 American Journal of Anal yt ical Chemistry, 2011, 2, 1-15 doi:10.4236/ajac.2011.228118 Published Online December 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Developments in Analytical Meth ods for Detection of Pesticides in Environmental Samples Rama Bhadekar*, Swanandi Pote, Vidya Tale, Bipinraj Nirichan Department of Microbial Biotechnology, Rajiv Gandhi Institute of Information Technology & Biotechnology, Bharati Vidyapeeth Deemed University, Pune, India E-mail: *neeta.bhadekar@gmail.com Received November 19, 2011; revised December 21, 2011; accepted December 28, 2011 Abstract The present review gives a survey of all the published methods along with their advantages and limitations. Traditional methods like thin layer chromatography, gas chromatography, liquid chromatography etc are still in use for this purpose. But some recent bio-analytical methods such as immunosensors, cell based sensors etc. have also gained equal importance. This article also overviews various electro-analytical methods and their applications as detection devices when combined with FIA and biosensors. Lastly nanoparticle based biosensors have also been discussed. The review concludes with futuristic approach to reduce the risks caused by pesticides. This scrutiny should provide concise evaluation of different techniques employed for pesticide detection in environmental samples. Keywords: Biosensors, Chromatography, Detection, Flow Injection Analysis, Nano Particles, Pesticides, Pollutants 1. Introduction People have contradictory ideas about the meaning of pesticides. The dictionary defines pesticide as a sub- stance for destroying harmful insects. The scientists are of the opinion that pesticides are chemical or biological substances that are designed to kill or retard the growth of pests interfering with the growth of crops, shrubs, trees, timber and other vegetation desired by humans. The term pesticide includes substances intended for use as plant growth regulators, defoliants, desiccants or agents for thinning fruit or preventing the premature fall of fruit. The substances applied to crops either before or after harvest to protect the commodity from deterioration dur- ing storage and transport also come under the category of pesticides [1]. Pesticides are broadly classified into two groups viz A) chemical pesticides and B) biopesticides. A) Chemical pesticides are conventionally synthetic materials that di- rectly kill or inactivate the pest. They are classified ac- cording to the type of organisms they act against as for example 1) insecticides, 2) herbicides, 3) fungicides, 4) rodenticide, 5) nematicides [2]. Insecticides include or- ganophosphates (TEPP, parathion. trimesters of phos- phates and phosphoric acids), carbamates (aldicarb), or- ganochlorines (dichlorodiphenyltrichloroethane, chlor- dane, aldrin, dielrin, lindane, endrin) and botanical insec- ticides (nicotine, rotenoids, pyrethrum). Herbicides are used to destroy other weeds that interfere with produc- tion of the desired crop. Based on their structure they are grouped into chlorophenoxy compounds (e.g.: 2,4-D, 2, 4,5-T), dinotrophenols like 2-methyl-4,6-dinitrophenol (DNOC), bipyridyl compounds like paraquot, carbamate herbicides, substituted urea, triazines and amide herbi- cides like alanine derivatives. Fungicides include a num- ber of structurally different chemicals like cap tan, folpet, pentachlorophenolziram, nambam etc. Fungicides con- taining mercury are known to cause nerve disorders [2]. Rhodenticides are designed to kill rodents, mice, squir- rels, gophers and other small animals. They vary from highly toxic one with the ability to kill an organism with one-time dose or less toxic ones requiring repeated in- gestion over a period of time. Nematicides act against nematodes like Meloidogyne incognita, Criconemella xenoplax etc. B) Biopesticides are pesticides derived from natural sources like animals, plants, bacteria, and certain miner- als. For example, canola oil and baking soda have pesti- cidal applications and are considered biopesticides. Bio- pesticides fall into three major classes:  R. BHADEKAR ET AL. 2 1) Microbial pesticides consist of microorganisms like bacteria, fungi, viruses or protozoa as the active ingredi- ents. They can control many different kinds of pests, although each with separate active ingredient that is rela- tively specific for its target pest(s). 2) Plant-Incorpo- rated-Protectants (PIPs) are pesticidal substances that are produced by genetically modified plants for example: introduction of Bt toxin gene in the cotton plants. 3) Biochemical pesticides are naturally occurring substances that control pests by non-toxic mechanisms (for e.g. in- sect sex pheromones that interfere with mating as well as various scented plant extracts). Biopesticides are envi- ronmentally safe and non toxic to plants and animals. However, their use is limited due to 1) less social aware- ness, 2) comparatively lower crop yields, 3) need for frequent applications, 4) less worked research area. On the contrary, application of chemical pesticides has proved to be economically beneficial and hence their use has increased globally especially after the advent of “Green Revolution”. The productivity of crop has been increased by use of suitable pesticide. They protect the crop from disease causing organisms, from plant patho- gens and also from vector borne diseases. Another im- portant advantage is reduction in cost of labor. Even though pesticides play significant role in agri- culture they are the most important environmental pol- lutants. This is due to their wide spread presence in water, soil, atmosphere and agricultural products. Currently it poses major threat not only to living organisms but also to environment specially ground and surface water. Syn- thetic pesticides affect the growth of plants. Chemical compounds in the pesticides are not biodegradable. This causes their sedimentation near plant roots making the supply of essential NPK inefficient. This inefficiency hinders growth of crops and their resistance to other harmful microbes. Pesticides percolate into the soil and get mixed with ground water. This causes draining of pesticides into the nearby stream or lake. This in turn adversely disturbs the aquatic eco system. Soil is another important component for plant growth. Pesticides ham- per the fertility of soil by inhibiting the storage of nitro- gen and other essentials in soil. Light and toxic com- pounds are suspended in air by pesticide spray. This causes air borne diseases and nasal infections. Besides all the environmental hazards; pesticides pose serious risk to mankind. Health hazards caused by some of the pesti- cides are summarized in Table 1. Different pesticides have different acceptable residual levels and these are set up by World Health Organization (WHO), European Community (EU), FAO (Food and Agricultural Organi- zation) of UN, US environmental protection agency (EPA) and the US National Institute for Occupational Safety and Health (NIOSH) [3-5]. The Toxicity of pesti- cides, made it essential to have accurate and reliable me- thods of monitoring their levels for safety purposes. Ear- lier techniques used for pesticide detection were chroma- tographic methods like Gas Chromatography (GC), High Performance Liquid Chromatography (HPLC) along with Mass Spectrometry (MS) etc. They were sensitive and reliable. However, they had limitations like 1) complex procedure, 2) time consuming sample treatments, 3) need of highly trained technicians, 4) inability to perform on site detection etc. To improve these methods newer tech- niques are being developed. The new techniques use more sensitive devices like chromatographic techniques with various detection methods, electro analytical tech- niques, chemical and biosensors, spectroscopic tech- niques and flow injection analysis (FIA). Sometimes a combination of one or more methods proved successful in detecting a particular class of pesticide. This article presents an all embracing survey of the classical methods along with update knowledge of recent advances in the techniques. 