Applied Mathematics
Vol.05 No.10(2014), Article ID:46590,10 pages
10.4236/am.2014.510147
Formulation of a Vector SIS Malaria Model in a Patchy Environment with Two Age Classes
Josephine Wairimu1,2*, Sallet Gauthier2, Wandera Ogana1
1School of mathematics, University of Nairobi, Nairobi, Kenya
2INRIA, Metz and University of Lorraine, Metz, France
Email: *jwndirangu@uonbi.ac.ke
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 March 2014; revised 6 April 2014; accepted 13 April 2014
ABSTRACT
We formulate an SIS model describing transmission of highland malaria in Western Kenya. The host population is classified as children, age 1- 5 years and adults, above 5 years. The susceptibility and infectivity of an individual depend on age class and residence. The large scale system with 6n equations is reduced into a compact form of 3n equations by a change of variables. Then 3n equations are vectorialized using the matrix theory to get a one dimension, compact form of the system, equation in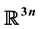 . Using Vidyasagar theorem [1] , the graph of the reduced system is shown to be strongly connected and the system is a monotone dynamical system. This means that circulation of malaria parasites among the species and among the patches is strongly connected, hence transmission is sustained. We show that for the n-dimensional age structured system the positive orthant is positively invariant for all positive values of the variables.
. Using Vidyasagar theorem [1] , the graph of the reduced system is shown to be strongly connected and the system is a monotone dynamical system. This means that circulation of malaria parasites among the species and among the patches is strongly connected, hence transmission is sustained. We show that for the n-dimensional age structured system the positive orthant is positively invariant for all positive values of the variables.
Keywords:
Highland Malaria, Differentiated Susceptibility and Infectivity, Monotone Dynamical Systems, Age Structure

