American Journal of Plant Sciences
Vol.4 No.8(2013), Article ID:35477,4 pages DOI:10.4236/ajps.2013.48203
Hairy Root Cultures and Plant Regeneration in Solidago nemoralis Transformed with Agrobacterium rhizogenes
![]()
1Kentucky Tobacco Research and Development Center, Lexington, USA; 2Department of Pharmaceutical Sciences, University of Kentucky, Lexington, USA; 3Naprogenix Inc., Lexington, USA.
Email: *skgunj2@uky.edu
Copyright © 2013 Samir Kumar Gunjan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 21st, 2013; revised June 22nd, 2013; accepted July 15th, 2013
Keywords: Agrobacterium rhizogenes; Hairy Root; Regeneration; Solidago nemoralis
ABSTRACT
By screening a native plant extract library we identified Solidago nemoralis as a novel source of agonists for alpha7 nicotinic receptors for acetylcholine with therapeutic potential. The next phase of our drug discovery strategy is to increase the yields of active compounds in the plant species by gain of function mutations in hairy root cultures [1]. Here we report a protocol for Agrobacterium rhizogenes-mediated genetic transformation of hairy root cultures of Solidago nemoralis which will enable this. Leaf explants of this species were successfully transformed with a frequency of 30% - 35% using A. rhizogenes strain R1000 harboring the binary vector pCambia 1301. Transformation was confirmed using the β-glucuronidase (GUS) histochemical assay. Transformed hairy roots showed spontaneous regeneration of adventitious shoots in media without the addition of cytokines, albeit at very low frequency. However, media supplementation with auxin (α-naphthaleneacetic acid, NAA) increased shoot regeneration frequency to 35% and resulted in viable adventitious shoots. Transformation was confirmed at all phases of plant regeneration by GUS staining. Hairy root transformation of Solidago altissima has been previously reported, but this is the first report of genetic transformation of S. nemoralis. The protocol will allow for a large population of activation tagged mutants of S. nemoralis to be generated which will be then screened for the presence of stable mutants which are over-producing metabolites with activity at alpha7 nicotinic receptors. These over-producing mutant cultures will then be regenerated into intact mutant plants.
1. Introduction
Plants have complex biosynthetic machineries that have allowed them to evolve bioactive, complex and multifunctional secondary metabolites as protection against stressors. One example is the complex alkaloid methyllycaconitine (MLA) in Delphinium species which deters herbivorous insects by targeting the insect nicotinic receptor for acetylcholine (nAChR), the most prevalent excitatory receptor in the insect CNS [2]. In addition to being a high affinity ligand for the insect nAChR, MLA is also a highly selective ligand for the alpha7-subtype of human nAChR [3]. Since this receptor is an emerging target for the treatment of neurodegenerative disorders [4] other plant metabolites with this selectivity would be of considerable therapeutic interest. We therefore screened a native plant library for this pharmacological activity using a “differential screening” approach [1] which identified Solidago nemoralis (“gray goldenrod”) as a prime candidate which has not previously been investigated for this activity. However, as for other plant species, long grow cycles, low yield and necessity to harvest large amounts of biomass, is likely to hinder the development and commercialisation of these metabolites. A number of bioengineering strategies to circumvent these issues have been described for known metabolites with known metabolic pathways [5]. However, in the case of unknown metabolites with unknown pathways for production (as here) an alternative strategy for optimizing plant production is required. The strategy developed in our laboratory relies on Agrobacteriummediated continuous hairy root culture together with random gain-of-function mutagenesis. Stable mutants over-producing the active metabolites are identified by pharmacological screening, and are then regenerated into intact mutant plants. However, for the Solidago genus, only one species, S. altissima, has been reported to be transformed by Agrobacterium [6]. Here we report an Agrobacterium-mediated transformation protocol for S. nemoralis, which will enable the application of our genomic optimization strategy to the potential therapeutic compounds contained in this species.
2. Materials and Methods
2.1. Plant Material & Culture Conditions
Solidago nemoralis seeds were washed with 70% ethanol for 2 min followed by surface sterilization in 30% commercial bleach for 20 min. Seeds were rinsed five times with sterile water and aseptically germinated in plates containing half-strength of Murashigue and Skoog (MS) media supplemented with 0.6% agar and 1% sucrose. For all media, the pH was adjusted to 5.8 and autoclaved. The temperature in the growth chamber was maintained at 25˚C ± 2˚C with a 12 h photoperiod and (light intensity 45 µmol·m−2·s−1).