2. Spectrophotometry This was a widely used method for the detection of pes- ticide residues from environmental samples. Spectro- Table 1. Harmful effects of pesticides on humans. Type of pesticide Effects observed Ref. Organophosphates Adversely affects nerve functioning, direct exposure can cause eye problems like blurring of vision, reddening, retardation in fetal growth etc. [6,7] Chlorides Disruption of dopamine transport in the brain, increased risk of lung and pancreatic cancer, neutrophil inflammation etc. [8] Methyl Bromide Increase in serum albumin level [9] Mercury containing fungicides Nerve disorders [10] Fungicides like atrazines, amides, etc. Irritation of skin and eyes, slowing of heart beats, weakness of muscles, central nervous system disorders etc. [11] Rhodenticides like Strychnine Sodium monofluoroacetate Thallium, etc. Complete loss of hair, paresthesias, nausea, vomiting and abdominal pain, pulmonary oedema bronchopneumonia, diaphoresis, blurred vision and severe symmetric extensor muscle spasms[12] Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL.3 photometry measures the amount of light absorbed by the analyte solution and this amount of light is directly proportional to the quantity of the analyte. The technique is based on two properties of light: 1) particle nature of light and 2) wave nature of light. The former gives rise to photoelectric effect and the latter results in formation of visible spectrum of light. Normally white or UV light is used as a source of light. The beam of light splits into its component wavelengths after passing through the prism. Light of different wavelengths is absorbed by different analyte solutions to different extent depending on analyte concentration. The analyte particles absorb photons and then the unabsorbed photons are converted into electrical signal by the phototube. The detection unit then records the difference in the intensity of light. The difference in the intensities of source beam and the beam coming out of the analyte determines the concentration of the analyte. The components of a spectrophotometer are 1) source of light, 2) cell containing analyte solution, 3) phototube, and 4) detection unit. Use of this technique for detection of atrazine and dicamba herbicides from water samples was described by Hernández et al., (2005). The authors reported detection limits (LOD) of 0.1 µg/ml for atrazine and 0.2 µg/ml for dicamba [13]. Moreover spectropho- tometric detection methods were also found suitable for detection of organopesticides such as malathion, phorate and dimethoate from food samples. The procedure was based on oxidation of organophosphoours pesticides with slight excess of N-bromosuccinimide. The unconsumed N-bromosuccinimide was then reacted with rhodamine B which was followed by spectrophotometric estimation of decrease in color at 550 nm. The sensitivity of the meth- ods was up to µg/g [14]. Even with limited success in these methods, some drawbacks were evident. They were 1) extensive sample preparation, 2) relatively slow and 3) could not be used for real time estimation. Hence these days spectropho- tometric methods are used only for detection of limited number of pesticides. Sometimes they are coupled with other systems as terminal detection devices to detect pes- ticides. 3. Electroanalytical Techniques Electroanalytical techniques have gained importance for analysis of environmental samples. Their main advan- tages are simplicity in operation, sensitivity, selectivity, portability and so on. Commonly used electroanalytical techniques are: potentiometry, conductometry, voltametry, amperometry etc [15]. The basic principles of these tech- niques are discussed below. 3.1. Potentiometry Potentiometry measures the potential of electrochemical cells. A potentiometric cell is composed of i) reference electrode ii) salt bridge iii) analyte solution and iv) indi- cator electrode. The commonly used reference electrodes are hydrogen electrodes, calomel electrodes or silver/ silver chloride electrodes. The indicator electrodes can be either metallic or ion selective. The salt bridge acts as a barrier between the standard electrode and the analyte solution. Potentiometric methods are governed by Nernst equation. The potential (E) is calculated as (1) [16,17]. E cellE indicatorE reference (1) 3.2. Conductometry It is based on the property of electrolyte solutions to dis- sociate into ions. It measures the change in electrical resistance of a solution. A conductometric cell consists of 1) two electrodes: Anode (positively charged) and cathode (negatively charged) 2) an electrolyte solution and 3) battery (current reading detection unit). The number of ions determines the amount of current gener- ated which indicates the concentration of electrolytes. The electrolytic properties of a conductor are described by Ohm’s law (2) and the conductance is given by (3) [18, 19]. VIR (2) Equation (2) is V (voltage), I (current), R (electrical resistance) G1R (3) Equation (3) is G (conductance). 3.3. Voltametry It measures the change in the current—potential charac- teristics of an electrochemical cell. This change is di- rectly proportional to the concentration of the analyte. The current—potential relationship is dependent on the mass transfer rate. It is the rate at which the electroactive species generated due to oxidation reduction reactions reach the electrode. This mass transfer can be due 1) ionic migration (formed due electrochemical gradient) 2) diffusion under a chemical potential difference or 3) bulk transfer. In voltametry the potential applied is usually varied as a function of time. Based on this voltametry is grouped into A) linear voltametry and B) cyclic Volta- metry. In former the potential applied to the electro- chemical cell is gradually increased. In latter, the poten- tial is varied between a fixed lower and upper value [20, 21]. Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL. 4 Amperometry Amperometry can be considered as a sub-class of volta- metry since both the procedures depend on the same principal. The only difference in voltametry and am- perometry is that in amperometry the potential applied across the cell is constant. It measures the current gene- rated due to the oxidation-reduction reactions taking place in the analyte solutions. The electroanalytical techniques are described in detail by Bard et al. [22]. Many variations in these techniques have been reported in literature. For instance amperome- try and potentiometry are coupled together for quantify- cation of analytes. One or more of these techniques are combined with other methods like chromatography, bio- sensors, flow injection analysis etc. for pesticide analysis from environmental samples. Applications of these tech- niques are discussed below. 4. Chromatographic Techniques Chromatographic techniques are among the first few techniques that were put to use for pesticide detection. As technology developed various modifications have been made in basic chromatography. However all forms of chromatography utilize the property of the analyte to distribute itself between two immiscible phases (X and Y). This co-efficient of distribution remains constant at a particular temperature and is given by (4) abCoefficient of distribution (4) Equation (4) is a = concentration of analyte in X, b = concentration of analyte in b. Every chromatographic system consists of two phases viz. stationary phase which is immobilized (solid, gel, liquid or mixture of solid and liquid) and a mobile phase which is passed over the stationary phase (gas, liquid). While performing the method the analytes continuously move between the two phases. They get separated from each other because of the difference in their distribution co-efficient. A typical chromatographic unit is made of stationary phase, mobile phase, a column, injector sys- tem, a detector, chart recorder and fraction collector. The performance of the system depends mainly on three fac- tors; 1) Retention time (T) (5) 2) retention factor which is the time taken by the analyte bound to the stationary phase to elute from the column relative to the time taken by the free analyte and 3) column height and resolution. T = Tx – Ty (5) Equation (5) is Tx: Time for which the stationary phase retains the analyte. Ty: Time taken by the analyte to bind to the stationary phase. Chromatographic analysis requires sample preparation. This makes the technique more time consuming. The main steps of sample preparation are: 1) solvent extrac- tion (for example by acetone or acetonitrile) or solid phase extraction, 2) column switching (beneficial for HPLC: here the analyte is adsorbed on a suitable ad- sorbtant. The impurities are washed and then the analyte is eluted with an appropriate organic solvent.), 3) super- critical fluid extraction (gases for example, liquid carbon dioxide is used for solvent extraction) and 4) sample de- rivatisation (involves covering of functional groups in the analyte, adversely affecting the chromatographic de- tection). After a pesticide has been extracted and isolated from the sample, it is further separated from other coex- tractives. It makes use of gas chromatography or liquid chromatography or, less frequently thin layer chroma- tography [23]. 