1. Introduction
In Kenya, malaria is the leading cause of morbidity and mortality. It accounts for 30% of all outpatient attendances and 19% of all admissions to health facilities. The infection ranges from intense in the lowland to endemic in the highlands causing havoc to the public health system. About 20% of all deaths in children under five result from malaria (Ministry of Health-Kenya, 2006). People living around the lake, the coast, and the Western highlands epidemic-prone districts have 20% risk to be infected.
In the Western Kenya highlands, the risk of infection is 70%. The climate and topography influence the epidemic magnitudes. Drainage quality and rainfall determine vector breeding. The U-shaped valleys are broad and with slow moving rivers with poor drainage. This favor mosquitoes and high malaria infection and incidence.
The V-shaped valleys with narrow bottoms and fast flowing rivers with good drainage are less favorable to mosquitoes. The plateaus are flat, but have good drainage. Their ecosystem resembles the V-shaped valleys without dams. The terrain can modify the transmission of malaria. The V-shaped and the U-shaped valleys are separated from each other. In Kenya malaria is a “traveling disease”. 80% percent of the people treated for malaria in Kibera had travelled out of Nairobi. The neighboring estate is inhabited by people who originate from Lake Victoria region where the disease is widespread. By migrating to Nairobi, they are less exposed to malaria, thus loose the semi-immunity they used to have. This is why they contract it easily when traveling upcountry.
Children between 1 and 5 years of age are easily infected [2] . They are not bitten in same way as adults, [3] [4] . Most deaths occur in infants and parasitemia levels of infected individuals decrease with age [5] . The large areas affected by malaria make the spraying of every house impossible, [6] . Hyman [7] , formulated a general differential susceptibility and differential infectivity model to prove that the disease free equilibrium is globally stable when 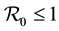 and unstable otherwise. When
and unstable otherwise. When 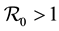 there exists an endemic equilibrium, which solutions approach asymptotically. The model can only be used for direct transmission. Pongsumpun [8] modelled the influence of age structure in an SIS model for dengue hemorrhagic fever (DHF). They showed that age structure reduces the periods of oscillations on the susceptible and infected human population and on the infected mosquito population. The difference with us is the metapopulation setting, the differentiated patch, age susceptibility and infectivity. Gao and Ruan [9] examined how population dispersal affects malaria spread between patches. The residents migrate to other patches instead of making short visits as we shall assume. There is no age structure. They showed that travel can lead the disease to become endemic in both patches, even though the disease dies out in each isolated patch. Auger [10] modified Ross [11] model to
there exists an endemic equilibrium, which solutions approach asymptotically. The model can only be used for direct transmission. Pongsumpun [8] modelled the influence of age structure in an SIS model for dengue hemorrhagic fever (DHF). They showed that age structure reduces the periods of oscillations on the susceptible and infected human population and on the infected mosquito population. The difference with us is the metapopulation setting, the differentiated patch, age susceptibility and infectivity. Gao and Ruan [9] examined how population dispersal affects malaria spread between patches. The residents migrate to other patches instead of making short visits as we shall assume. There is no age structure. They showed that travel can lead the disease to become endemic in both patches, even though the disease dies out in each isolated patch. Auger [10] modified Ross [11] model to  patches without vectors migration. They model assumed that susceptibility and infectivity are similar in all patches.
patches without vectors migration. They model assumed that susceptibility and infectivity are similar in all patches.
Motivated by the work of Auger [10] and Pongsumpun [8] , we formulate an age structured model of malaria with susceptibility and infectivity depending on residence patch.
The region is subdivided into homogenous patches, 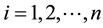 and we distinguish children, aged 1-5, from adults, over 5 years of age. The two age groups are allowed to visit other patches other than their residences. Children move with the same rate as adults. Mosquitoes fly between patches less than 2 km apart, the approximate distance a mosquito can travel, Lutambi [12] and Kelly [13] .
and we distinguish children, aged 1-5, from adults, over 5 years of age. The two age groups are allowed to visit other patches other than their residences. Children move with the same rate as adults. Mosquitoes fly between patches less than 2 km apart, the approximate distance a mosquito can travel, Lutambi [12] and Kelly [13] .
2. The Model
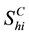 denotes the total number of susceptible children in patch
denotes the total number of susceptible children in patch ,
, 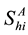 denotes the total number of susceptible adults in patch
denotes the total number of susceptible adults in patch .
.  denotes the total number of infected children in patch
denotes the total number of infected children in patch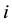 ,
, 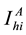 denotes the total number of susceptible adults in patch. The vector population is likewise identified by
denotes the total number of susceptible adults in patch. The vector population is likewise identified by 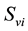 and
and 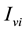 for the susceptible and infective vectors. The constant total human population comprises all hosts in all the patches:
for the susceptible and infective vectors. The constant total human population comprises all hosts in all the patches: . So that
. So that 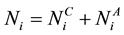 denotes the total host population in patch
denotes the total host population in patch








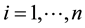
The total host population on patch 



Figure 1. Transfer diagram for the different epidemiological groups in different patches.
where 


We define the vectors





and the matrix 
The total population of mosquitoes 
If all the patches are sufficiently distant from each other, then mosquitoes do not migrate and
The dynamics of the total host and vector populations on patch 

where 
and the matrix 
The migration equation becomes

We denote
• 

• 


• 


• 

• 
• 
• 



• 
• 
• 



The total host and vector populations are constant in all the patches. The parameter 

For patches 


The term 

tions mosquitoes using frequency dependent transmission and a non constant host population in patch






We will assume in the sequel that 
The rationale for this assumption is that the rate of recovering, for an adult or a child, is considerably greater
than the mean sojourn time in the compartment of childhood. Actually 
The complete system is given by Equations (4) and (5).
3. Reduced System
It turns out that system given by Equation (5) can be rewritten in a triangular form so we need the following theorem to reduce such a system and thus study a smaller system.
Theorem 3.1 (Vidyasagar) Consider the following 

If 







To apply the above Vidyasagar theorem we would need to prove the stability analysis of first equation, then we would only have to test the stability of the infection equation.
From Equation (5) if we add the first and third equation together we get
Which gives
We note that the matrix
For a matrix 


