American Journal of Plant Sciences
Vol.4 No.3(2013), Article ID:29005,6 pages DOI:10.4236/ajps.2013.43078
Coastal Adaptation of Adenophora triphylla var. japonica (Campanulaceae)
![]()
1Graduate School of Integrated Arts and Sciences, Kochi University, Kochi, Japan; 2Faculty of Agriculture, Kochi University, Kochi, Japan; 3Faculty of Science, Yamagata University, Yamagata, Japan.
Email: *tfukuda@kochi-u.ac.jp
Received January 11th, 2013; revised February 4th, 2013; accepted February 18th, 2013
Keywords: Adaptation; Adenophora triphylla var. japonica; Coastal; Ecotype; Heterochronic; Leaf Thickness
ABSTRACT
The comparative morphology and anatomy of leaves between the coastal ecotype and the normal type of Adenophora triphylla (Thunb.) A.DC. var. japonica (Regel) H.Hara (Campanulaceae) were examined to clarify the differences in morphological characters between the 2 groups. Morphological and anatomical analyses revealed that the coastal ecotype had a thicker leaf than the normal type, because of the increased size of epidermal and spongy cells. Thus, the main morphological change from the normal type into the coastal ecotype of A. triphylla var. japonica is the increase in leaf size, suggesting that the coastal ecotype may have evolved from the normal type via a heterochronic process.
1. Introduction
Plant diversity has fascinated humans throughout history; the diversity is presumably plant adaptation to different natural environments, primarily due to the tremendous variations in morphological traits that exist in nature. The fitness effects of such naturally occurring variation present within or among species have driven plant evolution by natural selection [1]. In addition, considerable variation exists within many species, which likely reflects adaptations to different natural environments and is the origin of plant species differentiation [2].
One of the major challenges of plant biology is to describe and understand the morphological mechanisms behind naturally occurring variations for adaptation to various environments. Coastal regions are one type of specific environment that can lead to morphological modification. Plant species growing in coastal regions need to be adapted to an environment in which drought strongly affects plant growth [3]. Moreover, water availability is the main environmental factor limiting photosynthesis and growth even in plants well adapted to coastal conditions [3]. Another source of stress and an abiotic driver of natural selection in coastal plant communities is soil salinity [4,5]. Different levels of salt spray can result in vegetation zonation; plants adapted to salt spray grow close to the ocean and are replaced by less salt-resistant plants further inland [6,7]. Therefore, plants in coastal regions have developed various interesting morphological adaptations including succulent tissues to store water, a pubescent epidermis and a thick cuticle to reduce transpiration and water loss, belowground structures to withstand sand burial, and an annual habit [8].
Adenophora triphylla (Thunb.) A.DC. var. japonica (Regel) H.Hara belonged to Campanulaceae is distributed in Japan, Korea, and Sahalin, and includes distinct morphological variations [9] to adapt to various environments. For instance, the rheophytic ecotype of this variety is found along rivers [10]. This ecotype has a narrower leaf than the non-rheophytic type because of the decreased number and size of the leaf cells [11]. The serpentine ecotype of A. triphylla var. japonica also had narrow leaves, but the leaf thickness and stomatal density was significantly different from those of the normal plants, suggesting that there were different adaptation processes between the two ecotypes of this species [12]. From these studies, it appears that A. triphylla var. japonica can easily alter its morphology and has several ecotypes in various environments. We found the coastal ecotype of A. triphylla var. japonica, which has thick leaves and stems (Figure 1), in some coastal areas of Kochi Prefecture in Japan (Figure 2), where it grows along with other coastal taxa such as Cirsium maritimum Makino (Asteraceae), Dianthus japonicus Thunb. (Caryophyllaceae), Setaria viridis (L.) P.Beauv. var. pachystachys (Franch. et Sav.) Makino et Nemoto (Poaceae), and some CAM plants of Crassulaceae. It is very important to understand the morphological modifIcations to adapt to a specific environment by analysing ecotypic species. Therefore, we characterise the variation of their leaves by morphological and anatomical analyses of the coastal ecotype of A. triphylla var. japonica.
2. Materials and Methods
2.1. Plant Materials
All samples of A. triphylla var. japonica examined in this study were collected from the field between August and November 2011. A total of 130 individuals representing 4 populations were sampled from across the range of the species (Table 1, Figure 2).
2.2. Morphological Analyses
For morphological analysis, individuals were measured for the following continuous macro morphological variables of leaves and stems: 1) length and width of the leaf blade; 2) leaf thickness; 3) angle of the leaf base; 4) plant height; and 5) stem width at the middle point of plant height. Measurements were made using a digi matic cal-

Figure 1. Plants of Adenophora triphylla var. japonica. (a) normal type; (b) coastal ecotype. Bar = 1 cm.