4.1. Thin Layer Chromatography (TLC) In TLC the stationary phase is bound to a glass or a met- al plate. The sample is spot inoculated or applied as a thin band near the end of the plate. The mobile phase flows over the stationary phase by capillary action. Se- paration of analytes takes place by adsorption or partition or ion exchange or molecular exclusion depending on the type of stationary phase. The movement of the analyte depends on the retardation factor (6) Retardation factorxy (6) Equation (6) is x = distance traveled by analyte from start point, y = distance traveled by mobile phase from start point. TLC is usually followed by detection of compounds by i) examining the plate under UV, ii) spraying the plate with reagent which reacts with the compound to form coloured products, iii) use of fluorescent dye iv) by radio labeling the analytes and observing them by radiography. The separated compounds can be quantified with a preci- sion densitometer. A number of modifications in TLC technique are used to detect pesticides. They are listed below. 4.1.1. TLC Bioassay This technique described by N. K. B. Ardikaran et al. (2009) uses a TLC plate sprayed with spores of Ca- dosporium cladosporioides for detection of fungicides. Here the presence of pesticide is confirmed by absence of fungal growth around the sample spot [24]. Copyright © 2011 SciRes. AJAC 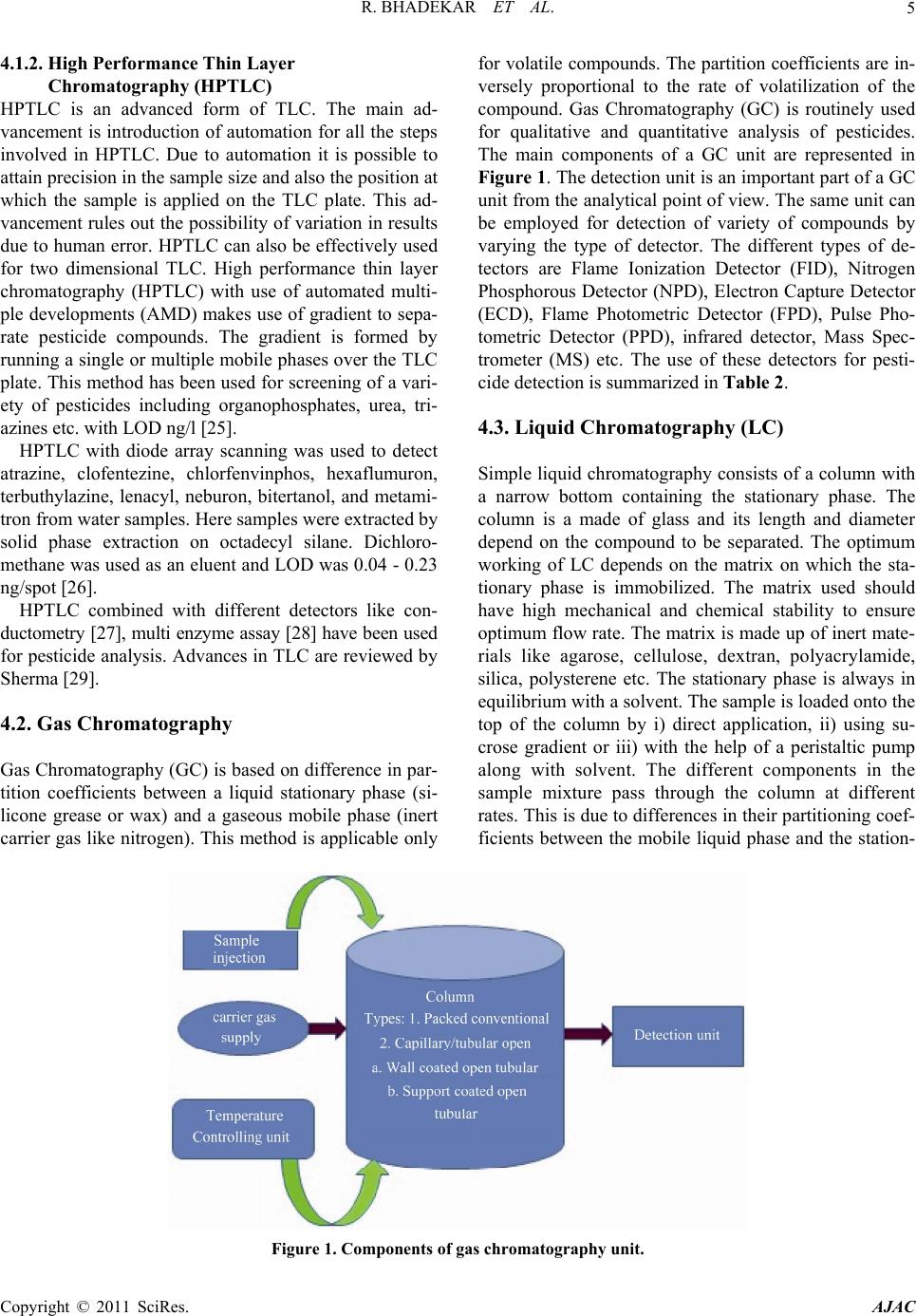 R. BHADEKAR ET AL. Copyright © 2011 SciRes. AJAC 5 4.1.2. High Performance Thin Layer Chromatography (HPTLC) HPTLC is an advanced form of TLC. The main ad- vancement is introduction of automation for all the steps involved in HPTLC. Due to automation it is possible to attain precision in the sample size and also the position at which the sample is applied on the TLC plate. This ad- vancement rules out the possibility of variation in results due to human error. HPTLC can also be effectively used for two dimensional TLC. High performance thin layer chromatography (HPTLC) with use of automated multi- ple developments (AMD) makes use of gradient to sepa- rate pesticide compounds. The gradient is formed by running a single or multiple mobile phases over the TLC plate. This method has been used for screening of a vari- ety of pesticides including organophosphates, urea, tri- azines etc. with LOD ng/l [25]. HPTLC with diode array scanning was used to detect atrazine, clofentezine, chlorfenvinphos, hexaflumuron, terbuthylazine, lenacyl, neburon, bitertanol, and metami- tron from water samples. Here samples were extracted by solid phase extraction on octadecyl silane. Dichloro- methane was used as an eluent and LOD was 0.04 - 0.23 ng/spot [26]. HPTLC combined with different detectors like con- ductometry [27], multi enzyme assay [28] have been used for pesticide analysis. Advances in TLC are reviewed by Sherma [29]. 4.2. Gas Chromatography Gas Chromatography (GC) is based on difference in par- tition coefficients between a liquid stationary phase (si- licone grease or wax) and a gaseous mobile phase (inert carrier gas like nitrogen). This method is applicable only for volatile compounds. The partition coefficients are in- versely proportional to the rate of volatilization of the compound. Gas Chromatography (GC) is routinely used for qualitative and quantitative analysis of pesticides. The main components of a GC unit are represented in Figure 1. The detection unit is an important part of a GC unit from the analytical point of view. The same unit can be employed for detection of variety of compounds by varying the type of detector. The different types of de- tectors are Flame Ionization Detector (FID), Nitrogen Phosphorous Detector (NPD), Electron Capture Detector (ECD), Flame Photometric Detector (FPD), Pulse Pho- tometric Detector (PPD), infrared detector, Mass Spec- trometer (MS) etc. The use of these detectors for pesti- cide detection is summarized in Table 2. 4.3. Liquid Chromatography (LC) Simple liquid chromatography consists of a column with a narrow bottom containing the stationary phase. The column is a made of glass and its length and diameter depend on the compound to be separated. The optimum working of LC depends on the matrix on which the sta- tionary phase is immobilized. The matrix used should have high mechanical and chemical stability to ensure optimum flow rate. The matrix is made up of inert mate- rials like agarose, cellulose, dextran, polyacrylamide, silica, polysterene etc. The stationary phase is always in equilibrium with a solvent. The sample is loaded onto the top of the column by i) direct application, ii) using su- crose gradient or iii) with the help of a peristaltic pump along with solvent. The different components in the sample mixture pass through the column at different rates. This is due to differences in their partitioning coef- ficients between the mobile liquid phase and the station- Figure 1. Components of gas chromatography unit.  R. BHADEKAR ET AL. 6 Table 2. Gas chromatography with various types of detectors. System Sample Type Pesticide Detected Detection RangeRef. GC-PFPD Food Acephate, Aldrin, Dicofol, Endrin, Captan etc. 0.003 - 0.2 ppm[31] GC-MS with large volume injection Food Trifluralin, Dicholoron etc. 100 ng/l [32] GC with microwave emission detector Food Parathion 0.5 ppb [33] GC with PFPD Food Organophosphates ppb [34] GC-ECD/FID and NPD Food, water, soilNitogen and phosphorous containing pesticides 380 mg/l [35] Capillary GC Water Organochlorines 6 - 300 µg/l [36] GC-MS Meconium Cypermethrin, malathion,cyfluthin etc. 0.01 - 4 - 15 µg/g[37] ary phase. The compounds are separated by collecting aliquots of the column eluent at different time intervals [23]. This chromatography is widely used in combination with MS for pesticide quantification [30]. Methods based on separation with MS detection are found to be ex- tremely useful as compared to GC-MS [38]. This tech- nique has been successfully been applied for detection of organophosphates, organochlorines etc. However certain modifications in the LC are essential. This is because many a time pesticides cannot be detected in one run due to interference of groups present in the pesticides. In order to overcome these problems dual LC-MS systems have been developed. In such a unit two types of ex- perimental conditions can be simultaneously applied for effective separation. High Performance Liquid Chromatography (HPLC) This type of chromatography has a better edge over other types of chromatography. The reason behind is the mate- rials used for making the column can withstand high pressure and flow rates. Here usually the columns are long (3 - 50 cm) in length and 1 - 4 mm in diameter. The HPLC unit consists of 1) stationary phase which is either in microporous, pellicular or bonded form, 2) mobile phase, 3) pumps for delivering the eluent and 4) detec- tors. The detectors used are: Variable wavelength length detectors, Scanning wavelength detectors fluorescence detectors, electrochemical detectors, mass spectrometer, NMR spectrometer, refractive index detector and evapo- rative light scattering detectors and so on. Vodeb et al. (2006) have used HPLC with a diode array detector to quantify β-cyfluthrin with reverse phase and normal phase types of column [39].HPLC combined with super- critical fluid extraction has been used to detect multiple pesticide residues from food samples in the method de- scribed by Kaihara et al. (2000). The authors have re- ported LOD of 0.005 - 0.1 ppm [40]. Application of re- verse phase HPLC with acetonirile gradient and UV dectector for detection of dalazion, malathion and sumu- thion is illustrated by Islam et al. (2009) [41]. HPLC with CD detector has also been used for detection of chiral pesticides. 5. Electrochemical Sensors and Biosensors Biosensors have been described as analytical machines coupled with bio recognition elements with various de- tection techniques. The biological components include enzymes, antibodies, microorganisms or DNA. The im- mobilized biocatalyst incorporated into the sensor allows continuous utilization of substrate. These methods have been reviewed extensively by Theveno et al. (2001) [42]. With the help of biosensors, on site analysis can be per- formed to understand the extent of pollution almost im- mediately [43-46]. The advantages of using biosensors are: 1) disposable, selective, reliable and economical 2) they can be produced in large quantities and can be miniaturized for efficient use for onsite detection, 3) re- quire less sample size and 4) easy to operate even by non skilled personnel [47-50]. In spite of their clear advan- tages, they have certain limitations. They have low re- sponse stability low mechanical stability, high diffusion resistance of substrate/bio component assembly; inter- fering signals form other compounds in real samples etc [51]. However, these drawbacks can be minimized by proper designing of the biosensor. For convenient use, biosensors are usually coupled with an electrochemical sensor. The sensors are potentiometer, amperometer, vol- tameter, conductimeter etc. This coupling gives the data in readable form. A number of electrochemical sensors are available commercially. Certain characters like selec- tivity, response time, and linear range, limit of detection, reproducibility, stability and lifetime of biosensors are compared with standard IUPAC protocols for their per- Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL.7 formance evaluation [52-54]. Different types of biosen- sors coupled with electrochemical devices are briefly described below. 