stage and similar blastocyst formation rate in both groups was evident, but patients whose oocytes were preincubated 4 - 6 h prior insemination procedure had higher implantation and pregnancy rates than those whose oocytes were incubated 1 - 3 h (P < 0.05), suggesting that the oocyte maturation is an important process required to achieve optimal oocyte quality and a subsequent genetically normal embryo. The aneuploidy rate has been reported to be lower for blastocysts compared with top quality Day 3 embryos, even when genetic abnormalities have not prevented development to the blastocyst stage [54-56].
To conclude, our data indicates that a period of preincubation ≥4 h before insemination improves oocyte quality, thus allowing adequate nuclear and cytoplasmic maturations to take place, which will be evident during embryo development and will be reflected in implantation and pregnancy rates. Additionally, we suggest more studies randomized controlled on the effect of preincubation time before the process of oocyte insemination with blastocyst transfer in cycles of IVF and ICSI.
![]()
![]()
REFERENCES
- Eppig, J.J., Schultz, R.M., O’Brien, M. and Chesnel, F. (1994) Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Development Biology, 164, 1-9. doi:10.1006/dbio.1994.1175
- First, N.L., Leibfried-Rutledge, M.L. and Sirard, M.N. (1988) Cytoplasmic control of oocyte maturation and species differences in the development of maturational competence. Progress in Clinical and Biological Research, 267, 1-46.
- Hunter, A.G. and Moor, R.M. (1987) Stage dependent effects of inhibiting ribonucleic acids and protein synthesis on meiotic maturation of bovine oocytes in vitro. Journal of Dairy Science, 70, 1646-1651. doi:10.3168/jds.S0022-0302(87)80192-3
- Kastrop, P.M., Bevers, M.M., Desetree, O.H.J. and Kruip, T.A.M. (1991) Protein synthesis and phosphorylation patterns of bovine oocytes matured in vivo. Molecular Reproduction and Development, 29, 271-275. doi:10.1002/mrd.1080290309
- Farin, C.E. and Yang, L. (1992) Inhibition of germinal vesicle breakdown by 5,6-dichlorobenzimidazole riboside in bovine oocytes maturated in vitro. Theriogenology, 37, 208. doi:10.1016/0093-691X(92)90277-X
- Sirard, M.A., Florman, H.M., Leibfried-Rutledge, M.L., Barnes, F.L., Sims, M.L. and First, N.L. (1989) Timing of nuclear progression and protein synthesis necessary for meiotic maturation of bovine oocytes. Biology of Reproduction, 40, 1257-1263. doi:10.1095/biolreprod40.6.1257
- Kruip, T.A.M., Cran, D.G., van Beneden, T.H. and Dieleman, S.J. (1983) Structural changes in bovine oocytes during final maturation in vivo. Gamete Research, 8, 29- 47. doi:10.1002/mrd.1120080105
- Hytell, P., Xa, K.P., Smith, S. and Greve, T. (1986) Ultrastructure of in vitro oocyte maturation in cattle. Journal of the Society for Reproduction and Fertility, 78, 615-625. doi:10.1530/jrf.0.0780615
- Levesque, J.T. and Sirard, M.A. (1995) Effects of different kinases and phosphatases on nuclear and cytoplasmic maturation of bovine oocytes. Molecular Reproduction and Development, 42, 114-121. doi:10.1002/mrd.1080420115
- Wickramasinghe, D. and Albertini, D.F. (1993) Current topics in developmental biology. Academic Press, San Diego, 125-153.
- Barron, D.J., Valdimarsson, G., Paul, D. and Kidder, G.M. (1989) Connexin 43, a gap junction protein, is a persistent oocyte product through preimplantation development. Development Genetics, 10, 318-323. doi:10.1002/dvg.1020100407
- Watson, A.J., Westhusin, E., De Sousa, P.A., Betts, D.H. and Barcroft, L.C. (1999) Gene expression regulating blastocyst formation. Theriogenology, 51, 117-133. doi:10.1016/S0093-691X(98)00236-2
- Trounson, A.O., Mohr, M.R. and Wood, C. (1982) Effect of delayed insemination on in vitro fertilization, culture and transfer of human embryos. Journal of the Reproduction and Fertility, 64, 285-294. doi:10.1530/jrf.0.0640285
- Falcone, P., Gambera, L., Pisoni, M., Lofiego, V., De Leo, V., Mencaglia, L. and Piomboni, P. (2008) Correlation between oocyte preincubation time and pregnancy rate after intracytoplasmic sperm injection. Gynecological Endocrinology, 24, 295-299. doi:10.1080/09513590802095613
- Rienzi, L., Ubaldi, F., Anniballo, R., Cerulo, G. and Greco, E. (1998) Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Human Reproduction, 13, 1014-1019. doi:10.1093/humrep/13.4.1014
- Ho, J.Y.-P., Chen, M., Yi, Y.-C., Guu, H.-F. and Ho, E.S.-C. (2003) The effect of preincubation period of oocytes on nuclear maturity, fertilization rate, embryo quality, and pregnancy outcome in IVF and ICSI. Journal of Assisted Reproduction and Genetics, 20, 358-364. doi:10.1023/A:1025476910771
- Gardner, D.K., Lane, M., Calderon, I. and Leeton, J. (1996) Environment of the preimplantation human embryo in vivo: Metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertility and Sterility, 65, 349-353.
