American Journal of Plant Sciences
Vol.07 No.15(2016), Article ID:72250,15 pages
10.4236/ajps.2016.715204
Characterization and Bioaccessibility of Minerals in Seeds of Salvia hispanica L.
Aline D. Barreto1, Érika M. R. Gutierrez2, Mauro R. Silva1, Fabiano O. Silva3, Nilton O. C. Silva3, Inayara C. A. Lacerda1, Renata A. Labanca1, Raquel L. B. Araújo1*
1Faculdade de Farmácia, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
2Faculdade de Tecnologia Deputado Roque Trevisan, Piracicaba, Brazil
3Fundação Ezequiel Dias, Belo Horizonte, Brazil

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 1, 2016; Accepted: November 21, 2016; Published: November 24, 2016
ABSTRACT
Salvia hispanica L. is a herbacia plant that originates from Mexico and Guatemala, and it is currently known by the popular name of chia. Currently, chia seeds have been considered to be of great importance for human health and nutrition because they have a high concentration of polyunsaturated fatty acids. They contain the largest known percentage of fatty α-linolenic acid (ALA) in plants―approximately 68%. Furthermore, they are an excellent source of protein, dietary fiber, calcium, magnesium, iron, vitamin B and phenolic compounds that have antioxidant properties. However, despite the high nutritional value present in the food and the possible health benefits of its nutrients, there is a need to evaluate the bioaccessibility of its micronutrients to measure their effectiveness. Thus, we evaluated the chemical composition of chia seeds from different producers, their lipid profiles and the bioaccessibility of some of their minerals.
Keywords:
Salvia hispanica L., Chemical Composition, Lipid Profile, Bioaccessibility

1. Introduction
Chia seeds (Salvia hispanica L.) are originally from Mexico and Southeast and Northeast Guatemala [1] [2] . Chia seed features high levels of polyunsaturated fatty acids, and it contains the largest percentage of a fatty acid known as α-linolenic acid (ALA) in vegetables―approximately 68% [3] [4] . Additionally, it has high concentrations of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which in proper proportions reduce the risks of developing chronic diseases (DCs) [5] .
Chia seeds are also an excellent source of other macro- and micronutrients that are essential to human health because they contain high nutritional value proteins, fiber, calcium, magnesium, iron, the vitamin B complex and phenolic bioactives that have antioxidant properties [6] [7] . However, knowledge of the mineral content in food is not sufficient to evaluate the nutritional quality because every mineral is not fully available to the body after intake. Anti-nutritional factors, such as phytic acid, tannins, oxalates and other components including fibers, may interfere with the bioaccessibility of nutrients [8] .
An important parameter to evaluate the actual availability of minerals is the bioaccessibility, which estimates the proportion of micronutrients that are actually released. This term refers to the assessment of the fraction of ingested nutrient that has the potential to meet physiological demands of target tissues [9] [10] .
Therefore, this study determined the chemical composition, mineral and bioaccessibility contents, including their lipid profile and the proportionality of the constituent fatty acids, of chia seeds from three different producers. From a comparison of the results, we analyzed how external factors, such as soil, nutrition, light, altitude and climate, influence the nutritional constitution of these grains.
2. Material and Methods
2.1. Material
Chia seeds were (Salvia hispanica L.) provided from three different trademark representatives (brands A, B and C). They were crushed in a porcelain pestle at the time of analysis to reduce their size and thickness and the occurrence of lipid oxidation.
The chemical characterization of the samples was performed in Food Analyses Laboratories, Department of Food (ALM) of the Pharmacy College (FAFAR) of the Federal University of Minas Gerais (UFMG). The assessment of the lipid profile via gas chromatography was conducted in the laboratory of Biochemistry and Experimental Analysis of Food of the Department of Agribusiness, Food and Nutrition (LAN) “Luiz de Queiroz”/University of São Paulo, and the determination of the mineral content was conducted at the Ezequiel Dias Foundation (FUNED) using an optical emission spectrometer using inductively coupled plasma (ICP-OES). All glassware were in common use at the laboratory, and the reagents were of analytical grade.
2.2. Methods
2.2.1. Determination of the Chemical Composition of the Chia Seeds
The chemical composition analysis of the samples was conducted according to the methods described in AOAC [11] . The moisture content was determined by drying in a ventilated oven (Quimis, Q31M242) at 105˚C to a constant weight. The determination of total lipids was conducted in a grease matter extractor (Quimis, Q308G26) using ethyl ether as the solvent extractor (ITB 2004). For quantification of the total proteins the micro-Kjeldahl technique by using a nitrogen conversion factor of 6.25. For the analysis of the total ash content, samples were incinerated in a muffle (Coel, UL 1400) at 550˚C. The soluble and insoluble fiber contents were determined using the gravimetric enzymatic method. The carbohydrate content was calculated via the percentage difference by subtracting the total sum of the moisture, ash, lipids, proteins and dietary fiber.