5.1. Cell Based Biosensors They make use of living microorganisms such as algae, bacteria, yeast and fungi as bio-catalytic elements. Their main advantage is that they are easy to develop and there is no need for isolating sub-cellular components like en- zymes, antibodies, antigens etc to detect pesticides. Va- rious examples reported in literature are summarized in Table 3. 5.2. Enzyme Based Biosensors These biosensors measure the activity of the enzyme or enzymes used in the system. The activity of the enzyme depends on the various factors. They are amount of sub- strate, time of incubation, presence of inhibitors, reac- tions conditions like pH, temperature etc. To make the system more cost effective, enzymes are immobilized using various methods [55-58]. Mostly such biosensors are based either on enzyme activity or enzyme inhibiton. Example of former is organophosphorus hydrolase (OPH) with broad substrate specificity. Biosensors of second type often make use of Choline estarese (CE), acid phos- phatase, tyrosinase, ascorbate oxidase, acetolactate syn- thase, aldehyde dehydrogenase etc. In such systems, ace- tylcholine esterase (ACE) immobilized on activated sil- ica gel is most commonly used. The method is based on enzyme inhibition since carbamate and organophosphte pesticides inhibit the activity of ACE. ACE primarily hydrolyses neurotransmitters producing choline and ace- tic acid. (7) Carbamate (C) pesticides reversibly inhibit this enzyme (8) whereas organophosphates (ORP) inhibit it irreversibly (9). ACE + H2O → Choline + Acetic acid (7) ACE + C ↔ ACE-C (8) ACE + ORP ↔ ACE-ORP (9) The production of acetic acid results in change of pH of the system. This can be easily monitored using spec- trophotometer [59] fluorescence indicator [60], potenti- ometer [61] or direct measurement by pH meter using glass electrode or change in conductance of medium. Research on enzyme based methods for detection is ex- tensively discussed in review by Van Dyk et al. [62]. Examples of both the types enzyme based sensors are summarized in Table 4. 5.3. Immunosensors These biosensors are based on the property of specific binding of two immunological molecules viz. antigen and antibodies. They are characterized by sensitivity, rapidity, specificity, low cost and ability to analyse large number of samples. Here pesticide specific antigen-an- tibody reactions are employed for their detection. For quantification purposes the antigen-antibody reactions are coupled with enzyme labels. Immunosensors are of two types: i) labeled type and ii) label free type. The first type makes use of different enzymes like glucose oxidase, horse raddish peroxidase, alkaline phosphatse etc. Two different methods viz: sandwhich assay and competitive assay are used with labeled type. Similarly labeled free types of sensors are grouped into direct and indirect types. The applications of immunoassay as pesticide de- tection method have been reviewed in many papers [63- 65]. Commercial immunoassay kits are also available in the market. In immunosensors, sensing element can be either an antibody (Ab) or an antigen (Ag) which is im- mobilized on a transducer. If Ab is immobilized, the binding of analyte can be measured directly. If Ag is immobilized, the detection is based on the competition between immobilized Ag, the analyte, and a fixed amount of Ab. Mainly four types of immunosensors are reported viz piezoelectric, optical, electrochemical or thermomet- ric. Piezoelectric immunosensors: are more common due to label free detection of atrazine, parathion etc [84-86]. A piezoelectric crystal can be coated with an Ag or Ab and the change in the mass by the binding of the analyte can be correlated to the concentration of the analyte [87]. Optical immunosensors: Main optical immunosensros Table 3. Use of whole cells for pesticide detection. Type of Cell Electrochemical sensor Pesticide Detected Detection limitRef Escherichia coli Potentiometric Paraoxon, Parathion, Methylparathion, Diazinon 3 µM [66] Pseudomonas putida Amperometric Paraoxon, Parathion, Methyl parathion 0.26 - 0.29 ppb[67] Moraxella sp Triazines, Parathion, Carbamates, Organophosphates 27.5 ppb [68] Chlorella vulgaris Conductometric Organophosphates 10 ppb [69] Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL. 8 Table 4. Biosensors based on enzymes. Type of Biosensor (enzyme and its mode of action) Pesticide Detected Transducer Detection limitsRef Catalytic activity Organophosphorus acid anhyrolase Fluorine containing organophosphates Potentiometry 12.5 µm [70] Organophosphorus hydrolase (OPH) Organophosphates Amperometry 20 nM [71] Organophosphates and their neurotoxin Amperometry and Photometry µm [72] Enzyme Inhibition Butyryl Choline Esterase Trichorfon Potentiometry Below 0.1 µm[73] Acetyl choline estarase Triazophos Amperometry 0.01 µm [74] Organophosphorous , carbamatesSPE 0.35 µm [75] Acelyl choline esterase and choline oxidase Aldicarb, Carbofuran, Carbamyl Amperometry µg/l [76] Cholinesterase,choline oxidase and peroxidase Trichlorfon Potentiometry 5 nM [77] Acid Phosphatase Organophosphates and CarbamateAmperometry 40 µg/l [78] Ascorbate oxidase Ethyl paraoxon, organophosphatesAmperometry ppm [79] Tyrosinase (competitive inhibition) Organophosphates, carbamates Potentiometry ppb [80] Tyrosinase Carbamates Amperometry μM/l [81] Acetolactate Synthatase Herbicides µM [82] Aldehyde Dehydrogenase Dithiocarbamate Amperometry ppb [83] SPE = Screen Printed Electrode. developed are based on Surface Plamon Resonance (SPR) device. In another type of optical immunosensor, the Ab is coated on the metal sheet causes a minute change in the refractive index when bound with the analyte and this change can be detected by the SPR device. Another op- tical immunosensor is based on total internal reflection fluorescence (TIRF). These biosensors are used to detect terbutryn, atrazine, parathion, polychlorophenol etc [88]. 5.4. Nucleic Acid Based Biosensors These biosensors utilize the oxidation property of the nucleic acid base guanine [89]. They are based on inter- action of DNA molecules with pesticides. Such reactions can be detected by monitoring the change in redox po- tential. For this purpose electrochemical sensors like voltametry and potentiometry are used (here DNA is immobilized on the electrodes). Sometimes the change in electroactive analytes that are intercalated on DNA layer is also monitored. Nucleic acid biosensors have been extensively reviewed in a review published by Fang et al. [90]. 5.5. Use of Nano Particles in Biosensors Recent developments in enzyme based biosensors in- clude use of gold nano particles to increase accuracy. Moreover these sensors have multiplexing facility which allows detection of trace amounts of pesticides. Because pesticides are present in trace amounts pre concentration and extraction steps are essential prior to detection. De- velopments in nano materials particularly applications of carbon nano tubes as sorbant in solid phase micro extrac- tion techniques has been elaborately discussed by Pyrzy- nska [91]. These particles increase the adsorption and stability of ACE on planar gold electrode surface [92]. Nanoparticle layer also improves the sensitivity and de- tection limit of the device. Slight change in the environ- ment can disturb the charge based distribution of such sensors affecting the detection of pesticides. However, new studies and developments in surface chemistry and material physics along with proteomics can overcome this hurdle. It delivers fine and accurate measurement of any environmental pollutant. Alvarez et al. [93] has shown the use of nanome- chanical biosensors for the real time detection of or- ganochlorine pesticides like DDT. In this method canti- levers are coated with DDT5 hapten molecules over a self assembled monolayer of alkanethiol with gold nano- partilce. Assay is performed by mixing the samples con- taining a fixed concentration of DDT monoclonal anti- body with DDT solutions at different concentrations. After the incubation only the free antibody couples with the bioreceptor on the cantilever. The difference in the Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL.9 deflection occurs due to change in the surface stress of the cantilever. It can be detected by a laser beam sensi- tive photodetector. Gan et al. [94] have developed a highly sensitive dis- posable enzyme biosensor based on composite magnetic nanoperticles modified screen printed carbon electrode (SPCE). Organophosphates