- Fanchin, R., Ayoubi, J.M., Righini, C., Olivennes, F., Schonauer, L.M. and Frydman, R. (2001) Uterine contractility decreases at the time of blastocyst transfers. Human Reproduction, 16, 1115-1119. doi:10.1093/humrep/16.6.1115
- Quea, G., Romero, K. and García-Velasco, J.A. (2007) Extended embryo culture to increase implantation rate. Reproductive Biomedicine Online, 14, 375-383. doi:10.1016/S1472-6483(10)60882-6
- Marek, D., Langley, M., Gardner, D., Phil, D., Confer, N., Doody, K.M. and Doody, K.J. (1999) Introduction of blastocyst culture and transfer for all patients in an in vitro fertilization program. Fertility and Sterility, 72, 1035- 1040. doi:10.1016/S0015-0282(99)00409-4
- Gardner, D.K., Phil, D., Lane, M., Stevens, J., Schlenker, T. and Schoolkraft, W.B. (2000) Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertility and Sterility, 73, 1155-1158. doi:10.1016/S0015-0282(00)00518-5
- Gardner, D.K., Phil, D., Surrey, E., Minjarez, D., Leitz, A., Stevens, J. and Schoolkraft, W.B. (2004) Single blastocyst transfer: A prospective randomized study. Fertility and Sterility, 81, 551-555. doi:10.1016/j.fertnstert.2003.07.023
- Henman, M., Catt, J.W., Wood, T., Bowman, M.C., De Boer, K.A. and Jansen, R. (2005) Elective transfer of single fresh blastocysts and later transfer of cryostored blastocysts reduces the twin pregnancy rate in younger women. Fertility and Sterility, 84, 1620-1627. doi:10.1016/j.fertnstert.2005.05.064
- Nilsson, S., Waldenström, U., Engström, A.B. and Hellberg, D. (2005) Promising results with 306 single blastocyst transfers. Fertility and Sterility, 83, 1849-1841. doi:10.1016/j.fertnstert.2004.11.079
- Papanikolaou, E.G., Camus, M., Kolibianakis, E.M., Van Landuyt, L., Van Steirteghem, A. and Devroey, P. (2006) In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. New England Journal of Medicine, 354, 1139-1146. doi:10.1056/NEJMoa053524
- Gardner, D.K. and Schoolcraft, W.B. (1999) In vitro culture of human blastocysts. In: Jansen, R. and Mortimer, D., Eds., Towards Reproductive Certainty: Infertility and Genetics Beyond 1999: The Plenary Proceedings of the 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics, Parthenon Press, Pearl River, 378-388.
- Mansour, R. (2005) Minimizing embryo expulsion after embryo transfer: A randomized controlled study. Human Reproduction, 20, 170-174. doi:10.1093/humrep/deh573
- Zenzes, M.T., Belkien, L., Bordt, J., Kan, I., Schneider, H.G. and Nieschlag, E. (1985) Cytological investigation of human in vitro fertilization failures. Fertility and Sterility, 43, 883-891.
- Kubiak, J.Z. (1989) Mouse oocytes gradually develop the capacity for activation during the metaphase II arrest. Developmental Biology, 136, 537-545. doi:10.1016/0012-1606(89)90279-0
- Eppig, J.J. and O’Brien, M.J. (1996) Development in vitro of mouse oocytes from primordial follicles. Biology of Reproduction, 54, 197-207. doi:10.1095/biolreprod54.1.197
- Heikinheimo, O. and Gibbons, W.E. (1998) The molecular mechanisms of oocytes maturation and early embryonic development are unveiling new insights into reproductive medicine. Molecular Human Reproduction, 4, 745-756. doi:10.1093/molehr/4.8.745
- Moor, R.M., Dai, Y., Lee, C. and Fulka Jr., J. (1998) Oocyte maturation and embryonic failure. Human Reproduction Update, 4, 223-236. doi:10.1093/humupd/4.3.223
- Trounson, A., Anderiesz, A. and Jones, G. (2001) Maturation of human oocytes in vitro and their developmental competence. Reproduction, 121, 51-75. doi:10.1530/rep.0.1210051
- Ebner, T., Moser, M., Sommergruber, M., Puchner, M., Wiesinger, R. and Tews, G. (2003) Developmental competence of oocytes showing increased cytoplasmic viscosity. Human Reproduction, 18, 1294-1298. doi:10.1093/humrep/deg232
- De Sutter, P., Dozortsev, D., Qian, C. and Dhont, M. (1996) Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Human Reproduction, 11, 595-597. doi:10.1093/HUMREP/11.3.595
- Alikani, M., Palermo, G., Adler, A., Bertoli, M., Blake, M. and Cohen, J. (1995) Intracytoplasmic sperm injection in dysmorphic human oocytes. Zygote, 3, 283-288. doi:10.1017/S0967199400002707
- Otsuki, J., Okada, A., Morimoto, K., Nagai, Y. and Kubo, H. (2004) The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Human Reproduction, 19, 1591-1597. doi:10.1093/humrep/deh258
- Serhal, P.F., Ranieri, D.M., Kinis, M., Marchant, S., Davies, M. and Khadum, I.M. (1997) Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Human Reproduction, 12, 1267-1270. doi:10.1093/humrep/12.6.1267
- Loutradis, D., Drakakis, P., Kallianidis, K., Milingos, S., Dendrinos, S. and Michalas, S. (1999) Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertility and Sterility, 72, 240-244. doi:10.1016/S0015-0282(99)00233-2
- Meriano, J.S., Alexis, J., Visram-Zaver, S., Cruz, M. and Casper, R.F. (2001) Tracking of oocytes dysmorphism for ICSI patients may prove relevant to the outcome in subsequent patient cycles. Human Reproduction, 16, 2118- 2123. doi:10.1093/humrep/16.10.2118
- Krisher, R.L. (2004) The effect of oocyte quality on development. Journal of Animal Science, 82, E14-E23.