2.2.2. Mineral Determination
The minerals that were analyzed included calcium, iron, phosphorus, sodium, potassium, copper and zinc, and we used the methodology described by the Adolf Lutz Institute [12] . Initially, an analytical curve was constructed for each mineral starting with a standard stock solution that had a concentration of 1000 mg/L (Sigma-Aldrich, St. Louis, MO), which was used for the preparation of standard working solutions at concentrations of 1.00; 2.00; 5.00; 7.50 and 10.00 mg/L in acidified aqueous 10% (v/v) with hydrochloric acid. The mineral standard solutions were analyzed directly using an optical emission spectrometer via inductively coupled plasma (ICP-OES) (Perkin Elmer, mod Optima 2000DV Sampler, mod As90plus) in the axial configuration, at a 1400 kw radio frequency power and a 0.60 L/min flow rate of gas. To verify the linearity of the response, we analyzed the R values for each calibration curve that was constructed for each studied mineral.
The Chia seed samples were subjected to chemical and thermal treatment to prepare the ashes and promote a greater release of mineral elements during the analysis by ICP-OES. Thus, we weighed in triplicate 1 g of each sample on an analytical balance (Shimadzu AUX 220-accurate to 0.01 mg) in porcelain crucibles, which were previously incinerated in a muffle at 550˚C (UL 1400, COEL, Brazil), dried and weighed. The crucibles that contained the samples were taken for direct combustion on the heater plate (Edutec, EEQ-9013) at 200˚C until complete carbonization of the material. Subsequently, the crucibles were placed in the furnace for incineration, according to the same heating scheme (5˚C/min for the first 2 h until a temperature of 250˚C and 10˚C/min for an additional 5 h until reaching a maximum temperature of 550˚C). Afterwards, the samples were removed from the oven, cooled in a desiccator and added to 1 ml of nitric acid. After complete evaporation of the reagent on the hot plate, the porcelain crucibles were returned to the furnace and were incinerated at 550˚C until white ashes were obtained. Later, we dissolved the mineral elements that were present in the samples. To each crucible, 1 mL of concentrated HCl and 2 mL of MilliQ water (Purifier Millipore Water, model rivers and gradient) were added, and the crucibles were heated slightly on a hot plate at 80˚C to facilitate solubilization of the sample. The samples were transferred to a volumetric flask, and MilliQ water was added to produce a total volume of 25 mL. The measurement using the ICP-OES was performed directly from this flask, and for each mineral, different dilutions were performed.
2.2.3. Determination of Mineral Bioaccessibility
To determine the mineral bioaccessibility, we used the in vitro simulated gastrointestinal digestion method proposed by Akhter et al. [13] . For this test, 5.0 g of chia seed were macerated in a porcelain mortar and transferred to 600 ml beakers. In each of these, 100 mL of deionized water was added. The solution was adjusted to pH 2 using aHCl solution of 6 mol/L. Subsequently, 5 mL of porcine pepsin solution (≥250 pcs./Mg Sigma) at 5% was added, and the mixture was incubated for 2 h under stirring in a water bath at 37˚C (Quimis, 226.11). For the in vitro intestinal digestion, semi-permeable membrane segments that were 25 cm long (Spectra/To-®, cutoff 6 to 8 kd, 23 mm thick, 1.7 mL/cm volume) and filled with 25 mL of MilliQ water (Millipore Water Purifier, Model: Rivers and gradient), adjusted to pH 7.0 with NaHCO3 solution 0.1 mol/L) were placed individually in each of the beakers and incubated again for 30 min at 37˚C in a stirred water bath. Afterwards, 50 mL of 5% bile solution/pancreatin (Synth Vetec) was added to each beaker, and it was allowed to continue to dialysis for a third incubation period in a water bath at 37˚C under stirring for 2 h. At the end of this process, the dialysis tubes were carefully removed, rinsed with deionized water and the dialyzed product was collected within the membrane and transferred to a porcelain crucible that was previously incinerated in a muffle at 550˚C (UL 1400, COEL, Brazil). These samples were dried for 8 h at 105˚C and analyzed for minerals, including calcium, iron, phosphorus, sodium, potassium, copper and zinc, using an atomic absorption spectrophotometer according to the methodology described in paragraph 2.2.1. The dialyzed minerals were calculated as:
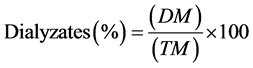
where the mineral fraction that was dialyzed is DM (mg mineral/100g Chia seeds), and TM is the total mineral content (mg mineral/100g of chia seeds).
The same procedure was performed for the whole chia seed samples to compare the differences in the bioaccessibility of whole and crushed seeds. Additionally, blank samples were analyzed to assess the presence of minerals in the reagents.
2.2.4. Determination of the Lipid Profile of the Chia Seeds
The lipids that were present in the samples were cold extracted by employing a combination of solvents that included chloroform and methanol, according to the methodology described by Bligh and Dyer [14] with modifications.
To this, 10 g samples were weighed on an analytical balance (Shimadzu AUX-220) and transferred to flasks, and 10 ml of chloroform and 20 ml of methanol were added. This mixture was stirred for 5 min in a mechanical shaker (TE 424, Tecnal) that was temperature-controlled at 25˚C. Then, an additional 10 mL of chloroform was added while stirring for 20 min. Finally, another 10 ml of chloroform was added, and the samples were again shaken for 10 min. The contents of each flask were filtered and transferred to a separatory funnel, and 10 mL of a KCl solution of 1% was added under gentle stirring. The flask was left to stand until total separation of the phases occurred. The solvent excess was evaporated on a rotary evaporator at 50˚C (Fisotom, 550), and the separated oil was used in the next step of esterification.