are detected by the inhibition of the acetyl cholinesterase catalyzed hydrolysis of ace- tylthiocholine. In this method the biosensor was fabri- cated by sythesysing acetylcholinesterase (ACE)-coated Fe3O4/Au (GMP) magnetic nanoparticulate (GMP-ACE). It is adsorbing on the surface of a SPCE modified by carbon nanotubes (CNTs)/nano-ZrO2/prussian blue (PB)/ Nafion (Nf) composite membrane by an external mag- netic field. The biosensor could detect dimethoate from Chinese cabbage with comparable accuracy. Moreover, according to Palchetti et al. [95] such electrochemical biosensors have some advantages over other analytical transducing systems. There advantages are possibility to operate in turbid media, comparable instrumental sensi- tivity, and possibility of miniaturization. Other pesticides like monocrotophos, methyl parathion and carbamyl could be detected using a sol-gel-derived silicate network containing nanoparticles. This arrange- ment created a biocompatible microenvironment around the enzyme molecule which aided not only in stabilizing its biological activity but also preventing its runoff from the system [96].For detection of malathion, planar gold electrode coated with chitosan hydrogel containg gold nano particles was formulated. Here thiocholine was used as an indicator and the system was based on che- misortion and desorption of the indicator with LOD of 0.03 ng/ml [97]. Though use of nanoparticles is a promising option in pesticide detection techniques more studies are essential to ensure proper standardization and increase in sensiti- vity. 6. Flow Injection Analysis Flow injection analysis is very sensitive, rapid and effi- cient tool used to detect presence of pesticides in differ- ent environmental samples. Other advantages of the technique are 1) low cost of instrumentation, 2) less la- bor cost and smaller sample size, 3) continuous sample injection, 4) better reproducibility and 5) high sampling rate with precision. This technique involves 3 steps viz 1) sample injection, 2) sample processing and 3) detection. The sample processing can be done by dilution, solvent extraction, medium exchange, enzymatic reactions, im- munoassays etc. The detection and estimation of sample makes use of mass spectrometry, spectrophotometery and measurement of fluorescence or change in pH, use of biosensors etc [98]. Following is the brief description of various quantification methods. Use of Biosensors Biosensors combined with FIA are reported for detection of carbamate insecticides in water samples [99] and for carbofuran in food samples [100]. In the latter method, ACE is incorporated in lipid films supported on a me- thylacrylate polymer. Similar enzyme system was used in the year 2009 for detection of organophosphorous pesticides. Here ACE is immobilized by adsorption on lead oxide which acts as an electrode. It catalyzes the oxidative degradation of thiocholine in the reactor. Change in the electrochemical gradient due to oxidation of choline corresponds to the amount of pesticide present in the sample [101]. Combination of biosensors with FIA overcomes limitations of biosensors. It also offers better option for standardization and optimization. The immobilized ACE-FIA coupled with Spectropho- tometry systems were used by by Andres and Nara- ya- naswamy and Xavier et al. (2000) for detection of pro- poxur, carbofuran and paraoxon. The detection limits were found to be 0.4 ng, 3.1 ppb and 24.7 ppb respec- tively [102,103]. The property of photolytic degradation of organo- phosphorous pesticides in presence of light has been uti- lized for screening the food samples for presence of or- ganophosphorous pesticides [104]. Photolysis can be due to absorption of UV or due to oxygen and hydrogen rad- icals. In this method FIA is used in combination with thermal lens spectrometry [105]. Similar technique has also been employed for detection of dithiocarbamate fungicides [101] in water samples. FIA in combination with amperometry can also be used for detection of or- ganophosphates [106]. FIA combined with immunochemilunisence assay to detect presence of atrazine in minute quantities (0.01 ng/ml) has been reported by Chouhan et al. (2010). The immuno-reactor consists of antibody (anti-antrazine) im- mobilized on protein-A sepharose matrix packed in a glass capillary column. This is then treated with atrazine and atrazine-horseradish peroxidase conjugate which fa- cilitates competitive binding. For generation of photons the reactants are treated with hydrogen peroxide and lu- minal. The amount of pesticide present is inversely pro- portional to the number of photons generated [107,108]. Photo induced fluorosence (PIF) has been used with FIA for determination of α-cypermethrin pesticide resi- dues in natural water samples [108]. In nature this pesti- cide has low fluorescence. It can be enhanced by treat- ment with UV radiation and cyclodextrins. The FIA-PIF technique is rapid and can detect this pesticide in con- centration range as low as ng/ml. Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL. 10 FIA combined with chemiluminescence has been used for carbofuran atrazine and similar triazines detection [109-111]. The method makes use of the property of the pesticides to get converted into methylamine upon ex- posure to UV. The methylamine generated is made to react with tris ruthenium. The light emitted in this reac- tion is proportional to the amount of pesticide present [110]. Similar method has been employed for detection of the herbicide simetryn by Waseem et al. (2008). The technique is based on the oxidation of luminol by the photoproducts of the simetryn in alkaline medium [110]. Rapid quantitative analysis of pesticide residues in food and water samples is reported using FIA-MS [112]. Samples were injected directly into a triple quadrpole instrument and data was obtained at the rate of 15 s/injection with accuracy limit of 0.01 ng/ml in food samples and 0.1 ng/ml of water samples with LOD of 0.003 mg/ml for food and 0.03 ng/ml for water samples. 7. Bioassay for Pesticide Detection Bioassay technique provides a rapid and sensitive assay for screening water samples for presence of herbicides. The method makes use of the property of herbicides to inhibit functioning of photo system II in Chlamydomonas reinhardtii. Briefly, C. reinhardtii grown on agar plates is incubated with samples that are dried on paper disks. The presence of herbicides is confirmed by observing the zone of inhibition around the disks. The advantage of the bioassay is that it can detect a wide range of herbi- cides including acifluorfen, chlorpropham, diclofopme- thyl (DFM), glyphosate, isoxaben, pinnacle, trifluralin dichlorobenzonitrile (DCB), 2,4-dichlorophenoxy-acteic acid (2,4-D), metobromuron, 2-ethyl-4-chlorophenoxya- cetic acid (MCPA), metribuzin, atrazine, hexazinone, norflurazon and terbacil [113]. Similar method has been described by Amutha et al. (2010) for detection of insec- ticides [114]. 8. Use of Capillary Electrophoresis (CE) Capillary electrophoresis can be employed for detection of certain pesticides [115]. The technique is useful for detection of chiral pesticides like propiconazole. This technique is a useful analytical tool for measuring the kinetics of biotransformation of stereoisomers of chiral pesticides and other pollutants from soil sediment. How- ever the sensitivity of the method is comparatively low. Hence more studies are essential before using this me- thod in routine practice. MS coupled with CE has high separation efficiency, low analysis time high resolution power, low consumption of samples and reagents [115]. 9. Enzyme Linked Immunosorbant Assay (ELISA) Use of ELISA for pesticide detection has been reported by Xu Zl et al. (2011). The authors have employed monoclonal Ab based indirect ELISA technique for de- tection of organophosphate pesticides. This method had LOD in ng/ml. However the method has broad specific- ity and hence can be used only for screening of organo- phosphates from water samples [116]. 10. Conclusions The persistence of pesticides in environmental samples is a global issue. With rules and regulations of organiza- tions like EPA, innumerable methods have been devel- oped to detect them. Modifications in the traditional me- thods help in detection of specific pesticides in trace quantities. Newer methods like biosensors and nano par- ticles, have overcome the limitations of classical meth- ods. Use of cell based biosensors, has opened a new avenue with possibility of exploiting different microor- ganisms for detection purposes. Another important de- velopment is use of ELISA and monoclonal Abs for de- tection purpose with remarkable specificity and sensitiv- ity. Taking this into account the authors are of the opin- ion that there should be 1) uniformity in permitted use of specific pesticides all over the world, 2) consensus among various organizations on MRL of these pesticides, 3) mandatory rules and regulations to abide by the estab- lished norms and most importantly 4) uniformity in the protocols for measurement of MRL in environmental samples, particularly edible products. In fact, biopesti- cides are the best alternative to chemical pesticides. How- ever, government support, technology innovations, in- crease in social awareness and enhancement in the exist- ing research and development are necessary to promote their use. All this will help in lowering the threats posed by the uncontrolled use of pesticides. 