Paper Menu >>
Journal Menu >>
 Vol.2, No.5, 395-399 (201 doi:10.4236/health.2010.25059 Copyright © 2010 SciRes. http://www.scirp.org/journalT / 0) Health Openly accessible at/HEAL H The effect of detergent as polluting agent on the photosynthetic activity and chlorophyll content in bean leaves Branislav R. Jovanić1*, Srdjan Bojović2, Bratimir Panić1, Božidar Radenković3, Marijana Despotović3 1Institute of Physics, Belgrade University, Zemun, Serbia; *Corresponding Author: branislav.jovanic@ipb.ac.rs 2Institute for Biological Research “Siniša Stanković”, Belgrade, Serbia 3Faculty of Organization Science, Belgrade, Serbia Received 28 October 2009; revised 10 January 2010; accepted 12 January 2010. ABSTRACT The paper investigates effects of detergent for domestic use on the photosynthetic activity and chlorophyll content in intact bean leaves. The plants were watered for 21 days with a solution of domestic washing powder of 0.60 g r/l. It was established that the activity of photosynthetic apparatus in the plant leaf PhACNorm [%] decrea- ses exponentially with the length of plant treat- ment/watering. At the end of the treatment (21st day) the activity of photosynthetic apparatus in the dosed plant leaf was no more than 45% of that in control plant (those which were not wa- tered with detergent solution). With increased plant treatment duration the changed chloro- phyll concentration ΔChlNorm [%] rose non-linearly in plant leaves. The highest change ΔChlNorm [%] was observed on the 21st day and amounted to 12%. Keywords: Chlorophyll; Detergent; Plant; Photosynthesis; Pollution; Water 1. INTRODUCTION High technologies and technological processes are al- ways accompanied with products which pollute the en- vironment to varying extents. Very few of these products are not pollutants. This is the reason why environmental study is becoming increasingly important for the sur- vival of plant and animal world and ultimately of hu- mankind itself. It should be noted that a culprit for envi- ronmental pollution should not be sought only in out- dated or new technologies. Sources of pollution can be, which is often ignored, some domestic processes in ur- ban environment, such as food preparation or personal hygiene. The subject of this study is an investigation into water pollution resulting from everyday domestic hygi- enic procedures. There are hardly any households with- out a washing machine connected with pipes to sewage for discharge of used water with detergent. Used water is discharged into the nearest river or a lake and together with it detergent. Undoubtedly, with time detergent con- centration in the river/lake goes up and the direct conse- quence of this is a dramatic change in the biosphere. Significant pollution in ground water was observed in Tehran [1]. There are other numerous examples of pol- luted rivers and lakes with industrial detergents. For example it was found out that the Caspian Sea waters and Volga Terek, and Sulak rivers were extremely pol- luted with a high detergent concentration [2]. The Аsa River in Nigeria is dramatically polluted with industrial detergents [3]. Likewise the Coastal Zone of the Sea of Okhotsk and Avacha Bay are polluted with detergents [4]. Regardless whether it is river or lake water, it is used in gardening for watering vegetables used for human consumption. Doubtless, this water will cause changes in vegetables which can have an adverse effect on people eating them. 2. MATERIAL AND METHODS 2.1. Methods Bean (Phaseolus vulgaris L.) seeds were grown for 3 weeks. They were placed in a