2.2. Agrobacterium Culture and Infection
The pCambia 1301 binary vector was mobilized into Agrobacterium rhizogenes strain R1000 by freeze-thaw method. Briefly, 1 µg of plasmid DNA was mixed with competent cells of A. rhizogenes and incubated on ice for 30 min. The DNA–bacterial mix was frozen in liquid nitrogen for 30 sec and incubated for 5 min in a water bath at 37˚C. After the heat shock, 600 µL of liquid Luria-Bertani (LB) media were added to the bacteria-DNA mix and shaken on rotary shaker set at 200 rpm for 4 h at 28˚C. Bacteria were pelleted down by centrifugation at 4000 rpm, resuspended in 100 μL LB and finally plated on solid LB media containing 50 mg/L kanamycin. The plate was incubated for two days at 28˚C which resulted in transformed colonies. A single colony was used to make transformed bacterial stock which was in turn aliquoted in 1.5 mL 60% glycerol suspensions and kept at −80˚C.
Five ml of liquid LB kan 50 media was inoculated with A. rhizogenes stock harboring pCambia 1301 and grown overnight at 28˚C. Two mL of this culture were used to inoculate 50 mL of LB plus kanamycin 50 mg/L liquid media and grown until the optical density (OD) reached to 0.6. Bacteria were then pelleted down by centrifugation at 4000 rpm and resuspended into 50 mL liquid MS media supplemented with 100 µM acetosyringone (3,5- dimethoxy-4-hydroxy-acetophenone).
Stem explants (4 week old plants), leaf explants (4 week old plants) and root-excised seedlings (2 week old plants) were tested for Agrobacterium infection. Leaf explants were excised from stems and stem explants were cut into 1 cm long sections. Explants or seedlings were placed in the Agrobacterium culture, wounded using a sterile needle and left to incubate for 20 min. Explants were then blot-dried on sterile filter paper, transferred onto solid MS media supplemented with vitamin B5 and 1% sucrose and incubated for 2 days in the dark in the growth chamber. After the 2-day co-cultivation period, explants were transferred onto MS media plate supplemented with 400 mg/L cefotaxime and 3% sucrose. Hairy roots appeared after 2 - 3 weeks at which point they were excised from explants, cut into 1 cm long sections and cultured and maintained on MS media supplemented with 250 mg/L cefotaxime and 3% sucrose.
2.3. Plant Regeneration from Hairy Roots
All media used in the regeneration process was supplemented with 250 mg/L cefotaxime in order to prevent undesired Agrobacterium growth. Hairy root cultures were transferred onto MS media alone or MS media supplemented with α-naphthaleneacetic acid (NAA) (0.1 or 1 mg/L) or MS media supplemented with NAA (0.1 or 1 mg/L) and 6-benzyladenine (BA) (2 or 5 mg/L). After 4 - 6 weeks adventitious shoots appeared. They were excised and transferred onto MS media for root formation. GUS staining was performed at all stages of hairy root culture and plant regeneration to confirm transformation.
2.4. β-Glucuronidase (GUS) Histochemical Assay
GUS staining buffer was prepared by adding 50 mM sodium phosphate (pH 7.0), 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 10 mM EDTA and 0.05% Triton X-100 into 150 ml of water. This buffer was dispensed into 15 ml aliquots and stored at −20˚C. For GUS staining, the stock buffer was thawed and diluted to a final volume of 100 ml in water. 35 mg of 5-bromo,4-chloro,3-indolyl-β-D-glucuronide (X-Gluc) was dissolved in 150 μl of dimethylformamide and subsequently added to the diluted buffer. Tissue samples were incubated in X-Gluc solution for 24-h at 37˚C and observed for blue staining indicative of GUS expression.
3. Results
S. nemoralis stem explants, leaf explants and seedlings were tested for their ability to be transformed by A. rhizogenes and produce hairy roots after infection. Leaf explants transformation produced the highest hairy root induction frequency (30% - 35%) as compared to stem explants (18% - 20%) and seedlings (6% - 9%). Therefore, further experiments on regeneration of transformed plants were undertaken with A. rhizogenes transformed leaf explants.
2 - 3 weeks after infection, hairy roots appeared on the site of infection. They were excised and cultured independently. When grown in solid MS media without the addition of phytohormones, adventitious shoot regeneration occurred via direct organogenesis. Indeed, once excised, several hairy roots turned green, produced green callus-like structures which ultimately turned into shoots after 4 - 6 weeks of culture (Figures 1(a) and (b)). However, the frequency of spontaneous shoot regeneration on simple MS media was low (only 2 - 3 shoots appeared on each hairy root). Therefore, in order to increase shoot regeneration frequency, media was supplemented with either BA/NAA or NAA alone.