The contents of each flask was filtered and transferred to a separatory funnel, 10 mL of a KCl solution of 1% was added under gentle stirring, and the flask was left to stand until total separation of the phases occurred. The solvent excess was evaporated on a rotary evaporator at 50˚C (Fisotom, 550), and the separated oil was used in the next step of esterification.
After extraction of the lipids, methylation was performed using the method described by Hartman and Lago [15] , using saponification reagent (methanol solution of NaOH 0.5 mol/L) and after esterification reagent (NH4Cl/methanol chromatographic grade/ H2SO4) was added to the tubes. Finally the methyl esters were collected and stored in glass vials and sealed under a vacuum atmosphere of gaseous nitrogen, and they were stored at −20˚C until analysis.
2.2.5. Chromatographic Analysis of the Methyl Esters of Fatty Acids
Chromatographic analysis of the methyl esters that were derived from the oils extracted from the samples was conducted using a gas chromatograph (Shimadzu GC-2010 equipped with a split injector and a flame ionization detector (FID). A Stabilwax column (30 m × 0.53 mm × 1 μm) with the following isothermal program was used: 180˚C/5min, increased by 5˚C/min to 210˚C and maintained for 15 min. The carrier gas was nitrogen. The injector temperature was maintained at 180˚C, and the detector temperature was 250˚C. A standard mix of saturated and unsaturated fatty acids that contained from 8 to 22 carbons (Supelco 37 FAME mix, Sigma) was used.
2.3. Statistical Analysis of the Chemical Composition
All experiments were performed in triplicate, and the results expressed as the mean ± standard deviation. We used analysis of variance (ANOVA single factor) and Tukey’s test at 5% probability to compare the values found in all analyses using SPSS-15.0 [16] .
2.4. Statistical Analysis of the Lipid Profile
In this step, the ratio found in the lipid profile of each sample was converted to the fatty acid content. The conversion was performed according to Equation (1) [16] :
 (1)
(1)
where:
Cl = concentration of fatty acid in g/100g;
%A = % on fatty acid;
%L = % lipid in the product;
fc = conversion factor (0.956).
The experiments were performed in duplicate, and the results are expressed as the mean ± standard deviation. The tests were performed in SPSS version 15.0. We used analysis of variance (ANOVA single factor) and the analysis of the independent variable Tukey’s test at 5% probability [16] .
3. Results and Discussion
3.1. Chemical Composition
The chemical composition results of the chia seed samples, as expressed on a dry basis, are presented in Table 1. The moisture levels that were determined for brands A, B and C were 7.4%, 6.2%, and 6.9%, respectively, and between the first two, there were no significant differences, which was also observed between the first and last brand.
Values that are similar to those observed in this study were presented by Dutra et al. [17] . They determined 7.8% of moisture in the same food. The statistical variation may be due to different soil compositions of the location of cultivation, different climatic conditions, or different stages of seed maturation [18] . The investigated brands had local planting differences, which explains the influence of environmental variations in the chemical composition.
With respect to the proteins, we found high levels of 23.2%, 21.2% and 21.0% in brands A, B and C, respectively. Significant differences were observed only when comparing samples B and C. Other authors, such as Capitani et al. [7] and Ayerza and Coates [19] , showed similar values to certain proteins in this work that ranged from 16.5 to 27 g/100g. Similar levels are also shown in the USDA Nutrient Database (Table 1), and the referenced percentage is equal to 16.5% [20] .
Different studies were conducted to evaluate the chia seeds grown at several sites where small changes in the protein levels were identified. According to these studies, an increase in elevation leads to a reduction in the protein concentration due to the inversely proportional relationship between altitude and temperature, i.e., the higher the temperature, the lower the altitude. Thus, hot temperatures favor metabolic processes and protein synthesis [3] [18] . A positive relationship of cause and effect between temperature and protein content in oil crops has also been reported in soybeans [21] .
Thus, the significant difference found in this study can be justified due to variations of the planting site because the seeds that were analyzed originated from different regions of the Americas: two brands originated in southern Brazil and the other in Central America.
A particular protein concentration in chia seeds is higher than other traditional grains, such as corn (14%), wheat (14%), rice (8.5%), oats (15.3%) and barley (9.2%), and chia seeds are considered to be of high biological value because its amino acid profile does not have limiting factors for an adult diet. For the percentage of carbohydrates,
Table 1. Chemical composition (dry basis) of chia seed samples (Salvia hispanica L.).
1The carbohydrate content was calculated via the difference. Mean values ± standard deviation (n = 18) with the same subscripts of abc on the same line are not significantly different (p ≤ 5; Tukey test). *Data obtained in this study; **Figures tabulated found in the reference table of the United States Department of Agricultural (USDA), 2014.
we found a mean of 7.2%. As described in the literature, there are values ranging from 3.7% to 14.9% [2] [7] [22] [23] .