growth chamber adjusted to the identical growing conditions (humidity, lighting, temperature, nutrition of soil). The seedlings were wa- tered daily during all investigated periods with tap water. After this period the plants were divided in two groups: control and stress. Growing conditions were also identi- cal for control and stressed samples and the only differ- ence was the presence or absence of detergent in soil. Concentration of domestic use detergent in the water 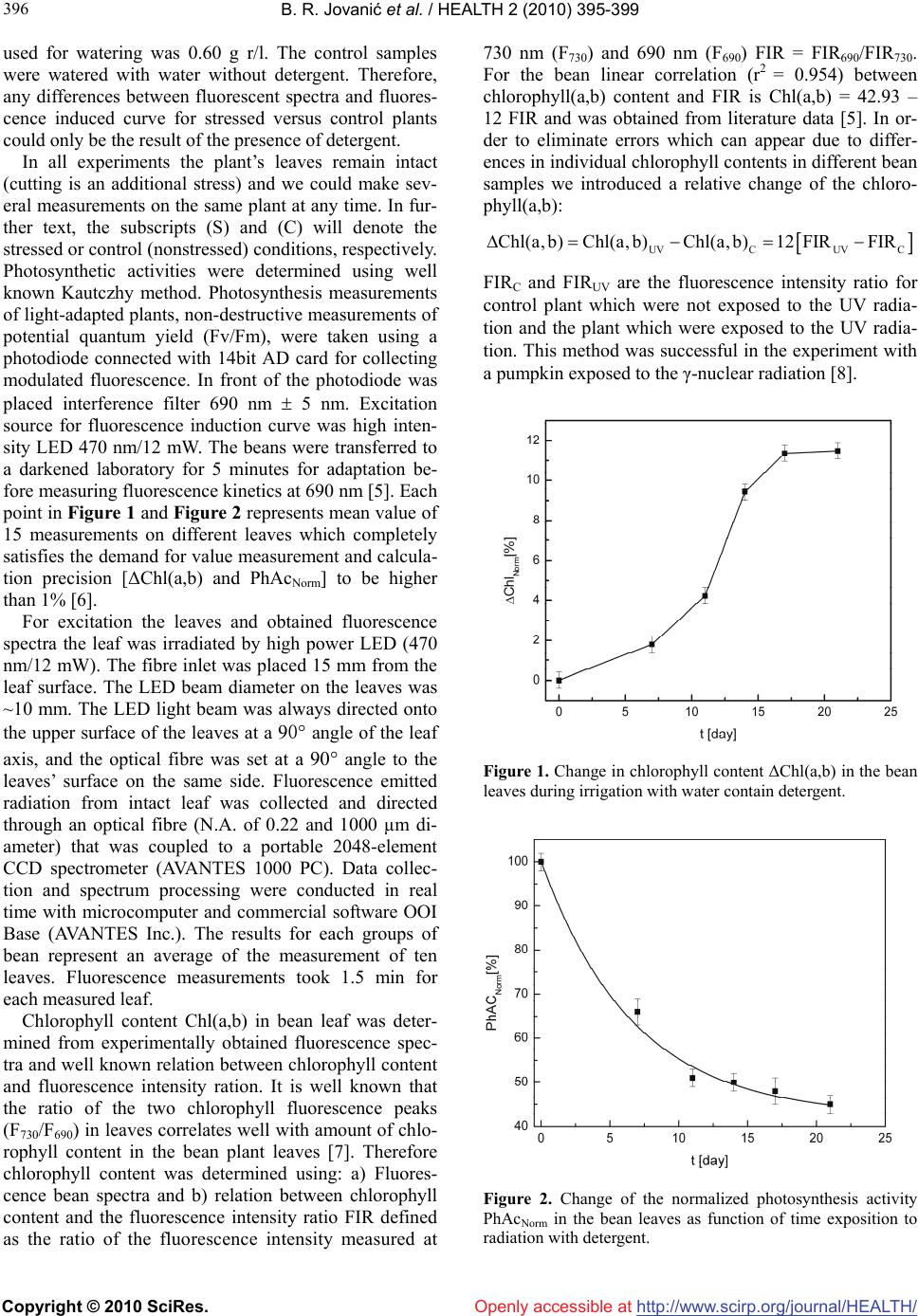 B. R. Jovanić et al. / HEALTH 2 (2010) 395-399 Copyright © 2010 SciRes. http://www.scirp.org/journalT / 396 Openly accessible at/HEALH used for watering was 0.60 g r/l. The control samples were watered with water without detergent. Therefore, any differences between fluorescent spectra and fluores- cence induced curve for stressed versus control plants could only be the result of the presence of detergent. In all experiments the plant’s leaves remain intact (cutting is an additional stress) and we could make sev- eral measurements on the same plant at any time. In fur- ther text, the subscripts (S) and (C) will denote the stressed or control (nonstressed) conditions, respectively. Photosynthetic activities were determined using well known Kautczhy method. Photosynthesis measurements of light-adapted plants, non-destructive measurements of potential quantum yield (Fv/Fm), were taken using a photodiode connected with 14bit AD card for collecting modulated fluorescence. In front of the photodiode was placed interference filter 690 nm 5 nm. Excitation source for fluorescence induction curve was high inten- sity LED 470 nm/12 mW. The beans were transferred to a darkened laboratory for 5 minutes for adaptation be- fore measuring fluorescence kinetics at 690 nm [5]. Each point in Figure 1 and Figure 2 represents mean value of 15 measurements on different leaves which completely satisfies the demand for value measurement and calcula- tion precision [ΔChl(a,b) and PhAcNorm] to be higher than 1% [6]. For excitation the leaves and obtained fluorescence spectra the leaf was irradiated by high power LED (470 nm/12 mW). The fibre inlet was placed 15 mm from the leaf surface. The LED beam diameter on the leaves was ~10 mm. The LED light beam was always directed onto the upper surface of the leaves at a 90 angle of the leaf axis, and the optical fibre was set at a 90 angle to the leaves’ surface on the same side. Fluorescence emitted radiation from intact leaf was collected and directed through an optical fibre (N.A. of 0.22 and 1000 µm di- ameter) that was coupled to a portable 2048-element CCD spectrometer (AVANTES 1000 PC). Data collec- tion and spectrum processing were conducted in real time with microcomputer and commercial software OOI Base (AVANTES Inc.). The results for each groups of bean represent an average of the measurement of ten leaves. Fluorescence measurements took 1.5 min for each measured leaf. Chlorophyll content Chl(a,b) in bean leaf was deter- mined from experimentally obtained fluorescence spec- tra and well known relation between chlorophyll content and fluorescence intensity ration. It is well known that the ratio of the two chlorophyll fluorescence peaks (F730/F690) in leaves correlates well with amount of chlo- rophyll content in the bean plant leaves [7]. Therefore chlorophyll content was determined using: a) Fluores- cence bean spectra and b) relation between chlorophyll content and the fluorescence intensity ratio FIR defined as the ratio of the fluorescence intensity measured at 730 nm (F730) and 690 