- Sirard, M.A., Desrosier, S. and Assidi, M. (2007) In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology, 68, S71-S76. doi:10.1016/j.theriogenology.2007.05.053
- Pavlok, A., Kopecny, V., Lucas-Hahn, A. and Niemann, H. (1993) Transcriptional activity and nuclear ultrastructure of 8-cell bovine embryos developed by in vitro maturation and fertilization of oocytes from different growth categories of antral follicles. Molecular Reproduction and Development, 35, 233-243. doi:10.1002/mrd.1080350304
- Lonergan, P., Monaghan, P., Rizos, D., Boland, M.P. and Gordon, I. (1994) Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization and culture in vitro. Molecular Reproduction and Development, 37, 48-53. doi:10.1002/mrd.1080370107
- De Sousa, P., Caveney, A., Westhusin, M.E. and Watson, A.J. (1998) Temporal patterns of embryonic gene expression and their dependence on oogenic factors. Theriogenology, 49, 115-128. doi:10.1016/S0093-691X(97)00406-8
- Fair, T., Murphy, M., Rizos, D., Moss, C., Martin, F., Boland, M.P. and Lonergan, P. (2004) Analysis of differential maternal nRNA expression in developmentally competent and incompetent bovine two-cell embryos. Molecular Reproduction and Development, 67, 136-144. doi:10.1002/mrd.10385
- Pennetier, S., Uzbekova, S., Perreau, C., Papiller, P., Mermillod, P. and Dalbiès-Tran, R. (2004) Spatio-temporal expression of germ cell marker genes MATER, ZAR1, GFD9, BMP15, and VASA in adult bovine tissues, oocytes, and preimplantation embryos. Biology of Reproduction, 71, 1359-1366. doi:10.1095/biolreprod.104.030288
- Alizadeh, Z., Kageyama, S.I. and Aoki, F. (2005) Degradation of maternal mRNA in mouse embryos: Selective degradation of specific mRNAs after fertilization. Molecular Reproduction and Development, 72, 281-290. doi:10.1002/mrd.20340
- Braude, P., Bolton, V. and Moore, S. (1988) Human gene expression first occurs between the four and eight-cell stages of preimplantation development. Nature, 332, 459- 461. doi:10.1038/332459a0
- Renard, J., Baldacci, P., Richoux-Duranthon, V., Pournin, S., Babinet, C. (1994) A maternal factor affecting mouse blastocyst formation. Development, 120, 797-802.
- Guerif, F., Le Gouge, A., Giraudeau, B., Poindron, J., Bidault, R., Gasnier, O. and Royere, D. (2007) Limited value of morphological assessment at days 1 and 2 to predict blastocyst development potential: A prospective study base don 4042 embryos. Human Reproduction, 362, 735-743.
- Rijnders, P.M. and Jansen, C.A. (1998) The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in vitro fertilization or cytoplasmic sperm injection. Human Reproduction, 13, 2869-2873. doi:10.1093/humrep/13.10.2869
- Milki, A.A., Hinckley, M.D., Gebhardt, J., Dasig, D., Westphal, L.M. and Behr, B. (2002) Accuracy of day 3 criteria for selecting the best embryo. Fertility and Sterility, 77, 1191-1195. doi:10.1016/S0015-0282(02)03104-7
- Magli, M.C., Jones, G.M., Gras, L., Gianaroli, L., Korman, I. and Trounson, A.O. (2000) Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Human Reproduction, 15, 1781-1786. doi:10.1093/humrep/15.8.1781
- Staessen, C., Platteau, P., Van Assche, E., Michiels, A., Tournaye, H., Camus, M., Devroey, P., Liebaers, I. and Van Steirteghem, A. (2004) Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: A prospective randomized controlled trial. Human Reproduction, 19, 2849-2858. doi:10.1093/humrep/deh536
- García, J.I., Noriega-Portella, L. and Noriega-Hoces, L. (2011) Effect of vitrification procedure on chromosomal status of embryos achieved from vitrified and fresh oocytes. Health, 3, 467-476. doi:10.4236/health.2011.37077