Figure 2. Sampling localities in this study. For other information, see Table 1.
liper (CD-15CXR; Mitutoyo, Kanagawa) and a digimatic outside micrometer (MDC-SB; Mitutoyo, Kanagawa). Leaf measurements were taken from a fully expanded leaf at the middle point of the plant height. The leaf size value was calculated by the following formula: leaf length × leaf width/2. The leaf index value was calculated as the ratio of the leaf length to the leaf width, according to [13].
2.3. Anatomical Analyses
For anatomical analysis, fully expanded leaves were collected from each individual. To count the number of cells on the blade, the adaxial surface of leaves were peeled off by Suzuki’s Universal Micro-Printing (SUMP) method. The number of epidermal cells was calculated by following formula: leaf size/cell size. Replicas of each leaf (1 cm2) were prepared to determine the stomatal density (number/mm2) and to measure the epidermal cell size of 10 cells per leaf. These copied SUMP images were examined once for each individual using a light microscope (CX41; OLYMPUS Co., Tokyo). The leaves were fixed overnight in a solution of formaldehyde, ethanol, and acetic acid (FAA). To observe the palisade and spongy cells, the fixed leaves were dehydrated by immersion in a graded ethanol series and then embedded in Histparaffin to section at 8-µm-thickness by using a rotary microtome (PR-50; Yamatokoki, Kanagawa). The widest part of the blade was analysed to determine the height of adaxial and abaxial epidermal cells and palisade and spongy cells (10 cells per leaf) (Figure 3). Slides were examined using a light microscope. Statistical analyses were performed using a Tukey’s honestly significant difference (HSD) test and Steel-Dwass test to compare the characteristics of the 2 ecotypes.
3. Results
The average heights of A. triphylla var. japonica from the 2 coastal populations, Muroto and Otsuki, and the 2 populations of the normal type, Higashisako and Hitsuzan, were 63.34 ± 13.23 cm, 56.96 ± 22.21 cm, 47.15 ± 9.50 cm, and 40.95 ± 13.60 cm, respectively, and the average widths were 2.40 ± 0.58 mm, 2.00 ± 0.57 mm, 1.54 ± 0.31 mm,

Figure 3. Transverse sections of the leaves of Adenophora triphylla var. japonica. (a) normal type; (b) coastal ecotype. Bar = 25 µm.
Table 1. Sampling localities in this study.
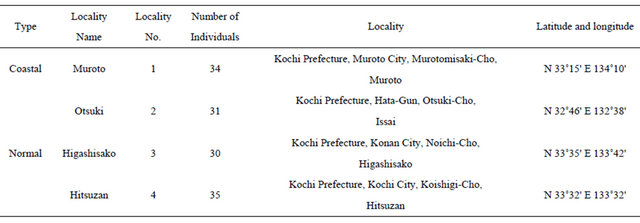
Locality numbers correspond to that shown in Figure 2.
and 1.71 ± 0.60 mm, respectively (Table 2). Although plant height was significantly different between the coastal ecotype and the normal type, the stem width was similar in each ecotype. The average leaf thickness was 230.10 ± 23.28 µm in Muroto, 228.90 ± 35.92 µm in Otsuki, 211.03 ± 19.00 µm in Higashisako, and 199.64 ± 23.28 µm in Hitsuzan. Although leaf thickness was significantly different between the coastal ecotype and the normal type, there were no significant differences in the leaf length, leaf width, or leaf size. Anatomical analyses of cell size, total number of cells, and stomatal density found no significant differences between the coastal ecotype and the normal type. Cross sections from a sample of leaves were examined to measure the height of epidermal, palisade, and spongy cells. The average heights of leaf epidermal cells of samples from Muroto, Otsuki, Higashisako, and Hitsuzan were 35.63 ± 4.99 µm, 38.38 ± 4.41 µm, 28.51 ± 1.98 µm, and 30.15 ± 3.61 µm, respectively, on the adaxial side; and 20.40 ± 1.28 µm, 21.98 ± 2.84 µm, 19.15 ± 1.78 µm, and 19.64 ± 2.90 µm, respectively, on the abaxial side. The heights of the palisade cells were 68.84 ± 19.40 µm, 96.92 ± 17.11 µm, 67.96 ± 13.36 µm, and 69.70 ± 16.79 µm, respectively, and the spongy cell height was 84.74 ± 6.81 µm, 85.52 ± 15.62 µm, 59.17 ± 8.82 µm, and 69.10 ± 11.05 µm, respectively. Of these, the adaxial epidermal cell and spongy cell heights were significantly different between the coastal ecotype and normal type.