nm (F690) FIR = FIR690/FIR730. For the bean linear correlation (r2 = 0.954) between chlorophyll(a,b) content and FIR is Chl(a,b) = 42.93 – 12 FIR and was obtained from literature data [5]. In or- der to eliminate errors which can appear due to differ- ences in individual chlorophyll contents in different bean samples we introduced a relative change of the chloro- phyll(a,b): UVCUV C Chl(a, b)Chl(a, b)Chl(a, b)12FIRFIR FIRC and FIRUV are the fluorescence intensity ratio for control plant which were not exposed to the UV radia- tion and the plant which were exposed to the UV radia- tion. This method was successful in the experiment with a pumpkin exposed to the γ-nuclear radiation [8]. Figure 1. Change in chlorophyll content ΔChl(a,b) in the bean leaves during irrigation with water contain detergent. Figure 2. Change of the normalized photosynthesis activity PhAcNorm in the bean leaves as function of time exposition to radiation with detergent.  B. R. Jovanić et al. / HEALTH 2 (2010) 395-399 Copyright © 2010 SciRes. http://www.scirp.org/journalT /Openly accessible at/HEALH 397 397 3. RESULTS To avoid effects of plant age on chlorophyll concentra- tion the control and dosed plants were of the same age, To avoid determining real chlorophyll concentration, relative chlorophyll concentration reduction was deter- mined: chlorophyll concentration in the dosed plants was marked ΔChlNorm as opposed to the control samples of the same age which were not treated with detergent. The reduction in chlorophyll concentration in the marked values ΔChlNorm over detergent treatment time is shown in Figure 1. Figure 1 shows that with time relative change of chlorophyll concentration increases quickly to reach its peak on the seventeenth day at about 11.5%. Obviously in this period the detergent has adverse effect on chlorophyll by destroying it constantly. After this the relative change of chlorophyll concentration remains steady at the same value. This pattern can be explained with a hypothesis that the plant adapted to the given un- favourable conditions and developed a mechanism to maintain the reduced chlorophyll concentration. When determining photosynthetic activity PhAc of photosynthetic apparatus in a plant leaf, equally when determining reduction in chlorophyll concentration in control and dosed plants, the plants were of the same age to avoid the effect of plant age. To avoid determining absolute photosynthetic activity, relative reduction of PhAc was determined. The PhAc in detergent treated plant was marked PhAcNorm to distinguish them from control samples of the same age which were not treated with detergent. The obtained changes in marked values PhAcNorm over the detergent treatment time are shown in Figure 2. Unlike chlorophyll concentration, photosyn- thetic activity PhAc constantly, exponentially decreases to reach only 45% of initial activity on the 21st day. This pattern indicates that this water has adverse effects on plants. 4. DISCUSSION Scientific papers offer data indicating different effects of different detergents on plants. In most cases detergents have adverse effects on plant pigments and morphology and inhibit metabolic processes. It was found the toxic effect of sodium dodecyl sulfate (SDS) and the house- hold synthetic detergents (HSDs) Kristall and Tix (0.1, 1, and 10 mg/l) on the diatom Alga Thalassiosira pseu- donana [9]. By the presence in water of detergents for wool domestic washings the native enzyme lost 50% of activity after 20 min of incubation [10]. The effect of detergent on plants varies depending