11. Acknowledgements The authors are indebted to Dr. S. S Kadam, Vice Chan- cellor Bharati Vidyapeeth Deemed University (BVDU), Pune, India and Dr. G. D Sharma, Principal, Rajiv Gan- dhi Institute of IT and Biotechnology (BVDU) for al- lowing them to undertake this work. 12. References [1] “International Code of Conduct on the Distribution and Use of Pesticides,” Hundred and Twenty-Third Session of the FAO Council, November 2002. Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL.11 [2] B. K. Sharma, “Environmental Chemistry,” Goel Pub- lication House, New Delhi, India, 2006. [3] “The EU Water Framework Directive—Integrated River Basin Management for Europe,” European Commission Environment, 2000. http://ec.europa.eu/environment/water/water-framework/i ndex_en.html [4] “Pesticides,” US Environmental Protection Agency, 2011. http://www.epa.gov/pesticides/ [5] “Pesticide Illnesses and Injury Surveillance,” Center for Disease Control and Prevention, 2011. http://www.cdc.gov/niosh/topics/pesticides/ [6] K. G. Harley, K. Huen, R. A. Schall, N. T. Holland, A. Bradman, D. B. Barr and B. Eskenazi, “Association of Organophosphate Pesticide Exposure and Paraoxonase with Birth Outcome in Mexican-American Women,” PLoS ONE, Vol. 6, No. 8, 2011. http://www.plosone.org/article/info%3Adoi%2F10.1371 %2Fjournal.pone.0023923 [7] “Potential Health Effects of Pesticide,” College of Agri- cultural Sciences, 2011. http://pubs.cas.psu.edu/freepubs/pdfs/uo198.pdf [8] W. J. Crinnion, “Chlorinated Pesticides: Threats to Health and Importance of Detection,” Alternative Medicine Re- view, Vol. 14, No. 4, 2009, pp. 347-359. [9] “Illness Associated with Exposure to Methyl Bromide- Fumigated Produce—California, 2010,” Morbidity and Mortality Weekly Report, Vol. 60, No. 27, 2011, pp. 923- 926. [10] “Mercury Compounds,” US Environmental Protection Agency, 2000. http://www.epa.gov/ttn/atw/hlthef/mercury.html [11] “Fungicides,” 2011. http://www.epa.gov/oppfead1/safety/healthcare/handbook /Chap15.pdf [12] W. Z. Azman and W. Abdullah, “General Classification Pesticides: Rodenticides,” 2011. http://www.prn.usm.my/old_website/mainsite/bulletin/su n/1997/sun12.html [13] J. A. Hernández, M. V-Manzanares, M. R. G.-Ortiz, B. H.-Carlos, M. P.-Torres and P. L. L.-de-Alba, “Simulta- neous Spectrophotometric Determination of Atrazine and Dicamba in Water by Partial Least Squares Regression,” Journal of Chilean Chemical Society, Vol. 50, No. 2, 2005, pp. 461-464. [14] S. B. Mathew, A. K. Pillai and V. K. Gupta, “A Rapid Spectrophotometric Assay of Some Organophosphorus Pesticides in Vegetable Samples,” Electronic Journal of Environmental, Agriculture and Food Chemistry, Vol. 5, No. 6, 2006, pp. 1604-1609. [15] A. Navaratne and N. Priyantha, “Chemically Modified Electrodes for Detection of Pesticides,” In: M. Stoytcheva Ed., Pesticides in the Modern World—Trends in Pesti- cides Analysis, 2011 http://www.intechopen.com/articles/show/title/chemically -modified-electrodes-for-detection-of-pesticides [16] “Potentiometry and Redox Titrations,” Chapter II, 2011. http://www.chem.ccu.edu.tw/~lkc/analytical%20chemistr y/AC1_Ch2_txt.pdf [17] “Potentiometry,” 2011. http://www.cem.msu.edu/~cem333/Week11.pdf [18] “Conductometry,” 2011. http://vedyadhara.ignou.ac.in/wiki/images/e/ed/Unit_4_C onductometric_Titrations.pdf [19] J. Gallová, “Conductometry,” 2011. http://www.fpharm.uniba.sk/fileadmin/ use r_u pload/ english /Fyzika/Determination_of_the_specific_conductance.pdf [20] S. P. Kounaves, “Voltammetric Techniques,” 2011. http://www.prenhall.com/settle/chapters/ch37.pdf [21] “Basics of Voltametry,” 2011. http://people.bath.ac.uk/chsataj/CH20016%202006/CH20 016%20Lecture%2013.pdf [22] A. J. Bard and L. R. Faulkner, “Electrochemical Methods: Fundamentals and Applications,” Wiley, Hoboken, 2000. [23] K. Wilson and J. Walker, “Priciples and Techniques of Biochemistry and Molecular Biology,” Cambridge Uni- versity Press, Cambridge, 2005. [24] H. M. C. K. Kanatiwela and N. K. B. Adikaram, “A TLC-Bioassay Based Method for Detection of Fungicide Residues on Harvested Fresh Produce,” Journal of the National Science Foundation of Sri Lanka, Vol. 37, No. 4, 2009, pp. 257-262. [25] S. Butz and H. J. Stan, “Screening of 265 Pesticides in Water by Thin-Layer Chromatography with Automated Multiple Development,” Analytical Chemistry, Vol. 67, No. 3, 1998, pp. 620-630. [26] T. Tuzimski, “Determination of Pesticides in Water Sam- ples from the Wieprz-Krzna Canal in the Leczyńsko- Włodawskie Lake District of Southeastern Poland by Thin-Layer Chromatography with Diode Array Scanning and High-Performance Column Liquid Chromatography with Diode Array Detection,” Journal of AOAC Interna- tional, Vol. 91, No. 5, 2009, pp. 1203-1209. [27] J. P. Lautié, V. Stankovic and G. Sinoquet, “Determina- tion of Chlormequat in Pears by High-Performance Thin Layer Chromatography and High-Performance Liqui Chro- matography with Conductimetric Detection,” Analusis, Vol. 28, No. 2, 2000, pp. 155-158. doi:10.1051/analusis:2000109 [28] R. Akkad, “Determination of Organophosphorus and Car- bamate Insecticides in Food Samples by High-Perfor- mance Thin-Layer Chromatography Multi-Enzyme Inhi- bition Assay,” PhD Dissertation, Institute of Food Chem- istry, University of Hohenheim, Stuttgart, Germany, 2011. [29] Joseph Sharma, “Recent Advances in Thin-Layer Chro- matography of Pesticides,” Journal of AOAC Interna- tional, Vol. 84, No. 4, 2001, pp. 993-1000. [30] W. M. Niessen, P. Manini and R. Andreoli, “Matrix Ef- fects in Quantitative Pesticide Analysis Using Liquid Chro- matography-Mass Spectrometry,” Mass Spectrometry Re- views, Vol. 25, No. 6, 2006, pp. 881-899. doi:10.1002/mas.20097 Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL. 12 [31] S.-H. TSeng, Y.-J. Lin, H.-F. Lee, S.-C. Su, S.-S. Chou and D.-F. Hwang, “A Multiresidue Method for Deter- mining 136 Pesticides and Metabolites in Fruits and Veg- etables: Application of Macroporous Diatomaceous Earth Column,” Journal of Food and Drug Analysis, Vol. 15, No. 3, 2007, pp. 316-324. [32] P. L. Wylie, “Trace Level Pesticide Analysis by GC/MS Using Large-Volume Injection,” 2011. http://cp.chem.agilent.com/Library/applications/5966121 4.pdf [33] H. A. Moye, “Improved Microwave Emission Gas Chro- matography Detector for Pesticide Residue Analysis,” 2011. http://pubs.acs.org/doi/abs/10.1021/ac60256a007 [34] L. V. Podhorniak, J. F. Negron and F. D. Griffith Jr., “Gas Chromatography with Pulsed Flame Photometric Detection Multiresidue Method for Organophosphate Pesticide and Metabolite Residues at the Parts-Per-Billion Level in Representatives Commodities of Fruits and Vegetable Crop Groups,” Journal of AOAC International, Vol. 84, No. 3, 2001, pp. 873-890. [35] S. Johnson, N. Saikia and A. Kumar, “Analysis of Pesti- cide Residues in Soft Drinks,” CSE Report, August, 2006. http://www.indiaenvironmentalportal.org.in/files/labrepor t/pdf [36] B. Du, H. Liu, et al., “Determination of Organochlorine Pesticide Residues in Herbs by Capillary Electrophore- sis,” Life Science Journal, Vol. 4, No.1, 2007, pp. 40-42. [37] D. Bielawski, E. Ostrea Jr., N. Posecion Jr., M. Corrion and J. Seagraves, “Detection of Several Classes of Pesti- cides and Metabolites in Meconium by Gas Chromatog- raphy-Mass Spectrometry,” Chromatographia, Vol. 62, No. 11-12, 2005, pp. 623-629. doi:10.1365/s10337-005-0668-7 [38] L. Alder, K. Greulich, G. Kempe and B. Vieth, “Residue Analysis of 500 High Priority Pesticides: Better by GC- MS or LC-MS/MS,” Mass Spectrometry Reviews, Vol. 25, No. 6, 2006, pp. 838-865. doi:10.1002/mas.20091 [39] L. Vodeb and B. Petanovska-Ilievska, “HPLC-DAD with Different Types of Column for Determination of β-Cy- fluthrin in Pesticide,” Acta Chromatographica, Vol. 17, 2006, pp. 188-201. [40] P. Vinas, N. Campillo, I. Lopez-Garcia, N. Aguinaga and M. Hernandez-Cordoba, “Capillary Gas Chromatography with Atomic Emission Detection for Pesticide Analysis in Soil Samples,” Journal of Agricultural and Food Che- mistry, Vol. 51, No. 3, 2003, pp. 3704-3708. doi:10.1021/jf021106b [41] S. Islam, M. S. Hossain, N. Nahar, M. Mosihuzzaman and M. I. R. Mamun, “Application of High Performance Liquid Chromatography to the Analysis of Pesticide Re- sidues in Eggplants,” Journal of Applied Sciences, Vol. 9, No. 5, 2009, pp. 