We have now
This proves that the stability modulus of 
implying that this Metzler matrix is non singular, which in turn implies the opposite, that is, its inverse is nonnegative [15] [16] . Therefore the equilibrium of this linear system is given by
and is globally asymptotically stable.
A similar result is obtained for the adult population with an equilibrium denoted by
and for the mosquito population with an equilibrium denoted by
Reduction Process
We will now give different expressions for the equation of our system. Depending on the case at hand we will use the most convenient form to give the properties of this system and the corresponding proofs.
Using



This system is clearly triangular if we consider the first variables

We set
Then Equation (8) can be written, in a vectorialized way, as

For another variable change, we set
Rewriting system (9) in terms of



where we define the matrices
Finally, we will make a final “vectorization” of the system
We observe that 




Indeed the stability modulus of 
where the minimum is taken over the components of the 3 positive vectors.
We have
Since the matrices involved are Metzler matrices, this implies the following inequality for the corresponding stability modulus
The relation 



Using the preceding matrices and the vector 

This system evolves on the unit cube of
4. Basic Properties of the Model
For any index 







Proposition 4.1 (Positively Invariant Set)
The unit cube
is positively invariant for system (10).
Proof
To show the invariance of the unit cube
On the patch 
If
implying that 
If 
The relation 


Taking this fact into account gives
or equivalently
which gives in turn, since
The equation for 

If 
if 

Finally the equation for 

If

and if 


The proposition is proved.
Proposition 4.2
If the matrix 
Proof
We utilise the theory of monotone dynamical systems introduced by [17] [18] , developed further in [19] and applied in [10] .
System (11) is monotone if its Jacobian is a Metzler matrix on the unit cube. The Jacobian of system (11) is given by
The Jacobian 

Next, we show that the Jacobian 





It is well known that a matrix is irreducible if its associated graph is strongly connected. Then only the off diagonal terms are concerned. Then it is sufficient to prove that the matrix
is irreducible. For the associated graph we distinguish three categories of vertices : the vertices corresponding to the 









To the matrix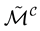





Since we have assumed that the matrix is irreducible, our graph is strongly connected, consequently the Jacobian is irreducible. We note that we are in a context of multiple species (i.e., childs, adults and mosquitoes) in a metapopulation model as conceptualized in [20] .
Our assumption simply means that the circulation of parasites is strongly connected.