on how the plant is exposed to it. For example, when bean was watered with 0.01% (w/v) solution the nondenaturing, zwitterionic detergent [3-{3-cholamidopropyl}-dimethylammonio]- 1-propane-sulfonate) 0.01% (w/v) it induced root hair deformation [11]. Also, in mungbean (Vigna radiata) seeds synthetic detergent induced reduction in dehydro- genase activity [12]. The leaves completely lost their turgor pressure and displayed chlorosis when they are treated with detergents [13]. The biophysical character- istics of the membrane were changed after detergent (Brij 58) treatment [14]. Detergent inhibited growth, metabolic activity took place only for 1 to 5 days, after which metabolic activity also ceased [15]. Also, high concentration of detergent might cause loss of the native configuration of β-carotene [16]. Cell growth and fission inhibition, as well as morphological changes and block- ing of chlorophyll a synthesis, were recorded at 10 mg/L concentration of detergent of household synthetic ab- stergent (HSA) [17]. When β-carotene is treated with a high concentration of detergent [18] this might cause loss of the native configuration [18]. Higher plant thyla- koid membranes can be fractionated with various deter- gents [19]. As can be seen from our data chlorophyll is sensitive to detergent which tallies with other research results. The plant treated with water content detergents showed high inhibitory effect on chlorophyll content in sunflower leaves [20]. The aggregation of chlorophyll is partly inhibited by detergent Triton X-100 [21]. In addition to reducing its concentration, detergents have other effects on chlorophyll. Studies on light harvesting complexes LHCs show that detergent-induced dissociation of LHCs and caused decline in bonding Chl b and Chl a [22]. The results on pigment-protein complexes of Pisum sativum thylakoids treated with detergent Triton X-100 and n-octyl β-D-glucopyranoside show that reversible disso- ciation of pigment-protein complexes occur [23]. Cell growth and fission inhibition on cryptophytic alga Chroomonas salina (Wils.) Butch. (Cryptophyta), as well as morphological changes and blocking of chlorophyll a synthesis, were recorded at 10 mg/L concentration of household synthetic abstergent (HSA) «Tix» [17] . In addition to the above discussed effect of detergent on chlorophyll, it was to be expected that similar possi- bly even identical effect will be observed on photosyn- thesis. Detergent-induced reversible denaturation of the photosystem reaction [24]. Detergents have strong effect on the fluorescence properties of the light-harvesting complexes of photosystem II [25]. Detergent (Triton X-100) has effect on relaxation dynamics of photosys- tem II [26]. Some results have shown that low concen- tration of nonionic detergent Triton X is sufficient for saturation of photosynthesis in terrestrial higher plants [27]. Even a short exposure to detergent effects causes extensive changes in photosynthesis. For example ex- posing for 10 min in water containing a few drops of liquid detergent induce the increase of photosyntesis [28]. It was concluded that detergent (Triton X-100) causes damage of the donor part of photosystem 2 in  B. R. Jovanić et al. / HEALTH 2 (2010) 395-399 Copyright © 2010 SciRes. http://www.scirp.org/journalT / 398 Openly accessible at/HEALH isolated chloroplasts [29]. Detergent treatment of the membranes resulted in loss of PS I activities [30]. Non-ionic detergent n-dodecyl-α, D-maltoside cause disintegration of the potosystem II (PS II) into separated PS II in stacked and unstacked thylakoid membranes from spinach [31]. The addition of the detergent Triton X-100 to the ‘chromatophore’ facilitated the photooxida- tive destruction of the antenna BChl [32]. In addition to inhibition of activities PHII detergent can cause mor- phological changes of these centres. Detergent treatment of stacked thylakoid or BBY membranes usually gives to PS II–LHC II varying size [31]. 