973-977. doi:10.3923/jas.2009.973.977 [42] D. R. Thevenot, K. Toth, R. A. Durst and G. S. Wilson, “Electrochemical Biosensors: Recommended Definitions and Classification,” Biosensors and Bioelectronics, Vol. 16, No. 1-2, 2001, pp. 121-131. [43] A. Hildebrandt, R. Bragos, S. Lacorte and J. L.Marty, “Performance of a Portable Biosensor for the Analysis of Organophosphorus and Carbamate Insecticides in Water and Food,” Sensors and Actuators B: Chemical, Vol. 133, No. 1, 2008, pp. 195-201. doi:10.1016/j.snb.2008.02.017 [44] J. Tschmelak, G. Proll, J. Riedt, J. Kaiser, P. Kraemmer, L. Barzaga, J. S. Wilkinson, P. Hua, J. P. Hole, R. Nudd, M. Jackson, R. Abuknesha, D. Barcelo, S. Rodriguez- Mozaz, M. J. Lopez de Alda, F. Sacher, J. Stien, J. Slo- bodnik, P. Oswald, H. Kozmenko, E. Korenkova, L. To- thova, Z. Krascsenits and G. Gauglitz, “Automated Water Analyser Computer Supported System (AWACSS) Part II: Intelligent, Remote-Controlled, Cost-Effective, On-line, Water Monitoring Measurement System,” Biosensors and Bioelectronics, Vol. 20, No. 8, 2005, pp. 1509-1519. doi:10.1016/j.bios.2004.07.033 [45] H. Alain, R. Jordi, B. Ramon, T. Marius and L. Silvia, “Development of a Portable Biosensor for Screening Neurotoxic Agents in Water Samples,” Talanta, Vol. 75, No. 5, pp. 1208-1213. [46] B. B. Dzantiev, E. V. Yazynina, A. V. Zherdev, Y. V. Plekhanova, A. N. Reshetilov, S. C. Chang and C. J. McNeil, “Determination of the Herbicide Chlorsulfuron by Amperometric Sensor Based on Separation-Free Bi- enzyme Immunoassay,” Sensors and Actuators B: Che- mical, Vol. 98, No. 2-3, 2004, pp. 254-261. doi:10.1016/j.snb.2003.10.021 [47] I. Palchetti, A. Cagnini, M. Del Carlo, C. Coppi, M. Mas- cini and A. P. F. Turner, “Determination of Acetylcholi- nesterase Pesticides in Real Samples Using a Disposable Biosensor,” Analytica Chimica Acta, Vol. 337, No. 3, 1997, pp. 315-321. doi:10.1016/S0003-2670(96)00418-7 [48] T. T. Bachmann, B. Leca, F. Villatte, J. L. Marty, D. Fournier and R. D. Schmid, “Improved Multianalyte De- tection of Organophosphate and Carbamate with Dispos- able Multielectrode Biosensors Using Recombinant Mu- tants of Drosophila Acetylcholinesterase and Artificial neutral Network,” Biosensors and Bioelectronics, Vol. 15, No. 3-4, 2000, pp. 193-201. doi:10.1016/S0956-5663(00)00055-5 [49] T. Montesinos, S. Perez-Munguia, F. Valdez and J. L. Marty, “Disposable Cholinesterase Biosensor for the De- tection of Pesticides in Water-Miscible Organic Sol- vents,” Analytica Chimica Acta, Vol. 431, No. 2, 2001, pp. 231-237. doi:10.1016/S0003-2670(00)01235-6 [50] K. A. Joshi, J. Tang, R. Haddon, J. Wang, W. Chen and A. Mulchaldani, “A Disposable Biosensors for Organo- phosphorus Nerve Agents Based on Carbon Nanotubes Modified Thick Film Strip Electrodes,” Electroanalysis, Vol. 17, No. 1, 2005, pp. 54-58. doi:10.1002/elan.200403118 [51] B. Prieto-Simón, M. Campàs, S. Andreescu and J.-L. Marty, “Trends in Flow-Based Biosensing Systems for Pesticide Assessment,” Sensors, Vol. 6, No. 10, 2006, pp. 1161-1186. doi:10.3390/s6101161 [52] C. Tran-Minh, “Biosensors in Flow-Injection Systems for Biomedical Analysis, Process and Environmental Moni- toring,” Journal of Molecular Recognition, Vol. 9, No. 5-6, 1996, pp. 658-663. doi:10.1002/(SICI)1099-1352(199634/12)9:5/6<658::AI Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL.13 D-JMR317>3.0.CO;2-M [53] M. P. Marco, S. Gee and B. D. Hammock, “Immuno- chemical Techniques for Environmental Analysis I: Im- munosensors,” TrAC Trends in Analytical Chemistry, Vol. 14, No. 7, 1995, pp. 341-350. doi:10.1016/0165-9936(95)97062-6 [54] B. Hock, A. Dankwardt, K. Kramer and A. Marx, “Im- munochemical Techniques: Antibody Production for Pes- ticide Analysis,” Analytica Chimica Acta, Vol. 311, No. 3, 1995, pp. 393-405. doi:10.1016/0003-2670(95)00148-S [55] M. A. González-Martínez, J. Penalva, R. Puchades, A. Maquieira, B. Ballesteros, M. P. Marco and D. Barceló, “An Immunosensor for the Automatic Determination of the Antifouling Agent Irgarol 1051 in Natural Waters,” Environmental Science & Technology, Vol. 32, No. 21, 1998, pp. 3442-3447. doi:10.1021/es980120v [56] E. Mallat, C. Barzen, A. Klotz, A. Brecht, G. Gauglitz and D. Barceló, “River Analyzer for Chlorotriazines with a Direct Optical Immunosensor,” Environmental Science & Technology, Vol. 33, No. 6, 1999, pp. 965-971. doi:10.1021/es980866t [57] M. A. González-Martínez, S. Morais, R. Puchades, A. Maquieira, A. Abad and A. Montoya, “Monoclonal An- tibody-Based Flow-Through Immunosensor for Analysis of Carbaryl,” Analytical Chemistry, Vol. 69, No. 14, 1997, pp. 2812-2818. doi:10.1021/ac961068t [58] C. G. Bauer, A. V. Eremenko, E. Ehrentreich-Fŏrster, F. F. Bier, A. Makower, H. B. Halsall, W. R. Heineman and F. W. Scheller, “Zeptomole-Detecting Biosensor for Al- kaline Phosphatase in an Electrochemical Immunoassay for 2,4-Dichlorophenoxyacetic Acid,” Analytical Chemi- stry, Vol. 68, No. 15, 1996, pp. 2453-2458. doi:10.1021/ac960218x [59] R. T. Andres and R. Narayanaswamy, “Fibre-Optic Pesti- cide Biosensor Based on Covalently Immobilized Ace- tylcholinesterase and Thymol Blue,” Talanta, Vol. 44, No. 8, 1997, pp. 1335-1352. doi:10.1016/S0039-9140(96)02071-1 [60] R.-A Doong, H.-M. Shih and S.-H. Lee, “Sol-Gel-De- rived Array DNA Biosensor for the Detection of Poly- cyclic Aromatic Hydrocarbons in Water and Biological Samples,” Sensors and Actuators B, Vol. 111-112, No. 110, 2005, pp. 323-330. [61] H.-S. Lee, Y. A. Kim, Y. A. Cho and Y. T. Lee, “Oxida- tion of Organophosphorus Pesticides for the Sensitive Detection by a Cholinesterase-based Biosensor,” Chemo- sphere, Vol. 46, No. 4, 2002, pp. 571-576. doi:10.1016/S0045-6535(01)00005-4 [62] J. S. Van Dyk and B. Pletschke, “Review on the Use of Enzymes for the Detection of Organochlorine, Organo- phosphate and Carbamate Pesticides in the Environment,” Vol. 82, No. 3, 2011, pp. 291-307. [63] J. P. Sherry, “Environmental Chemistry: The Immunoas- say Option,” Critical Reviews in Analytical Chemistry, Vol. 23, No. 4, 1992, pp. 217-300. doi:10.1080/10408349208050856 [64] E. P. Meulenberg, W. H. Mulder and P. G. Stoks, “Im- munoassays for Pesticides,” Environmental Science Tech- nolology, Vol. 29, No. 3, 1995, pp. 553-561. doi:10.1021/es00003a001 [65] O. A. Sadik and J. M. Van Emon, “Application of Elec- trochemical Immunosensors to Environmental Monitor- ing,” Biosensors and Bioelectronics, Vol. 11, No. 8, 1996, pp. 1-11. doi:10.1016/0956-5663(96)85936-7 [66] A. Mulchandani, P. Mulchandani, S. Chauhan, I. Kaneva and W. Chen, “A Potentiometric Microbial Biosensor for Direct Determination of Organophosphate Nerve Agents,” Electroanalysis, Vol. 10, No. 11, 1998, pp. 733-737. doi:10.1002/(SICI)1521-4109(199809)10:11<733::AID-E LAN733>3.0.CO;2-X [67] Y. Lei, P. Mulchandani, J. Wang, W. Chen, W. Chen and A. Mulchandani, “Highly Sensitive and Selective Am- perometric Microbial Biosensor for Direct Determination of p-Nitrophenyl-Substituted Organophosphate Nerve Agents,” Environmental Science & Technology, Vol. 39, No. 22, 2005, pp. 8853-8857. doi:10.1021/es050720b [68] M. Priti, C. Wilfred and M. Ashok, “Microbial Biosensor for Direct Determination of Nitrophenyl-Substituted Or- ganophosphate Nerve Agents Using Genetically Modified Moraxella sp.,” Analytica Chimica Acta, Vol. 568, No. 1-2, 2006, pp. 217-221. doi:10.1016/j.aca.2005.11.063 [69] C. Chouteau, S. Dzyadevych, C. Durrieu and J. M. Cho- velon, “A Bienzymatic Whole Cell Conductometric Bio- sensor for Heavy Metal Ions and Pesticides Detection in Water Samples,” Biosensors and Bioelectronics, Vol. 21, No. 2, 2005, pp. 273-281. doi:10.1016/j.bios.2004.09.032 [70] A. L. Simonian, J. K. Grimsley, A. W. Flounders, J. S. Schoeniger, T. C. Cheng, J. J. DeFrank and J. R. Wild, “Enzyme-Based Biosensor for the Direct Detection of Fluorine-Containing Organophosphates,” Analytica Chi- mica Acta, Vol. 442, No. 1, 2001, pp. 15-23. doi:10.1016/S0003-2670(01)01131-X [71] P. Mulchandani, W. Chen and A. Mulchandani, “Flow- Injection Amperometric Enzyme Biosensor for Direct Determination of Organophosphate Nerve Agents,” En- vironmental Science & Technology, Vol. 35, No. 12, 2001, pp. 2562-2565. doi:10.1021/es001773q [72] M. J. Schoening, R. Krause, K. Block, M. Musahmen, A. Mulchandani and J. Wang, “A Dual Amperometric/Po- tentiometric FIA-Based Biosensor for the Distinctive De- tection of Organophosphorus Pesticides,” Sensors and Ac- tuators B: Chemical, Vol. 95, No. 1-3, 2003, pp. 291- 296. doi:10.1016/S0925-4005(03)00426-X [73] J. Wang, R. Krause, K. Block, M. Musameh, A. Mul- chandani, P. Mulchandani, W. Chen and M. J. Schoening, “Dual Amperometric Potentiometric Biosensor Detection System for Monitoring Organophosphorus Neurotoxins,” Analytica Chimica Acta, Vol. 469, No. 2, 2002, pp. 197- 203. doi:10.1016/S0003-2670(02)00666-9 [74] K. Reybier, S. Zairi and N. Jaffrezic-Renault, “The Use of Polyethylenimine for Fabrication of Potentiometric Cholinesterase Biosensors,” Talanta, Vol. 56, No. 6, pp. 1015-1020. doi:10.1016/S0039-9140(01)00588-4 [75] D. Du, X. Huang, J. Cai and A.