Table 2 shows the data of the total dietary fiber content and its soluble and insoluble fractions that were present in the three brands studied.
The three chia seed brands that were evaluated showed high concentrations of dietary fiber at 30.4%, 32.8%, and 31.7% for brands A, B and C, respectively. In all samples, there was a predominance of insoluble dietary fiber (28.7%) with a significant difference only between brands A and B. The same variation was also found in other total dietary fiber research. The average soluble fiber content obtained was 2.9%, and these values were not significantly different from each other.
Thus, the seeds can be considered an important source of dietary fiber, as previously reported in the literature with yields ranging from 18% to 41.7% [24] [25] [26] [27] [28] . By relating the dietary fiber found in these grain content with other foods, we can state that there were higher percentages in this food because the oats had a concentration of 9.1%, wheat was at 13.1%, soy was at 16.5%, peanut was at 8%, and walnut and almond were at 7.2% and 11.6%, respectively [20] [25] [28] .
3.2. Mineral Determination
For the ash content, the average value found was 4.8%, and significant differences between the samples were observed. Similar values were reported in the literature (4.8 to 5.1%), which confirms the suitability of this methodology [17] [20] .
Comparing our results with similar foods found in the literature, such as flaxseed (3.5%), walnut (2.1%) and peanuts (2.2%), we observed superior levels of these micro-nutrients in the seeds of chia. Thus, we conclude that this seed has a significant amount of minerals at concentrations that are higher than the aforementioned oil that is typically consumed by Brazilians.
Therefore, we suggest the incorporation of this food in meals along with other grains and oilseeds as a method of complementing the mineral contents that are present in food [7] [27] [28] . The quantification of the minerals in whole chia seeds is presented in Table 3.
Minerals that were found in greater amounts included phosphorus (779.8 mg/100g), potassium (635.2 mg/100g) and calcium (566.6 mg/100g). The USDA Food Table [20] showed lower values for potassium (407 mg/100g) and higher values for calcium (631
Table 2. Soluble, insoluble and total dietary fiber (dry basis) values present in four different samples of chia seed (Salvia hispanica L.).
Mean values ± standard deviation (n = 3) with subscripts of abc on the same line are not significantly different (p ≤ 5; Tukey test).
Table 3. Mineral contents (mg/100g) (dry basis) of the chia seed samples (Salvia hispanica L.).
Mean values ± standard deviation (n = 18) with the same subscripts of abc on the same line are not significantly different (p ≤ 5; Tukey test). Calcium (Ca), potassium (K), copper (Cu), sodium (Na), phosphorus (P), iron (Fe), zinc (Zn).
mg/100g) and phosphorus (860 mg/100g). Dutra [17] analyzed ground chia seeds and found values similar to those reported here: 659 mg/100g for phosphorus, 663 mg/100g for potassium, and 525 mg/100g for calcium.
There were significant differences between brands A and B for Ca, Na and Fe. For zinc, the results differed significantly between the three evaluated brands. These variations among the different brands can be explained by distinct plant growing conditions, such as soil type, nutrition, light, altitude and climate, because the investigated seeds originate from different locations in the Americas [3] [18] .
For Ca and P, an inter-relationship study on the metabolism of these two elements have shown that changes in Ca metabolism may be caused by variations in the levels of P and vice versa. Additionally, problems occur in P absorption when the Ca/P ratio is less than 1. An improved assimilation by the organism occurs with a Ca:P ratio of 1:1; thus, the absorption of Ca and P is proportional to the consumption of these two elements, and if there is a higher calcium content in the diet, the P absorption efficiency will be lower [29] .
In chia seeds, the ratio of Ca:P obtained in our analysis was 0.7:0.8. These values are similar to those reported by Capitani et al. [7] (from 0.5 to 0.8) and are equivalent to a normal diet in the US and Canada [30] . Thus, based on the fact that the ratio of Ca:P should be within the range 1:1 to 2:1 to prevent eating disorders, these grains can be used as a supplement along with other cereals due to the high content of minerals and adequate Ca:P ratio that is present in the seed [7] [30] .
When comparing the chia seed with other foods that are consumed daily by the Brazilian population, such as rice, flax, oats, wheat flour, soybeans, among others, there was a higher mineral content present in the chia seeds, demonstrating the nutritional benefit for incorporating this food into the population’s diet [7] [28] .
Another important mineral with high concentrations in chia seed is potassium. According to TACO [28] , silver banana has 358 mg/100g of this mineral, i.e., the seeds of chia (635.2 mg/100g) have approximately twice the concentration of this micronutrient.
3.3. Analysis of the in Vitro Bioaccessibility of Minerals
When we investigated the efficiency of the membrane to allow diffusion of the minerals through, we observed recoverable amounts of these minerals at nearly 100% (Table 4).
A small variation above and below 100% can be due to the analytical method employed, which was adapted so that the concentrations of standard patterns would fit into the linear range of the calibration curve for the analyzed mineral.