4. Discussion
Coastal ecotypes of plants have modifications of various characters to adapt to stresses in the environment. Flavonoids are effective UV-B screening compounds that are synthesised in plants [14,15]. For the coastal ecotype of A. triphylla var. japonica, [16] indicated that the flavonoid composition was not significantly different in quality but was lower in quantity than that in the normal
(inland) type, based on chemical analyses. Further, leaf morphology rather than flavonoid composition may play an important role in adaptation to the coastal environment. In this study, the leaves of the coastal ecotype were thicker than those of the normal ecotype (Table 2). Moreover, the epidermal cells and spongy tissues of the coastal ecotype of A. triphylla var. japonica were larger than those of the normal type of this variety, indicating that the thick leaf of the coastal ecotype strongly contributed to the variation in epidermal cells and spongy tissues. Thus, in this ecotype, increase in leaf thickness because of the large-sized epidermal cells and photosynthetic organs such as spongy tissue might be more important for adapting to the coastal environment than that by the differences in chemical compositions. Although a previous anatomical study showed that the epidermal cells of the coastal variety of Aster hispidus Thunb. var. insularis (Makino) Okuyama (Asteraceae), were larger in size but fewer in number than those of Aster hispidus var. hispidus [17], our results indicated that the number of epidermal cells in the coastal ecotype in our study species was not lower than in the normal type. To clarify the general tendency of anatomical processes of adaptation in coastal areas, further comparative studies using another coastal ecotype need to be conducted.
In our results, the greatest morphological change from the normal type to the coastal ecotype of A. triphylla var. japonica was the increase in size. Such differences were occasionally involved in the heterochronic process, which is a model using morphological size and shape as independent variables to account for morphological differences among related taxa [18-20]. For instance, differences in leaf size and shape between the rheophytic Osmunda lancea Thunb. (Osmundaceae) and the closely related inland species O. japonica Thunb. may be related to the heterochronic process of progenesis [21]. Moreover, the leaf shapes of the species of Marsileaceae indi-
Table 2. Morphological and anatomical measurements (average ± standard deviation) of Adenophora triphylla var. japonica.

Columns marked by different letters differ significantly according to the Tukey’s HSD test (p < 0.05). 1Nonparametric pairwise comparison was conducted “Steel-Dwass test”.
cate that heterochrony plays a crucial role in leaf-shape development [22]. As for A. triphylla var. japonica, the growing areas of the coastal ecotype were warmer than those of the normal ecotype because of the proximity to the Pacific Ocean. Therefore, these differences between the coastal ecotype and normal type suggested that the coastal ecotype of A. triphylla var. japonica may have evolved from the normal type via a heterochronic process leading to pre-displacement, which is the earlier initiation of a developmental process.
Some recent studies have provided evidence for adaptation to local environments using the genetic model plant, Arabidopsis thaliana (L.) Heynh. [23-25]. This analysis has begun only recently thanks to the availability of whole genome sequences, which allows for the development of genomic tools to identify gene functions and the mechanistic basis of phenotypes in model plants [26-28]. Patterns of phenotypic diversity across environmental gradients can be indicative of adaptive responses to selection, and evaluating these patterns could lead to the identification of the genetic polymorphisms underlying these adaptive responses. As for the coastal environment, for example, a weak allele of AtHKT1;1 that drives elevated leaf Na+ in this population has been previously linked to elevated salinity tolerance using genome-wide association mapping, genetic complementation, and gene expression analyses; and the geographical distribution of this allele revealed its significant enrichment in populations associated with the coast and saline soils [29]. Therefore, similar genomic comparative analyses of this allele between the coastal ecotype and the normal type of A. triphylla var. japonica will extend the study of coastal adaptation in this species. In addition, a comparison of the molecular mechanisms involved will provide an integrated functional perspective on the coastal adaptation of A. triphylla var. japonica.
Our study, along with [11,12], indicated that A. triphylla var. japonica can be categorised into serpentine, rheophytic, and coastal ecotypes in addition to the normal type. A. triphylla var. japonica f. violacea (H.Hara) T.Shimizu was distributed in the mountain areas of northern Honshu and Hokkaido, Japan [9]. Therefore, it will be very interesting to conduct comparative morphological and anatomical analyses of A. triphylla var. japonica f. violacea to understand its adaptations to mountain areas in the next stage of research.
