The Ecology of Keratella cochlearis in Lake Kinneret (Israel) 5
Table 3. Comparative analysis (ANOVA) between popula-
tion dynamics values (see text) and two periods: 1) 1972-
1985, and 2) 1986-2000. S = significant, NS = not significant.
Parameter Difference probability (p)
Death rate 1 < 2 <0.0001
Birth rate 1 < 2 <0.0001
Rate of change NS
Turnover time 1 > 2 0.0041
Eggs/Female 1 < 2 <0.0001
Females w. Egg NS
Individuals/L 1 > 2 0.0139
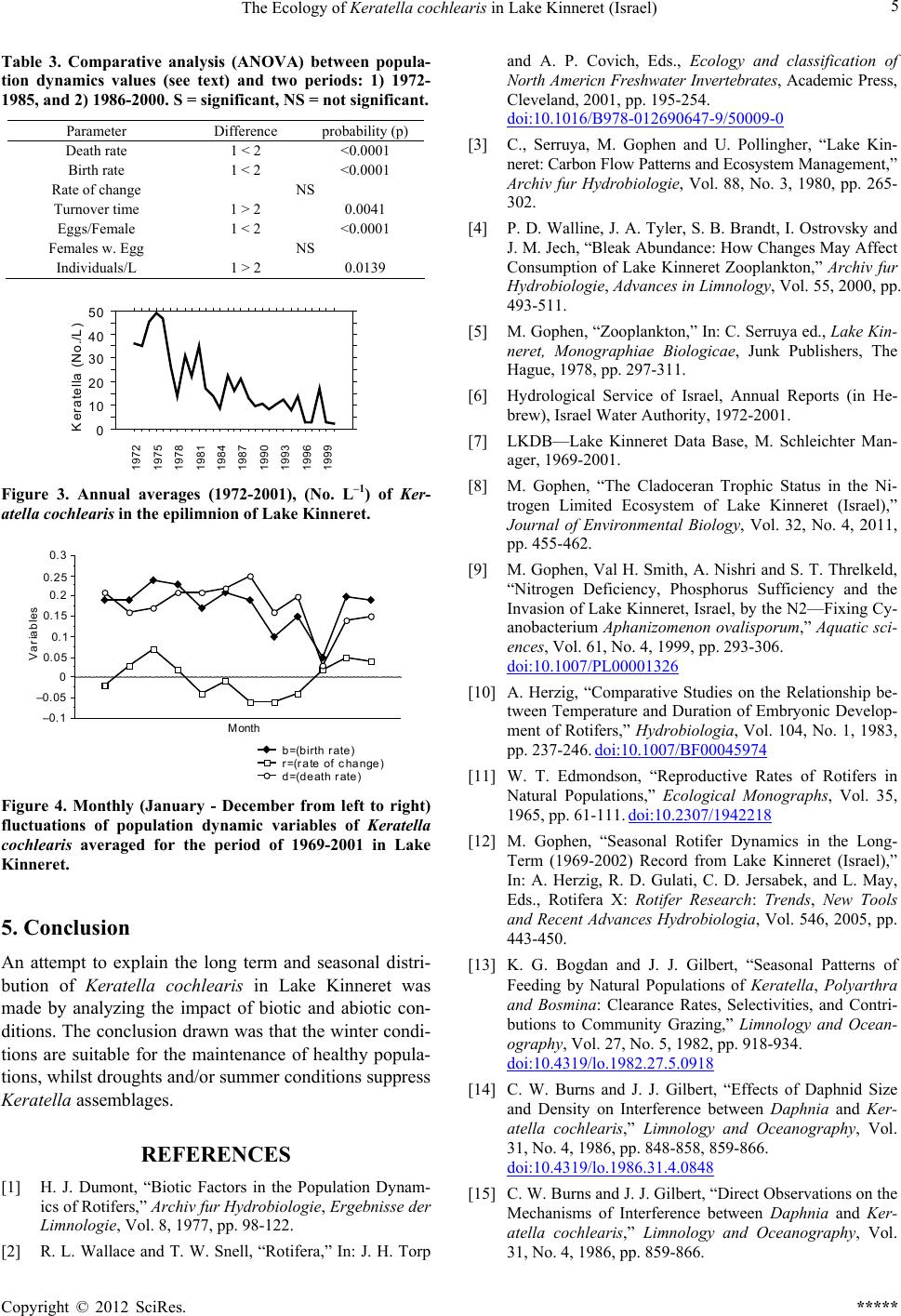
0
10
20
30
40
50
Keratella(No./L)
1972
1975
1978
1981
1984
1987
1990
1993
1996
1999
Figure 3. Annual averages (1972-2001), (No. L–1) of Ker-
atella cochlearis in the epilimnion of Lake Kinneret.