5. Conclusions
In this study, we formulated an infinite model representing the spread of malaria in a heterogenous population classified. This population is classified as children (0 - 5 years) and adults (above 5 years). The infectivity and susceptibility of the population to highland malaria depend on: 1) the age class and; 2) the type of ecosystem the individual inhabits. The study captures the role played by the age of the individual and the ecosystem hetero- geneity in malaria epidemically spreading in the regions called patches. Hats are defined depending on their topography. Model properties are described to show that the solution set exists in the positive orthant which is positively invariant. The system is reduced to a single equation in
Acknowledgements
We wish to acknowledge of the Inria Metz, UMMISCO(IRD), the French Embassy in Nairobi and the Uni- versity of Nairobi, Kenya, for their financial, logistic and moral support during the writing of this article. We are very grateful to Dr. Githeko, KEMRI Kisumu for the great insight and literature he gave us during this study.
References
- Vidyasagar, M. (1980) Decomposition Techniques for Large Scale Sytems with Nonadditive Interactions: Stability and Stabilization. IEEE Transactions on Automatic Control, 25, 773-779. http://dx.doi.org/10.1109/TAC.1980.1102422
- Githeko, A.K., Ayisi, J.M., Odada, P.K., Atieli, F.K., Ndenga, B.A., Githure, J.I. and Yan, G. (2006) Topography and Malaria Transmission Heterogeneity in Western Kenya Highlands: Prospects for Focal Vector Control. Malaria Jour- nal, 5, 107. http://dx.doi.org/10.1186/1475-2875-5-107
- Wolfgang, M.R., Willem, T., Richard, C. and Bart, K. (2002) Host-Specific Cues Cause Differential Attractiveness of Kenyan Men to the African Malaria Vector Anopheles Gambiae. Malaria Journal, 1, 17. http://dx.doi.org/10.1186/1475-2875-1-17
- Smith, D.L., Guerra, C.A., Snow, R.W. and Simon, H.I. (2007) Standardizing Estimates of the Plasmodium Falciparum Parasite Rate. Malaria Journal, 6, 131. http://dx.doi.org/10.1186/1475-2875-6-131
- Tumwiine, J., Mugisha, J.Y.T. and Luboobi, L.S. (2007) On Oscillatory Pattern of Malaria Dynamics in a Population with Temporary Immunity. Computational and Mathematical Methods in Medicine, 8, 191-203.
- Wanjala, C.L., Waitumbi, J., Zhou, G. and Githeko, A.K. (2011) Identification of Malaria Transmission and Epidemic Hotspots in the Western Kenya Highlands: Its Application to Malaria Epidemic Prediction. Parasites and Vectors, 4, 81. http://dx.doi.org/10.1186/1756-3305-4-81
- Hyman, J.M. and Li, J. (2006) Differential Susceptibility and Infectivity Epidemic Models. Mathematical Biosciences and Engineering, 3, 89-100. http://dx.doi.org/10.3934/mbe.2006.3.89
- Pongsumpun, P. and Tang, I.M. (2003) Transmission of Dengue Haemorrhagic Fever in an Age Structured Population. Mathematical and Computer Modeling, 37, 949-961. http://dx.doi.org/10.1016/S0895-7177(03)00111-0
- Gao, D. and Ruan, S. (2012) A Multipatch Malaria Model with Logistic Growth Populations. SIAM: SIAM Journal on Applied Mathematics, 72, 819-841. http://dx.doi.org/10.1137/110850761
- Auger, P., Kouokam, E., Sallet, G., Tchuente, M. and Tsanou, B. (2008) The Ross-Macdonald Mode in a Patchy Envi- ronment. Mathematical Biosciences, 216, 123-131. http://dx.doi.org/10.1016/j.mbs.2008.08.010
- Ross, R. (1911) The Prevention of Malaria. Springer-Verlag, Berlin.
- Lutambi, A.M., Penny, M.A., Smith, N. and Chitnis N. (2013) Mathematical Modeling of Mosquito Dispersal in a He- terogenous Environment. Mathematical Biosciences, 241, 198-216.
- Kelly, D.W. and Thompson, C.E. (2000) Epidemiology and Optimal Foraging: Modelling the Ideal Free Distribution of Insect Vectors. Parasitology, 120, 319-327.
- Arino, J, Davis, J.R., Hartley, D., Jordan R., Miller, J. and van den Driessche, P. (2005) A Multispecies Epidemic Model with Spatial Dynamics. Mathematical Medical Biology, 22, 129-142.
- Berman, A.P. and Robert, J. (1979) Nonnegative Matrices in the Mathematical Sciences, Volume 9 of Classics in Ap- plied Mathematics. Society for Industrial and Applied Mathematics (SIAM), Philadelphia. Revised Reprint of the 1979 Original.
- Smith, H.L. (1995) Monotone Dynamical Systems: An Introduction to the Theory of Competitive and Cooperative Systems, Volume 41. Mathematical Surveys and Monographs.
- Hirsch, M.W. (1982) Systems of Differential Equations That Are Competitive or Cooperative I: Limit Sets. SIAM: SIAM Journal on Applied Mathematics, 13, 167-179. http://dx.doi.org/10.1137/0513013
- Hirsch, M.W. (1988) Systems of Differential Equations That Are Competitive or Cooperative III: Competing Species. SIAM: SIAM Journal on Applied Mathematics, 1, 51-71.
- Hirsch, M.W. and Smith, H.L. (2005) Monotone Dynamical Systems. Handbook of Differential Equations: Ordinary Differential Equations. Vol. II, Elsevier B. V., Amsterdam, 239-357.
- Arino, J. (2009) Diseases in Metapopulations. In: Ma, Z., Zhou, Y. and Wu, J., Eds., Modeling and Dynamics of Infec- tious Diseases in Series in Contemporary Applied Mathematics, World Scientific, Singapore City, 65-123. Also CDM Preprint Series Report 2008-04.
NOTES
*Corresponding author.




























