The bioaccessibility results of the mineral content in mg/100g in the chia seeds are shown in Table 5. Samples from ground chia seeds had higher bioaccessibility for the studied minerals, except for iron, when compared to the results obtained for the whole seeds. The result likely was due to the larger contact surface between the ground seeds, which facilitated the activities of digestive enzymes and subsequent release of these minerals from the food matrix to the intestinal lumen [17] .
Monroy-Torres et al. [31] evaluated various treatments of chia seeds (raw seed, toasted, hydrated, raw seed meal and toasted) and variations of protein bioaccessibility. They found that the best bioaccessibility of the grains was obtained when it is in the form of flour (80%), and the seed full presented a bioaccessibility of 29%. This may occur because the grinding exposes all kernel components, which facilitates enzyme action that is involved in digestion.
Additionally, we found that potassium showed a higher bioaccessibility content in our study (55.4 mg/100g-seed; 57.5 mg/100g-ground), and it is possible that this occurred
Table 4. Membrane efficiency percentage in the bioaccessibility analysis of the minerals in the chia seeds (Salvia hispanica L.).
Table 5. Mineral bioaccessibility (mg/100g) levels (dry basis) of the chia seed samples (Salvia hispanica L.).
Mean ± standard deviation (n = 18).
due to its high concentration in the matrix food (635.2 mg/100g) of the grain. Phosphorus, a chemical element in larger quantities in food, did not follow this trend, and there were significant differences in its contents between the whole and ground seeds.
The mineral bioaccessibility percentage followed the same pattern of results in mg/ 100g (Table 6). The element with the highest percentage of bioaccessibility was potassium, followed by calcium and phosphorus. With the exception of iron, all percentages of bioaccessibility in the ground seeds were greater than the whole. The match showed a low percentage of bioaccessibility, and we observed a wide variation between the results of whole and ground seeds. Zinc exhibited much higher results than the other elements, at 60.3% of bioaccessibility for the wholesome food and 61.6% for the ground material.
Thus, these low percentages can be attributed to the presence of tannins and phytic acid in the chia seeds. Such substances may, for example, form insoluble complexes with calcium, thereby reducing its absorption. They are thus considered to be antinutritional factors, or they may react reversible or irreversibly with other food components, impairing its absorption (bioaccessibility and bioavailability) and thus decreasing its nutritional value [32] [33] .
Tannin-type polyphenols bind to components that are present in the food matrix and form complexes through hydrogen bonds that damage the bioaccessibility of these elements, thereby reducing the nutritional value of the food [17] [34] .
Dutra et al. [17] described that chia defatted seeds showed average values of 5 mg/g tannin. Thus, the presence of this anti-nutritional factor even at low quantities, which is associated with a high percentage in these grain fibers, can interfere with the bioaccessibility of minerals.
Phytic acid is an organic acid that has chelating agents that binds minerals, such as calcium, magnesium, iron and zinc, and interfere with biological availability. It is mainly found in nut shells, seeds and grains [35] . According to Dutra et al. [17] chia defatted seeds have a mean value of 7 mg/g with a specific amount within the cereal and oil seeds (1.9 to 9.7 mg/g), and this value is smaller than the concentrations that were determined for linseed (13.4 mg/g) [36] and common beans (the 7.3 to 10.8 mg/g) [32] . Thus, as tannins, these components, when present even in low amounts and associated with high concentrations of fibers, such as the creaky of grains, can act synergistically to
Table 6. Bioaccessibility percentage of the minerals in the chia seeds (Salvia hispanica L.).
hinder the release of minerals from the food matrix. However, despite being characterized as an anti-nutritional factor, some studies indicate that phytic acid can be considered an antioxidant, acting in a beneficial lipid oxidation for diabetes and chronic disorders, such as cardiovascular disease and cancer [37] [38] .
Additionally, other features, such as the aforementioned fiber content and nature of the proteins that are present in a food, can significantly influence the bioaccessibility percentages of minerals. Thus, a plausible explanation for the observed behaviors in P, Ca, Fe, K and Cu is that these elements have a higher tendency to form coordination complexes with phytates, tannins, fibers and proteins present in these foods. However, the Zn mineral should not have this predisposition because it is more easily released into the intestinal lumen for absorption [39] .
3.4. Lipids and Lipid Profile
Regarding the percentage of lipids, significant differences were found between samples A(35.4%) and C (35.5%); however, this range was observed between B (33.5%) and brands A and C. As with proteins, the discrepancy in the results can be explained by the differences in the cultivation as mentioned above. In contrast to the above proteins, in general, the lipid content in the chia seeds tends to increase with the altitude of the planting site. Thus, it is possible to conclude that cold temperatures favor increased lipid concentration in the grains. Other factors, such as light, soil type and its chemical composition, are reported in the same manner to account for the variation in the total amount of oil present [3] [18] .
Regarding the lipid profile of chia seed, we found capric, lauric, myristic, myristoleic, palmitic, palmitoleic, stearic, oleic, linoleic, linolenic, and eicosanoic acids in the samples. Of these, five are saturated (capric, lauric, myristic, palmitic and stearic) and six are unsaturated (palmitic, myristoleic, oleic, linoleic, linolenic and eicosanoic). They have 10 to 20 carbon atoms, which characterizes them as short-chain fatty acids (TCC) that are of a medium (TCM) length.