5. Acknowledgements
We wish to thank Drs. Matsuyama K, Yoshimi Y, Yokoyama N, Isomoto S, Miyata H, Tsuchiya Y, Takei S, Sunami T, Kakimoto N, and Kumekawa Y for their help. This study was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (to TF).
REFERENCES
- Q. C. Cronk, “Plant Evolution and Development in a Post-Genomic Context,” Nature Reviews Genetics, Vol. 2, No. 8, 2001, pp. 607-619. doi:10.1038/35084556
- Y. B. Linhart and M. C. Grant, “Evolutionary Significance of Local Genetic Differentiation in Plants,” Annual Review of Ecology and Systematics, Vol. 27, 1996, pp. 237-277. doi:10.1146/annurev.ecolsys.27.1.237
- H. Greenway and R. Munns, “Mechanisms of Salt Tolerance in Nonhalophytes,” Annual Review of Plant Physiology, Vol. 31, 1980, pp. 149-190. doi:10.1146/annurev.pp.31.060180.001053
- M. J. Barbour, “Salt Spray as a Microenvironmental Factor in the Distribution of Beach Plants at Point Reyes, California,” Oecologia, Vol. 32, No. 2, 1978, pp. 213-224. doi:10.1007/BF00366073
- J. Rozema, F. Bijl, T. Dueck and H. Wesselman, “SaltSpray Stimulated Growth in Strandline Species,” Physiologia Plantarum, Vol. 56, No. 2, 1982, pp. 204-210. doi:10.1111/j.1399-3054.1982.tb00326.x
- R. Ignaciuk and J. A. Lee, “The Germination of Four Annual Strand-Line Species,” New Phytologist, Vol. 84, No. 4, 1980, pp. 581-591. doi:10.1111/j.1469-8137.1980.tb04772.x
- D. L. Erickson and D. R. Young, “Salinity Response, Distribution, and Possible Dispersal of a Barrier Island Strand Glycophyte, Strophostyles umbellata (Fabaceae),” Bulletin of the Torrey Botanical Club, Vol. 122, No. 2, 1995, pp. 95-100. doi:10.2307/2996447
- P. A. Hesp, “Ecological Processes and Plant Adaptations on Coastal Dunes,” Journal of arid environments, Vol. 21, No. 2, 1991, pp. 165-191.
- J. Okazaki, “Adenophora Fisch,” In: K. Iwatsuki, T. Yamazaki, E. B. David and H. Ohba, Eds., Flora of Japan Volume IIIa. Angiospermae Dictyedoneae Sympetalae (a), Kodansha, Tokyo, 1993, pp. 406-410.
- T. Yamanaka and K. Takezaki, “Distribution and Ecology of Rhododendron ripense Makino, with Reference to the Vegetation and Flora on Rocky River-Bank,” Journal of Japanese Botany, Vol. 34, 1959, pp. 215-224.
- K. Ohga, M. Muroi, H. Hayakawa, K. Ito, J. Yokoyama, S. Tebayashi, R. Arakawa and T. Fukuda, “Comparative Morphology and Anatomy of Non-Rheophytic and Rheophytic Types of Adenophora triphylla var. japonica (Campanulaceae),” American Journal of Plant Science, Vol. 3, No. 6, 2012, pp. 805-809. doi:10.4236/ajps.2012.36097
- K. Ohga, M. Muroi, H. Hayakawa, K. Ito, J. Yokoyama, S. Tebayashi, R. Arakawa and T. Fukuda, “Morphological and Anatomical Analyses of the Serpentine Ecotype of Adenophora triphylla var. japonica (Campanulaceae),” Journal of Plant Studies, Vol. 1, No. 2, 2012, pp. 180-187. doi:10.5539/jps.v1n2p180
- H. Tsukaya, “The Leaf Index: Heteroblasty, Natural Variation and the Genetic Control of Polar Processes of Leaf Expansion,” Plant and Cell Physiology, Vol. 43, No. 4, 2002, pp. 372-378. doi:10.1093/pcp/pcf051
- M. M. Caldwell, R. Robberecht and S. D. Flint, “Internal Filters: Prospects for UV-Acclimation in Higher Plants,” Physiologia Plantarum, Vol. 58, No. 3, 1983, pp. 445- 450. doi:10.1111/j.1399-3054.1983.tb04206.x
- C. S. Cockell and J. Knowland, “Ultraviolet Radiation Screening Compounds,” Biological reviews of the Cambridge Philosophical Society, Vol. 74, No. 3, 1999, pp. 311-345. doi:10.1017/S0006323199005356
- K. Hashiba, T. Iwashina and S. Matsumoto, “Variation in the Quality and Quantity of Flavonoids in the Leaves of Coastal and Inland Populations of Adenophora triphylla var. japonica,” Annals of the Tsukuba Botanical Garden, Vol. 24, 2005, pp. 43-52.