Mont h
–0 .1
–0 .0 5
0
0.05
0. 1
0.15
0. 2
0.25
0. 3
Variables
d=(death rate)
r=(rateof change)
b=(birth rate)
Figure 4. Monthly (January - December from left to right)
fluctuations of population dynamic variables of Keratella
cochlearis averaged for the period of 1969-2001 in Lake
Kinneret.
5. Conclusion
An attempt to explain the long term and seasonal distri-
bution of Keratella cochlearis in Lake Kinneret was
made by analyzing the impact of biotic and abiotic con-
ditions. The conclusion drawn was that the winter condi-
tions are suitable for the maintenance of healthy popula-
tions, whilst droughts and/or summer conditions suppress
Keratella assemblages.
REFERENCES
[1] H. J. Dumont, “Biotic Factors in the Population Dynam-
ics of Rotifers,” Archiv fur Hydrobiologie, Ergebnisse der
Limnologie, Vol. 8, 1977, pp. 98-122.
[2] R. L. Wallace and T. W. Snell, “Rotifera,” In: J. H. Torp
and A. P. Covich, Eds., Ecology and classification of
North Americn F reshwater Invertebrates, Academic Press,
Cleveland, 2001, pp. 195-254.
doi:10.1016/B978-012690647-9/50009-0
[3] C., Serruya, M. Gophen and U. Pollingher, “Lake Kin-
neret: Carbon Flow Patterns and Ecosystem Management,”
Archiv fur Hydrobiologie, Vol. 88, No. 3, 1980, pp. 265-
302.
[4] P. D. Walline, J. A. Tyler, S. B. Brandt, I. Ostrovsky and
J. M. Jech, “Bleak Abundance: How Changes May Affect
Consumption of Lake Kinneret Zooplankton,” Archiv fur
Hydrobiologie, Advances in Limnology, Vol. 55, 2000, pp.
493-511.
[5] M. Gophen, “Zooplankton,” In: C. Serruya ed., Lake Kin-
neret, Monographiae Biologicae, Junk Publishers, The
Hague, 1978, pp. 297-311.
[6] Hydrological Service of Israel, Annual Reports (in He-
brew), Israel Water Authority, 1972-2001.
[7] LKDB—Lake Kinneret Data Base, M. Schleichter Man-
ager, 1969-2001.
[8] M. Gophen, “The Cladoceran Trophic Status in the Ni-
trogen Limited Ecosystem of Lake Kinneret (Israel),”
Journal of Environmental Biology, Vol. 32, No. 4, 2011,
pp. 455-462.
[9] M. Gophen, Val H. Smith, A. Nishri and S. T. Threlkeld,
“Nitrogen Deficiency, Phosphorus Sufficiency and the
Invasion of Lake Kinneret, Israel, by the N2—Fixing Cy-
anobacterium Aphanizomenon ovalisporum,” Aquatic sci-
ences, Vol. 61, No. 4, 1999, pp. 293-306.
doi:10.1007/PL00001326
[10] A. Herzig, “Comparative Studies on the Relationship be-
tween Temperature and Duration of Embryonic Develop-
ment of Rotifers,” Hydrobiologia, Vol. 104, No. 1, 1983,
pp. 237-246. doi:10.1007/BF00045974
[11] W. T. Edmondson, “Reproductive Rates of Rotifers in
Natural Populations,” Ecological Monographs, Vol. 35,
1965, pp. 61-111. doi:10.2307/1942218
[12] M. Gophen, “Seasonal Rotifer Dynamics in the Long-
Term (1969-2002) Record from Lake Kinneret (Israel),”
In: A. Herzig, R. D. Gulati, C. D. Jersabek, and L. May,
Eds., Rotifera X: Rotifer Research: Trends, New Tools
and Recent Advances Hydrobiologia, Vol. 546, 2005, pp.
443-450.
[13] K. G. Bogdan and J. J. Gilbert, “Seasonal Patterns of
Feeding by Natural Populations of Keratella, Polyarthra
and Bosmina: Clearance Rates, Selectivities, and Contri-
butions to Community Grazing,” Limnology and Ocean-
ography, Vol. 27, No. 5, 1982, pp. 918-934.
doi:10.4319/lo.1982.27.5.0918
[14] C. W. Burns and J. J. Gilbert, “Effects of Daphnid Size
and Density on Interference between Daphnia and Ker-
atella cochlearis,” Limnology and Oceanography, Vol.
31, No. 4, 1986, pp. 848-858, 859-866.
doi:10.4319/lo.1986.31.4.0848
[15] C. W. Burns and J. J. Gilbert, “Direct Observations on the
Mechanisms of Interference between Daphnia and Ker-
atella cochlearis,” Limnology and Oceanography, Vol.
31, No. 4, 1986, pp. 859-866.
Copyright © 2012 SciRes. *****