Significant differences were observed only between the levels of the palmitic fatty acid. These variations were found between brands A and B/C.
According to Table 7, we found that the lipid fraction of chia seed is composed primarily of poly-unsaturated fatty acids, and linolenic acid (C18:3 n − 3) was the main one with an average value of 64.4%. Second, linoleic acid (C18:6, n − 6) averages at 19.5%. Ayerza et al. [3] found similar values at 63.8%, 64.8% (C18:3), 18.9%, and 22.5%, respectively (C18:6). The polyunsaturated linolenic fatty acids and linoleic acids are considered essential nutrients as humans and other mammals must obtain them via food intake [17] .
The consumption of these fatty acids (FAs) are associated with improved insulin sensitivity and diabetes prevention, as well as to reduce the risk of cardiovascular disease and to reduce anti-inflammatory effects. Moreover, they also act on the fluidity and permeability of cell membranes, in neurological functioning, nerve impulse transmission, in the transfer of atmospheric oxygen to the blood plasma, in hemoglobin
Table 7. Fatty acids (g/100g sample) contents (dry basis) of the chia seed samples (Salvia hispanica L.).
Mean values ± standard deviation (n = 18) with the same subscripts of abc on the same line are not significantly different (p ≤ 5; Tukey’s test). Values not detected (ND) = values ≤ 0.01.
synthesis and in cell division [34] [40] .
The most common sources of the type n − 3 polyunsaturated fatty acids include fish seafood, such as salmon, herring, sardines, and mackerel; however, chia seeds are also recognized as an excellent source of this constituent because they are the highest plant source of AG α-linolenic acid [40] . These fatty acids can be bioconverted in the human body, and they are the main representatives of the series omega-3 fatty acids, including EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) [40] .
The oils present in Chia seeds are therefore a good source of ALA, and they can significantly increase ALA levels of EPA and liver DHA by producing a ratio of n − 6/n − 3 that is much smaller [5] . Thus, the grains can be used as an aid in reducing dyslipidemia and to improve HDL-cholesterol, and they can act as a factor to help prevent chronic diseases [41] .
In this study, we found low levels of the n − 6 ratio: with n − 3 being, respectively, 0.31, 0.29, and 0.31. Dutra et al. [17] reported similar levels that correspond to 0.30. Thus, there is an average ratio of n6:n3 1:3.29, which is very interesting because western diets are rich mainly in providing n − 6 and ratios up to 15:1. This low ratio of n − 6: n − 3 makes the chia seed a relevant food that should be added to the Brazilian diet as an omega-3 source because various institutions recommend an intake of n6:n3 between 2:1 to 10:1.
4. Conclusion
The evaluated chia seeds showed high nutritional value and confirmed that supplementation with chia provides an interesting reduction of dyslipidemias and reduces the risk of cardiovascular diseases. Besides this, chia seeds have a concentration of phosphorus, potassium and calcium minerals that are higher than that of other cereals, such as rice, oats, wheat flour and soy. This demonstrates that the inclusion of these grains in Brazilian food can be interesting, modifying people’s consumption habits. However, we observed a low bioaccessibility content of the evaluated minerals, which may be due to the high concentration of fibers and presence of anti-nutritional factors.
Funding
The authors received financial support of the FAPEMIG, CAPES and PRPQ/UFMG for the research.
Conflicts of Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Cite this paper
Barreto, A.D., Gutierrez, É.M.R., Silva, M.R., Silva, F.O., Silva, N.O.C., Lacerda, I.C.A., Labanca, R.A. and Araújo, R.L.B. (2016) Characterization and Bioaccessibility of Minerals in Seeds of Sal- via hispanica L. American Journal of Plant Sciences, 7, 2323-2337. http://dx.doi.org/10.4236/ajps.2016.715204
References
- 1. Jamboonsri, W., Phillips, T.D., Geneve, R.L., Cahill, J.P. and Hildebrand, D.F. (2012) Extending the Range of an Ancient Crop, Salvia hispanica L.—A New ω3 Source. Genetic Resources and Crop Evolution, 59, 171-178.
https://doi.org/10.1007/s10722-011-9673-x - 2. Sandoval-Oliveiros, M. and Paredes-López, O. (2013) Isolation and Characterization of Proteins from Chia Seeds (Salvia hispanica L.). Journal of Agricultural and Food Chemistry, 61, 193-201.
https://doi.org/10.1021/jf3034978 - 3. Ayerza, R. and Coates, W. (2011) Protein Content, Oil Content and Fatty Acid Profiles as Potential Criteria to Determine the Origin of Commercially Grown Chia (Salvia hispanica L.). Industrial Crops Products, 34, 1366-1371.
https://doi.org/10.1016/j.indcrop.2010.12.007 - 4. Borneo, R., Aguirre, A. and León, A.E. (2010) Chia (Salvia hispanica L.) Gel Can Be Used as Egg or Oil Replacer in Cake Formulations. Journal of the American Dietetic Association, 110, 946-949.