5. CONCLUSIONS Detergents in the water for watering plants have adverse effect. In the bean plants which were watered with de- tergent water, significant changes were observed. Chlo- rophyll concentration dropped by 12%. The activity of photosynthesis apparatus in leaves decreased by around 45%. 6. ACKNOWLEDGEMENTS This work was supported by Grants No. 141007 of MNRS. Authors want to thank Mr. P. Hiddinga on kindness in supporting equipments. REFERENCES [1] Imandel, K., Razeghi, N. and Samar, P. (1978) Tehran ground water pollution by detergent. Water, Air, & Soil Pollution, 9, 119-122. [2] Korshenko, A. and GasimGul, A. (2005) Pollution of the Caspian Sea. Handbook of Environmental Chemistry, Springer-Verlag Berlin Heidelberg, 5, Part P, 109-142. [3] Adekola, B.N. and Eletta, O.A.A. (2007) A study of heavy metal pollution of Asa River, Ilorin. Nigeria; trace metal monitoring and geochemistry. Environmental Monitoring and Assessment, 12, 157-163. [4] Zhuravel, V.E., Bezverbnaya, I.P. and Buzoleva, S.L. (2004) Microbian indication of pollution of the coastal zone of the sea of Okhotsk and Avacha Bay. Russian Journal of Marine Biology, 30(2), 121-126. [5] Burdon, F., Bouchaud, J.P., Tannoudji, A. and Levy, C.C. (2002) Statistics & Laser Cooling (Paperback), Cam- bridge University Press, UK. [6] Lichthenthaler, K.H. and Buschmann, C. (1987) Chloro- phyll Fluorescence Spectra of Green Bean Leaves. Jour- nal of Plant Physiology, 129(1-2), 137-147. [7] Lichtenthaler, K.H. and Riderle, U. (1988) The role of the chlorophyll fluorescence in the detection of stress conditions in plants. CRC Critical Reviews in Analytical Chemistry, 19, S29-S85. [8] Jovanić, R.B. and Dramićanin, D.M. (2003) In vivo monitoring of chlorophyll fluorescence response to low-dose γ-irradiation in Pumpkin (Cucurbita pepo). Lu- minescence, 18, 274-277. [9] Aizdaicher, N.A. and Reunova, Yu. A. (2002) Effects of detergents on in vitro growth of diatom alga thalassiosira pseudonana. Russian Journal of Marine Biology, 28(5), 324-328. [10] Vasconcelos, A., Silva, C.J.S.M., Schroeder, M., Guebitz, G.M. and Cavaco-Paulo, A. (2006) Detergent formula- tions for wool domestic washings containing immobi- lized enzymes. Biotechnology Letters, 28(10), 725-731. [11] Ca´rdenas, L., Vidali, L., Domı´nguez, J., Pe´rez, H., Sa´nchez, F., Hepler, K.P. and Carmen Quinto, C. (1998) Rearrangement of actin microfilaments in plant root hairs responding to rhizobium etli nodulation signals. Plant Physiology, 116(3), 871-877. [12] Nand, L. and Richa, M. (2003) Synthetic detergent in- duced changes in the seed inhibition pattern and dehy- drogenese activity in mungbean (Vigna radiata). EcoEn- vConserv, 9(3), 379-383. [13] Park, J., Gu, Y., Lee, Y., Yang, Z. and Lee, Y. (2004) Phosphatidic acid induces leaf cell death in arabidopsis by activating the rho-related small G protein GTPase- mediated pathway of reactive oxygen species generation. Plant Physiology, 134(1), 129-136. [14] Behzadipou, M., Kluge, M. and Liithjea, S. (2001) Changes in plasma membrane fluidity of corn (Zea mays L.) roots after Brij 58 treatment. Protoplasma, 217, 65- 69. [15] Brandt, K.K., Hesseloy, M.E., Rosloev, E.P., Enriksen, K. and