A combination of BA (2 or 5 mg/L) and NAA (1 mg/L) did not increase shoot regeneration because hairy roots cultured in this particular medium produced white callus like structures that did not turn into shoot buds (Figure 1(c)). However, media with NAA alone (0.1 or 1 mg/L) did produce shoots in 4 weeks of culture (Figure 1(d)) after going through both white and green callus like structure stages. Regeneration efficiency was increased by NAA supplemented media which afforded an adventitious shoot budding frequency of ~35%, much higher than in non-supplemented media.
Since the transformation of S. nemoralis was performed with A. rhizogenes strain R1000 harboring pCambia 1301 which has the GUS gene as visible marker, all stages of hairy root development and shoot regeneration were tested by GUS histochemical assay to ensure transformation events (Figures 2(a)-(c)). The coding region of the gusA gene is interrupted by a catalase intron to ensure that the transcription and post-transcription splicing to form mature mRNA occurs by utilizing the plant cell machinery rather than the Agrobacterium protein synthesis machinery [7].
4. Discussion
A. rhizogenes-mediated genetic transformation in plants is a well established procedure which produces hairy roots at the site of infection by altering the endogenous auxin:cytokinin ratio in the plant cell [8,9]. Additionally, it is well documented that, once established, hairy roots can be cultured on hormone-free medium [10]. S. nemoralis hairy roots were easily established and then cultured both in solid and liquid hormone free medium.
Although shoot regeneration occurred spontaneously in hormone-free media, it occurred only at low frequency. Additionally, introducing exogenous NAA and BA led to no improvement in shoot regeneration. However, application of NAA alone on S. nemoralis hairy root cultures showed a positive effect on shoot regeneration. These results go against the notion that higher levels of cytokinin (in the auxin:cytokinin ratio) are necessary for shoot regeneration although it has been reported that A. rhizogenes transformed plants can regenerate shoots in the presence of only auxin, presumably due to the cytokinin mimetic effect of the over expression of the rolC gene, present on A. rhizogenes Ri plasmid [11].