https://doi.org/10.1016/j.jada.2010.03.011 - 5. Valenzuela, B.R., Masson, S., Gormaz, J.G., et al. (2012) Evaluation of the Hepatic Bioconversion of α-Linolenic Acid (ALA) to Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) in Rats Fed with Oils from Chia (Salvia hispanica) or Rosamosqueta (Rosa rubiginosa). Grasas y Aceites, 63, 61-69.
https://doi.org/10.3989/gya.057111 - 6. Ali, N.M., Yeap, S.K., Ho, W.Y., Beh, B.K., Tan, S.W. and Tan, S.G. (2012) The Promising Future of Chia, Salvia hispanica L. Journal of Biomedicine Biotechnology, 2012, 1-9.
https://doi.org/10.1155/2012/828139 - 7. Capitani, M.I., Spotornoc, V., Nolascoa, S.M. and Tomásb, M.C. (2012) Physicochemical and Functional Characterization of By-Products from Chia (Salvia hispanica L.) Seeds of Argentina. Food Science and Technology, 45, 94-102.
https://doi.org/10.1016/j.lwt.2011.07.012 - 8. Benevides, C.M.J., Souza, M.V., Souza, R.D.B. and Lopes, M.V. (2011) Fatores antinutricionais em alimentos: Revisao. Seguranca Alimentar e Nutricional, Campinas, Sao Paulo, 18, 67-79.
- 9. Miller, D.D., Schricker, B.R., Rasmussen, R.R. and Van Campen, D. (1981) An in Vitro Method for Estimation of Iron Availability from Meals. The American Journal of Clinical Nutrition, 34, 2248-2256.
- 10. Cozzolino, S.M.F. (1997) Biodisponibilidade de minerais. Revista de Nutricao, 10, 87-98.
- 11. Horwitz, W. and Latimer Jr., G.W. (Eds.) (2012) Official Methods of Analysis of the AOAC International. 19th Edition, AOAC, Gaithersburg.
- 12. IAL (Instituto Adolfo Lutz) (2004) Normas Analíticas do Instituto Adolfo Lutz. 4th Edition, Instituto Adolfo Lutz, Sao Paulo, 1032 p.
- 13. Akhter, S., Saeedb, A., Irfana, M. and Malika, K.A. (2012) In Vitro Dephytinization and Bioavailability of Essential Minerals in Several Wheat Varieties. Journal of Cereal Science, 56, 741-746.
https://doi.org/10.1016/j.jcs.2012.08.017 - 14. Bligh, E.G. and Dyer, W.J. (1959) A Rapid Method for Total Lipid Extraction and Purification. Canadian Journal Biochemistry and Physiology, 37, 911-917.
https://doi.org/10.1139/o59-099 - 15. Hartman, L. and Lago, R.C.A. (1973) Rapid Preparation of Fatty Acid Methyl Esters from Lipids. LaboratoryPractices, 22, 475-476.
- 16. Pimentel-Gomes, P. (1990) Curso Estatística de Estatística Experimental. Nobel SA, Piracicaba, 467 p.
- 17. Dutra, T.B.F., Salgado, J.M. and Risso, E.M. (2015) Caracterizacao nutricional e funcional da farinha de chia (Salviahispanica) e sua aplicao no desenvolvimento de paes. Novas Edicoes Acadêmicas, 116 p.
- 18. Ayerza, R. and Coates, W. (2009) Influence of Environment on Growing Period and yield, Protein, Oil and α-Linolenic Content of Three Chia (Salvia hipanica L.) Selections. Industrial Crops Products, 30, 321-324.
https://doi.org/10.1016/j.indcrop.2009.03.009 - 19. Ayerza, R. and Coates, W. (2005) Ground Chia seed and Chia Oil Effects on Plasma Lipids and Fatty Acids in the Rat. Nutrition Research, 25, 995-1003.
https://doi.org/10.1016/j.nutres.2005.09.013 - 20. US Department of Agriculture, Agricultural Research Service (2014) USDA National Nutrient Database for Standard Reference, Release 27.
http://www.ars.usda.gov/ba/bhnrc/ndl - 21. Kumar, V., Rani, A., Solanki, S. and Hussain, S.M. (2006) Influence of Growing Environment on the Biochemical Composition and Physical Characteristics of Soybean Seed. Journal of Food Composition and Analysis, 19, 188-195.
https://doi.org/10.1016/j.jfca.2005.06.005 - 22. Olivos-Lugo, B.L., Valdivia-López, M.A. and Tecante, A. (2010) Thermal and Physicochemical Properties and Nutritional Value of the Protein Fraction of Mexican Chia Seed (Salvia hispanica L.). Food Science and Technology International, 16, 89-96.
https://doi.org/10.1177/1082013209353087 - 23. Jin, F., Nieman, D.C., Sha, W., Xie, G., Qiu, Y. and Jia, W. (2009) Supplementation of Milled Chia Seeds Increases Plasma ALA and EPA in Postmenopausal Women. Plant Foods for Human Nutrition, 67, 105-110.
https://doi.org/10.1007/s11130-012-0286-0 - 24. Ayerza, R. (1995) Oil Content and Fatty Acid Composition of Chia (Salvia hispanica L.) from Five Northwestern Locations in Argentina. Journal of the American Oil Chemists Society, 75, 1079-1081.
https://doi.org/10.1007/BF02660727 - 25. Philippi, S.T. (2002) Tabela de composicao de alimentos: suporte para decisao nutricional. Editora Metha, Sao Paulo, 135 p.