Oyrensen, J.S. (2001) Toxic effects of linear alkyl- benzene sulfonate on metabolic activity, growth rate, and microcolony formation of nitrosomonas and nitrosospira strains. Applied and Environmental Microbiology, 67(6), 2489-2498. [16] Nanba, O. and Satoh, K. (1987), Proceedings of the Na- tional Academy of Sciences, USA. 84, 109-112. [17] Reunova, Y.A. and Ayzdaycher, N.A. (2003) Effects of detergent on chlorophyll a content and quantity dynamics of microalga Chroomonas salina (Wils.) Butch. (Crypto- phyta). International Journal on Algae, 5, 106-110. [18] Mimuro, M. and Katoh, T. (1991) Carotenoids in photo- synthesis: Absorption, transfer and dissipation of light energy. Pure and Applied Chemistry, 63(1), 123-130. [19] Green, B.R. (1988) The chlorophyll-protein complexes of higher plant photosynthetic membranes or Just what green band is that? Photosynthesis Research, 15(1), 30- 32. [20] Gadallah, M.A.A. (2004) Phytotoxic effects of industrial and sewage waste waters on growth, chlorophyll content, transpiration rate and relative water content of potted sunflower plants. Water, Air, & Soil Pollution, 89(1-2), 33-47. [21] Szabad, J., Lehoczki, E., Szalay, L. and Csatorday, K. (1984) Lutein-chlorophyll-a energy transfer in detergent micelles, Biophysics of Structure & Mechanism, 1(1), 65- 74. [22] Eggink, L.L., Park, H. and Hoober, J.K. (2001) The role of chlorophyll b in photosynthesis: Hypothesis, BioMed Central. [23] Murphy, D.J. and Woodrow, I.E. (1984) The effects of Triton X-100 and n-octyl f-D-glucopyranoside on energy transfer in photosynthetic membranes. Biochemical Journal, 224(3), 989-993. [24] Liu, S., Dong, F.Q., Tang, C.Q., Kuang, T.Y., Li, L.B. and Liu, Y. (2006) Photodamage to pigment in the pho-  B. R. Jovanić et al. / HEALTH 2 (2010) 395-399 Copyright © 2010 SciRes. http://www.scirp.org/journalT / Openly accessible at /HEALH 399 399 tosystem reaction center D1/D2/Cytochrome b559 com- plex. Journal of Integrated Plant Biology, 48(7), 800- 806. [25] Moya, I., Silvestri, M., Vallon, O., Cinque, G. and Bassi, R. (2001) Time-resolved fluorescence analysis of the photosystem II antenna proteins in detergent micelles and liposomes. American Chemical Society. [26] Tang, D., Jankowiak, R., Seibert, M. and Small, G.J. (1991) Effects of detergent on the excited state structure and relaxation dynamics of the photosystem II reaction center: A high resolution hole burning study. Journal of Photosynthesis Research, 27(1), 19-29. [27] Ivanov, B.N., Ignatova, L.K. and Romanova, A.K. (2007) Diversity in forms and functions of carbonic anhydrase in terrestrial higher plants. Russian Journal of Plant Physiology, 54(2), 143-162. [28] Santacruz-Ruvalcaba, F., Gutiérrez-Pulido, H. and Rodríguez-Garay, B. (1999) Efficient in vitro propaga- tion of agave parrasana berger. Plant Cell, Tissue and Organ Culture, 56(3), 163-167. [29] Klimov, V.V., Karapetian, N.V. and Krasnovskiĭ, A.A. (1975) The effect of detergent Triton X = 100 on the light induced changes in the fluorescence yield of chloroplasts. Journal of Molecular Biology (Mosk), 9, 219-226. [30] Katoh, S. (2003) Early research on the role of plastocya- nin in photosynthesis. Photosynthesis Research, 76(1-3), 255-261. [31] Dekker, J.P., Germano, M., Roon, H. and Boekema, E,J. (2002) Photosystem II solubilizes as a monomer by mild detergent treatment of unstacked thylakoid membranes. Photosynthesis Research, 72(2), 203-210. [32] Vernon, L.P. (2003) Photosynthesis and the Charles F. Kettering Research Laboratory. Photosynthesis Research, 76(1-3), 379-388. |