Figure 1. Agrobacterium rhizogenes mediated transformation of S. nemoralis. (a) Hairy root culture and their spontaneous regeneration into shoot; (b) Hairy root turned green after two weeks of culture and produced shoots on their surface; (c) Hairy root treated with BA produced white callus which failed to regenerate when culture continued on the same medium; (d) Shoot regeneration increased by application of NAA (0.1 mg/L).


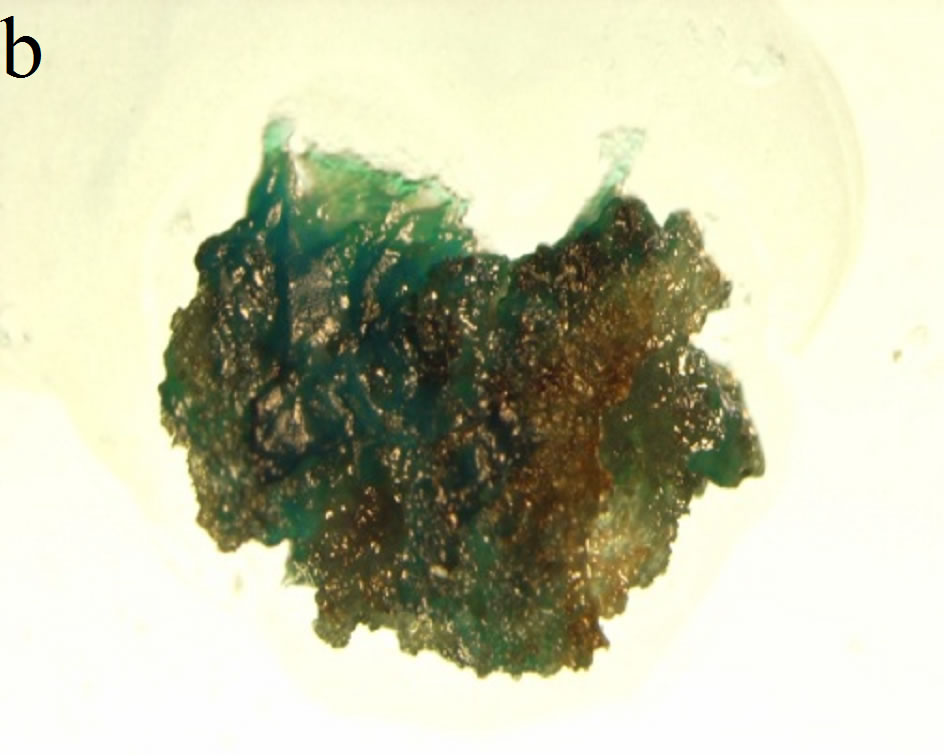
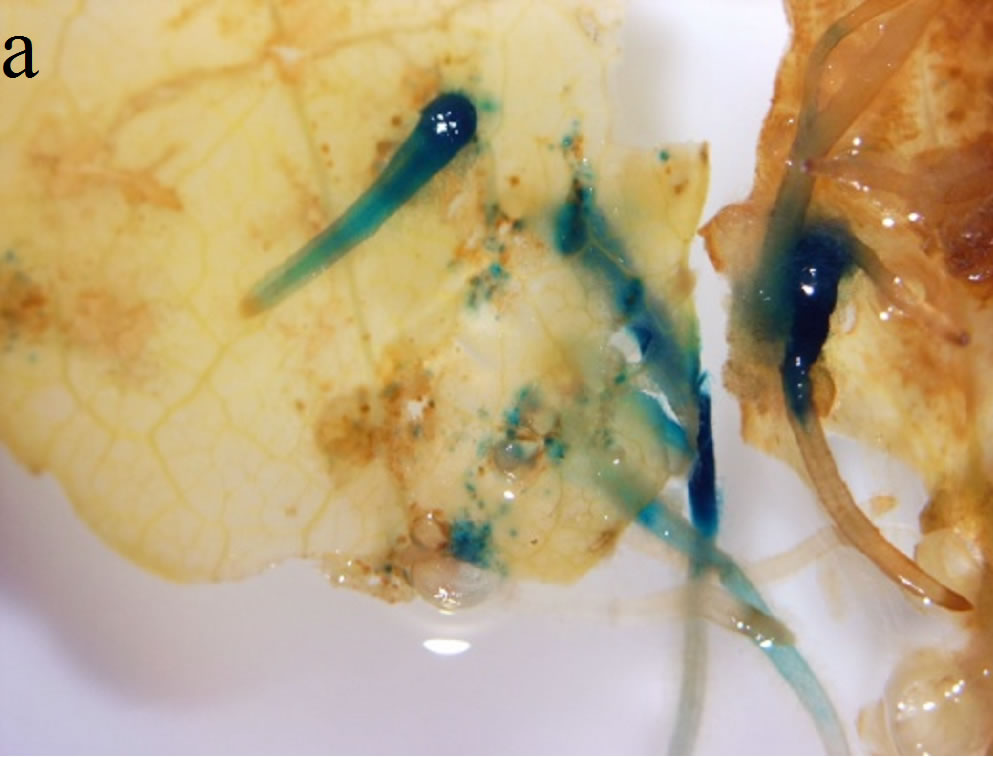
Figure 2. GUS histochemical assays were performed to ensure transformation events. Samples were treated in the x-Gluc solution overnight followed by washing with 70% ethanol. (a) Hairy roots generation on the leaf explants; (b) Transformed hairy root turned into callus during regeneration process; (c) Regenerated shoots showed blue GUS stain in their leaves; (d) Adverse effect of high concentration of sucrose in the culture medium—the regenerated shoots turned brown and did not elongate further when HR were cultured on 6% sucrose containing medium.
The effect of auxin application for shoot regeneration may be different for each hairy root and might be linked with the expression of the rolC gene [12]. A similar result has been previously reported in Solanum khasianum hairy roots where application of IAA and kinetin affected the growth and regeneration capacity of the plant [13].
Additionally, high concentrations of sucrose appear to have an adverse effect on root culture and shoot organogenesis. S. nemoralis hairy root grew better in media containing only 1% - 2% sucrose. On the other hand, media with more than 4% sucrose turned the root culture and regenerated shoots deep brown in color and abrogated growth (Figure 2(d)). Similar findings on the effect of sucrose concentration on growth and shoot regeneration have previously been reported for Hypericum perforatum [14].
In conclusion, we report a protocol for culture and transformation of S. nemoralis which should enable the genome of this species to be altered so as to optimize production of the potentially valuable pharmacologically active metabolites it contains.