- 26. Reyes-Caudillo, E., Tecante, A. and Valdivia-López, M.A. (2008) Dietary Fibre Content and Antioxidant Activity of Phenolic Compounds Present in Mexican chia (Salvia hispanica L.) Seeds. Food Chemistry, 107, 656-663.
https://doi.org/10.1016/j.foodchem.2007.08.062 - 27. Vuksan, V., Whitham, D., Sievenpiper, J.L., Jenkins, A.L., Rogovik, A.L., Bazinet, R.P., Vidgen, E. and Hanna, A. (2007) Supplementation of Conventional Therapy with the Novel Grain Salba (Salvia hipanica L.) Improves Major and Emerging Cardiovascula Risk Factors in Type 2 Diabetes. Diabetes Care, 30, 2804-2810.
https://doi.org/10.2337/dc07-1144 - 28. TACO (2011) Tabela Brasileira de Composicao Química dos Alimentos. 4th Edition, Nepa/Unicamp, Campinas, 104 p.
- 29. Salviano, L.M.C. and Vitti, D.M.S.S. (1997) Influência da proporcao de cálcio e fósforo na dieta, nas perdas endógenas e na absorcao de fósforo em ovinos. Embrapa, informacao tecnológica. Pernambuco, Petrolina.
- 30. Mota-Blancas, E. and Perales-Caldera, E. (1999) Los mecanismos de absorción de calcio y los modificadores de absorción como base para laelaboración de uma dieta de bajo costo para pacientes osteoporóticas. Gacetilla Médica de México, 135, 291-304.
- 31. Monroy-Torres, R., Mancilla-Escobar, M.L., Gallaga-Solórzano, J.C., Medina-Godoy, S. and Santiago-García, E.J. (2008) Protein Digestibility of Chia Seed (Salvia hispanica L.). Revista de la Facultad de Salud Pública y Nutricíon, 9, 1-9.
- 32. Guazmán-Maldonado, S.H., Acosta-Gallegos, J. and Paredes-López, O. (2000) Protein and Mineral Content of a Novel Collection of Wild and Weedy Commom Bean (Phaseolus vulgares L.). Journal of the Science of the Food and Agriculture, 13, 1874-1881.
https://doi.org/10.1002/1097-0010(200010)80:13<1874::AID-JSFA722>3.0.CO;2-X - 33. Escapa, A., Gonzales, M.C. (2001) An Overview of Analytical Chemistry of Phenolic Compounds in Foods. Critical Revews in Analytical Chemistry, 31, 57-139.
https://doi.org/10.1080/20014091076695 - 34. Santos, R.D. et.al. (2012) I Diretriz sobre o consumo de Gorduras e Saúde Cardiovascular. Arquivos Brasileiros de Cardiologia, 99, 1-40.
https://doi.org/10.5935/abc.20120202 - 35. Hurrell, F.R. (2003) Influence of Vegetable Protein Sources on Trace Element and Mineral Bioavailability. The American Society for NutritionalSciences, 133, 2973-2977.
- 36. Moura, N.C. (2008) Características físico-químicas, nutricionais e sensoriais de pao de forma com adicao de graos de linhaca (Linum usitatissimum). Dissertacao 108 (Mestrado em Ciências e Tecnologia de Alimentos), Universidade de Sao Paulo, Sao Paulo, 95 p.
- 37. Kunyanga, C.N., Imungi, J.K., Okoth, M.W., Biesalski, H.K. and Vadivel, V. (2011) Antioxidant and Type 2 Diabets Related Functional Properties of Phitic Acid Extracted from Keyan Local Food Ingredients: Effect of 107 Traditional Processing Methods. Ecology of Food and Nutrition, 50, 452-471.
https://doi.org/10.1080/03670244.2011.604588 - 38. Nawrocka-Musial, D. and Latocha, M. (2012) Phytic Acid—Anticancer Nutriceutic. Polsky Merkuriusz Lekarski, 33, 43-47.
- 39. Tognon, A.L. Quantificao e avaliacao da bioacessibilidade in Vitro, de micro e macroelementos em frutas, hortalicas e cereais. Ribeirao Preto, Sao Paulo, 111 p.
- 40. Izar, M.C.O. (2014) Monografia. Corene 3. Universidade Federal de Sao Paulo, Sao Paulo.
- 41. Chicco, A.G., D’Alessandro, M.E., Hein, G.J., Oliva, M.E. and Lombardo, Y.B. (2009) Dietary Chia Seed (Salvia hispanica L.) Rich in α-Linolenic acid Improves Adiposity and Normalises Hypertriacylglycerolaemia and Insulin Resistance in Dyslipaemicrats. British Journal of Nutrition, 101, 41-50.
https://doi.org/10.1017/S000711450899053X