- Tunala, H. Hayakawa, Y. Minamiya, S. W. Gale, J. Yokoyama, R. Arakawa and T. Fukuda, “Foliar Adaptations in Aster hispidus var. insularis (Asteraceae),” Journal of Plant Studies, Vol. 1, No. 2, 2012, pp. 19-25. doi:10.5539/jps.v1n2p19
- P. Alberch, S. J. Gould, G. F. Oster and D. B. Wake, “Size and Shape in Ontogeny and Phylogeny,” Paleobiology, Vol. 5, No. 3, 1979, pp. 296-317. doi:10.1111/j.1558-5646.2007.00297.x
- E. O. Guerrant Jr., “Neotenic Evolution of Delphinium nudicaule (Ranunculaceae): A Hummingbird-Pollinated Larkspur,” Evolution, Vol. 36, No. 4, 1982, pp. 699-712. doi:10.2307/2407883
- E. M. Lord, “Floral Morphogenesis in Lamium amplexicaule L. (Labiatae) with a Model for the Evolution of the Cleistogamous Flower,” Botanical Gazette, Vol. 143, No. 1, 1982, pp. 63-72. doi:10.1086/337271
- R. Imaichi and M. Kato, “Speciation and Morphological Evolution in Rheophytes,” In: K. Iwatsuki and P. H. Raven, Eds., Evolution and Diversification of Landplants, Springer-Verlag, Tokyo, 1997, pp. 309-318. doi:10.1007/978-4-431-65918-1_15
- K. M. Pryer and D. J. Hearn, “Evolution of Leaf Form in Marsileaceous Ferns: Evidence for Heterochrony,” Evolution, Vol. 63, No. 2, 2009, pp. 498-513. doi:10.1111/j.1558-5646.2008.00562.x
- A. L. Caicedo, J. R. Stinchcombe, K. M. Olsen, J. Schmitt and M. D. Purugganan, “Epistatic Interaction between Arabidopsis FRI and FLC Flowering Time Genes Generates a Latitudinal Cline in a Life History Trait,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 101, No. 44, 2004, pp. 15670- 15675. doi:10.1073/pnas.0406232101
- J. R. Stinchcombe, C. Weinig, M. Ungerer, K. M. Olsen, C. Mays, S. S. Halldorsdottir, M. D. Purugganan and J. Schmitt, “A Latitudinal Cline in Flowering Time in Arabidopsis thaliana Modulated by the Flowering Time gene FRIGIDA,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 101, No. 13, 2004, pp. 4712-4717. doi:10.1073/pnas.0306401101
- A. Wilczek, J. Roe, M. Knapp, M. Cooper, C. M. LopezGallego, L. Martin, C. Muir, S. Sim, A. Walker, J. Anderson, J. F. Egan, B. T. Moyers, R. Petipas, A. Giakountis, E. Charbit, G. Coupland, S. M. Welch and J. Schmitt, “Effects of Genetic Perturbation on Seasonal Life History Plasticity,” Science, Vol. 323, No. 5916, 2009, pp. 930-934. doi:10.1126/science.1165826
- K. A. Shepard and M. D. Purugganan, “The Genetics of Plant Morphological Evolution,” Current Opinion in Plant Biology, Vol. 5, No. 1, 2002, pp. 49-55. doi:10.1016/S1369-5266(01)00227-8
- V. F. Irish and P. N. Benfey, “Beyond Arabidopsis. Translational Biology Meets Evolutionary Developmental Biology,” Plant Physiology, Vol. 135, No. 2, 2004, pp. 611- 614. doi:10.1104/pp.104.041632
- E. A. Kellogg, “Evolution of Developmental Traits,” Current Opinion in Plant Biology, Vol. 7, No. 1, 2004, pp. 92-98. doi:10.1016/j.pbi.2003.11.004
- I. Baxter, N. Jessica, J. N. Brazelton, D. Yu, Y. S. Huang, B. Lahner, E. Yakubova, Y. Li, J. Bergelson, J. O. Borevitz, M. Nordborg, O. Vitek and D. E. Salt, “A Coastal Cline in Sodium Accumulation in Arabidopsis thaliana Is Driven by Natural Variation of the Sodium Transporter AtHKT1;1,” PLoS Genetics, Vol. 6, No. 11, 2012, Article ID: e1001193. doi:10.1371/journal.pgen.1001193
NOTES
*Corresponding author.