5. Acknowledgements
This work was supported by two NIH (National Institutes of Health) grants (# 5R42AA015475-05 and 1R21AA- 020188-01) awarded to Dr. John Littleton as Principal Investigator.
REFERENCES
- J. Littleton, T. Rogers, et al., “Novel Approaches to Plant Drug Discovery Based on High Throughput Pharmacological Screening and Genetic Manipulation,” Life Sciences, Vol. 78, No. 5, 2005, pp. 467-475. doi:10.1016/j.lfs.2005.09.013
- H. Baylis, D. Sattelle, et al., “Genetic Analysis of Cholinergic Nerve Terminal Function in Invertebrates,” Journal of Neurocytology, Vol. 25, No. 1, 1996, pp. 747-762. doi:10.1007/BF02284839
- A. Drasdo, M. Caulfield, et al., “Methyl Lycaconitine: A Novel Nicotinic Antagonist,” Molecular and Cellular Neuroscience, Vol. 3, No. 3, 1992, pp. 237-243. doi:10.1016/1044-7431(92)90043-2
- M. R. Picciotto and M. Zoli, “Neuroprotection via nAChRs: The Role of nAChRs in Neurodegenerative Disorders Such as Alzheimer’s and Parkinson’s Disease,” Frontiers in Bioscience, Vol. 13, 2008, pp. 492-504. doi:10.2741/2695
- L. B. Pickens, Y. Tang, et al., “Metabolic Engineering for the Production of Natural Products,” Annual Review of Chemical and Biomolecular Engineering, Vol. 2, No. 1, 2011, pp. 211-236. doi:10.1146/annurev-chembioeng-061010-114209
- M. Inoguchi, S. Ogawa, et al., “Production of an Allelopathic Polyacetylene in Hairy Root Cultures of Goldenrod (Solidago altissima L.),” Bioscience, Biotechnology, and Biochemistry, Vol. 67, No. 4, 2003, pp. 863-868. doi:10.1271/bbb.67.863
- S. Ohta, S. Mita, T. Hattori and K. Nakamura, “Construction and Expression in Tobacco of a β-Glucuronidase (GUS) Reporter Gene Containing an Intron within the Coding Sequence,” Plant and Cell Physiology, Vol. 31, No. 6, 1990, pp. 805-813.
- M. Faiss, M. Strnad, et al., “Chemically Induced Expression of the rolC-Encoded β-Glucosidase in Transgenic Tobacco Plants and Analysis of Cytokinin Metabolism: rolC Does Not Hydrolyze Endogenous Cytokinin Glucosides in Planta,” The Plant Journal, Vol. 10, No. 1, 1996, pp. 33-46. doi:10.1046/j.1365-313X.1996.10010033.x
- J. Palazón, R. M. Cusidó, et al., “Expression of the rolC Gene and Nicotine Production in Transgenic Roots and Their Regenerated Plants,” Plant Cell Reports, Vol. 17, No. 5, 1998, pp. 384-390. doi:10.1007/s002990050411
- M. Christey, “Use of ri-Mediated Transformation for Production of Transgenic Plants,” In Vitro Cellular & Developmental Biology—Plant, Vol. 37, No. 6, 2001, pp. 687-700.
- E. Casanova, A. Zuker, et al., “The rolC Gene in Carnation Exhibits Cytokininand Auxin-Like Activities,” Scientia Horticulturae, Vol. 97, No. 3-4, 2003, pp. 321-331. doi:10.1016/S0304-4238(02)00155-3
- T. Saitou, H. Kamada, et al., “Involvement of Phytohormones in Light-Induced Adventitious Shoot Formation of Horseradish Hairy Roots,” Plant Science, Vol. 86, No. 2, 1992, pp. 161-166. doi:10.1016/0168-9452(92)90162-F
- A. Jacob, and N. Malpathak, “Plantlet Regeneration Enhances Solasodine Productivity in Hairy Root Cultures of Solanum khasianum Clarke,” In Vitro Cellular & Developmental Biology—Plant, Vol. 41, No. 3, 2005, pp. 291- 295. doi:10.1079/IVP2005637
- B. Vinterhalter, S. Ninković, et al., “Shoot and Root Culture of Hypericum perforatum L. Transformed with Agrobacterium rhizogenes A4M70GUS,” Biologia Plantarum, Vol. 50, No. 4, 2006, pp. 767-770. doi:10.1007/s10535-006-0127-9
NOTES
*Corresponding author.
†Authors provided equal contributions.