-D. Zhang, “Amperomet- Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL. 14 ric Detection of Triazophos Pesticide Using Acetylcholi- nesterase Biosensor Based on Multiwall Carbon Nano- tube-Chitosan Matrix,” Sensors and Actuators B: Chemi- cal, Vol. 127, No. 2, pp. 531-535. [76] E. V. Gogol, G. A. Evtugyn, J. L. Marty, H. C. Budnikov and V. G. Winter, “Amperometric Biosensors Based on Nafion-Coated Screenprinted Electrodes for the Deter- mination of Cholinesterase Inhibitors,” Talanta, Vol. 53, No. 2, pp. 379-389. doi:10.1016/S0039-9140(00)00507-5 [77] T. T. Bachmann, B. Leca, F. Villatte, J.-L.Marty, D. Fournier and R. D. Schmid, “Improved Multianalyte De- tection of Organophosphate and Carbamate with Dispos- able Multielectrode Biosensors Using Recombinant Mu- tants of Drosophila Acetylcholinesterase and Artificial Neutral Network,” Biosensors and Bioelectronics, Vol. 15, No. 3-4, pp. 193-201. doi:10.1016/S0956-5663(00)00055-5 [78] F. Mazzei, F. Botre and C. Botre, “Acid Phosphata- se/Glucose Oxidase Based Biosensors for the Determina- tion of Pesticide,” Analytica Chimica Acta, Vol. 336, No. 1-3, 1996, pp. 67-75. doi:10.1016/S0003-2670(96)00378-9 [79] K. Rekha, M. D. Gouda, M. S. Thakur and N. G. Karanth, “Ascorbate Oxidase Based Amperometric Biosensor for Organophosphorus Pesticide Monitoring,” Biosensors and Bioelectronics, Vol. 15, No. 9-10, 2000, pp. 499-502. doi:10.1016/S0956-5663(00)00077-4 [80] Y. D. De Albuquerque and L. F. Ferreira, “Amperometric Biosensing of Carbamate and Organophosphate Pesti- cides Utilizing Screenprinted Tyrosinase-Modified Elec- trodes,” Analytica Chimica Acta, Vol. 596, No. 2, 2007, pp. 210-221. doi:10.1016/j.aca.2007.06.013 [81] M. T. Perez-Pita, A. J. Reviejo, F. J. Manuel-de-Villena and J. M. Pingarron, “Amperometric Selective Biosens- ing of Dimethyl- and Diethyldithiocarbamates Based on Inhibition Processes in a Medium of Reversed Micelles,” Analytica Chimica Acta, Vol. 340, No. 1-3, 1997, pp. 89-97. doi:10.1016/S0003-2670(96)00552-1 [82] A. Seki, F. Ortega and J. L. Marty, “Enzyme Sensor for the Detection of Herbicides Inhibiting Acetolactate Syn- thase,” Analytical Letters, Vol. 29, No. 8, 1996, pp. 1259- 1271. doi:10.1080/00032719608001479 [83] T. Noguer and J. L. Marty, “High Sensitive Bienzymic Sensor for the Detection of Dithiocarbamate Fungicides,” Analytica Chimica Acta, Vol. 347, No. 1-2, 1997, pp. 63- 70. doi:10.1016/S0003-2670(97)00127-X [84] J. Halamek, M. Hepel and P. Skladal, “Investigation of Highly Sensitive Piezoelectric Immunosensors for 2,4-Di- chlorophenoxyacetic Acid,” Biosensors and Bioelectron- ics, Vol. 16, No. 4-5, 2001, pp. 253-260. doi:10.1016/S0956-5663(01)00132-4 [85] J. Pribyl, M. Hepel, J. Halámek and P. Skladal, “Devel- opment of Piezoelectric Immunosensors for Competitive and Direct Determination of Atrazine,” Sensors and Ac- tuators B: Chemical, Vol. 91, No. 1-3, 2003, pp.333-341. doi:10.1016/S0925-4005(03)00107-2 [86] J. Halamek, J. Pribyl, A. Makower, P. Skladal and F. W. Scheller, “Sensitive Detection of Organophosphates in River Water by Means of a Piezoelectric Biosensor,” Analytical and Bioanalytical Chemistry, Vol. 382, No. 8, 2005, pp.1904-1911. doi:10.1007/s00216-005-3260-y [87] G. G. Guilbault, B. Hock and R. Schmid, “A Piezoelec- tric Immunosensor for Atrazine in Drinking Water,” Bio- sensors and Bioelectronics, Vol. 7, No. 6, 1992, pp. 411- 419. doi:10.1016/0956-5663(92)85040-H [88] D. Erickson, S. Mandal, H. Allen, J. Yang and B. Cor- dovez, “Nanobiosensors: Optofluidic, Electrical and Me- chanical Approaches to Biomolecular Detection at the Nanoscale,” Microfluid Nanofluidics, Vol. 4, No. 1-2, 2008, pp. 33-52. doi:10.1007/s10404-007-0198-8 [89] J. Wang, G. Rivas, E. Cai, P. Palecek, H. Nielsen, N. Shiraishi, D. Dontha, C. Luo, M. Parrado, P. A. M. Chi- carro, F. S. Farias, D. H. Valera, M. Grant, M. Ozsoz and M. N. Flair, “DNA Electrochemical Biosensors for Envi- ronmental Monitoring. A Review,” Analytica Chimica Acta, Vol. 347, No. 1-2, 1997, pp.1-8. doi:10.1016/S0003-2670(96)00598-3 [90] P. G. He, Y. Xu and Y. Z. Fang, “A Review: Electro- chemical DNA Biosensors for Sequence Recognition,” Analytical Letters, Vol. 38, No. 15, 2005, pp. 2597-2623. doi:10.1080/00032710500369794 [91] Krystyna Pyrzynska, “Carbon Nanotubes as Sorbents in the Analysis of Pesticides,” Chemosphere, Vol. 83, No. 11, 2011, pp.1407-1413. doi:10.1016/j.chemosphere.2011.01.057 [92] S. Olga and R. K. Jon, “An Acetylcholinesterase Enzyme Electrode Stabilized by an Electrodeposited Gold Nano- particle Layer,” Electrochemistry Communications, Vol. 9, No. 5, 2007, pp. 935-940. doi:10.1016/j.elecom.2006.11.021 [93] M. Alvarez, A. Calle, J. Tamayo, J. L. M. Lechuga, A. Abad and A. Montoya, “Development of Nanomechani- cal Biosensors for Detection of the Pesticide DDT,” Bio- sensor Bioelectronics, Vol. 18, No. 5-6, 2003, pp. 649- 653. doi:10.1016/S0956-5663(03)00035-6 [94] N. Gan, X. Yang, D. Xie, Y. Wu and W. Wen, “A Dis- posable Organophosphorus Pesticides Enzyme Biosensor Based on Magnetic Composite Nano-Particles Modified Screen Printed Carbon Electrode,” Sensors, Vol. 10, No. 1, 2010, pp. 625-638. doi:10.3390/s100100625 [95] I. Palchetti, S. Laschi and M. Mascini, “Electrochemical Biosensor Technology: Application to Pesticide Detec- tion,” Electrochemical and Mechanical Detectors, lateral Flow and Ligands for Biosensors, Human Press, Springer, LLC, USA, 2009, p. 115. [96] D. Du, S.-Z. Chen, J. Cai and A.-D. Zhang, “Electro- chemical Pesticide Sensitivity Test Using Acetylcholi- nesterase Biosensor Based on Colloidal Gold Nanoparti- cle Modified Sol-Gel Interface,” Talanta, Vol. 74, No. 4, 2008, pp. 766-772. [97] D. Du, J.-W. Ding, Y. Tao and X. Chen, “Application of Chemisorption/Desorption Process of Thiocholine for Pe- sticide Detection Based on Acetylcholinesterase Biosen- sor,” Sensors and Actuators B: Che m ic a l, Vol. 134, No. 2, Copyright © 2011 SciRes. AJAC  R. BHADEKAR ET AL. Copyright © 2011 SciRes. AJAC 15 2008, pp. 908-912. [98] A. Parikh, K. Patel, C. Patel and B. N. Patel, “Flow Injec- tion: A New Approach in Analysis,” Journal of Chemi- cal and Pharmaceutical Research, Vol. 2, No. 2, 2010, pp. 118-125. [99] S. Suwansa-ard, P. Kanatharana, P. Asawatreratanakul, C. Limsakul, B. Wongkittisuksa and P. Thavarungkul, “Semi Disposable Reactor Biosensors for Detecting Carbamate Pesticides in Water,” 2005. http://www.sciencedirect.com/science/article/pii/S095656 6304005512 [100] D. P. Nikolelis, M. G. Simantiraki, C. G. Siontorou and K. Toth, “Flow Injection Analysis of Carbofuran in Foods Using Air Stable Lipid Film Based Acetylcholinesterase Biosensor,” Analytica Chimica Acta, Vol. 537, No. 1-2, 2005, pp. 169-177. [101] Y.-Y. Wei, Y. Li, Y.-H. Qu, F. Xiao, G.-Y. Shi and L.-T. Jin, “A Novel Biosensor Based on Photoelectro-Syner- gistic Catalysis for Flow-Injection Analysis System/Am- perometric Detection of Organophosphorous Pesticides,” Analytica Chimica Acta, Vol. 643, No. 1-2, 2009, pp. 13-18. doi:10.1016/j.aca.2009.03.045 [102] M. P. Xavier, B. Vallejo, M. D. Marazuela, M. C. Mo- reno-Bondi, F. Baldini and A. Falai, “Fiber Optic Moni- toring of Carbamate Pesticides Using Porous Glass with Covalently Bound Chlorophenolred,” Biosensors and Bio- electronics, Vol. 14, No. 12, 2000, pp. 895-905. doi:10.1016/S0956-5663(99)00066-4 [103] M. Franko, M. Sarakha, A. Cibej, A. Boskin, M. Bavcon and P. Trebse, “Photodegradation of Pesticides and Ap- plication of Bioanalytical Methods for Their Detection,” Pure and Applied Chemistry, Vol. 77, No. 10, 2005, pp. 1727-1736. doi:10.1351/pac200577101727 [104] R. S. Chouhan, K. V. Rana, C. R. Suri, R. K. Thampi and M. S. Thakur, “Trace-Level Detection of Atrazine Using Immuno-Chemiluminescence: Dipstick and Automated Flow Injection Analyses Formats,” Journal of AOAC In- ternational, Vol. 93, No. 1, 2010, pp. 28-35. [105] A. Waseem, M. Yaqoob and A. Nabi, “Photodegradation and Flow-Injection Determination of Dithiocarbamate Fungicides in Natural Water with Chemiluminescence Detection,” Analytical Sciences, Vol. 25, No, 3, 2009, pp. 395-400. doi:10.2116/analsci.25.395 [106] H.-Y. Hu, X.-Y. Liu, F. Jiang, X. Yao and X.-C. Cui, “A Novel Chemiluminescence Assay of Organophosphorous Pesticide Quinalphos Residue in Vegetable with Luminol Detection,” Chemistry Central Journal, Vol. 4, No. 13, 2010, p. 13. [107] L. Pogacknic and M. Franko, “Photothermal Bioanalyti- cal Methods for Pesticide Toxicity Testing,” Arhiv Za Higijenu Rada I Toksikologiju, Vol. 54, No. 3, 2003, pp. 197-205. [108] J.-J. Aaron, M. Mbaye and M. D. G. Seye, “Determina- tion of A-Cypermethrin Insecticide Residues in Senegal Waters by a Flow Injection Analysis-Photochemically Induced Fluorescence (FIA-PIF) Method,” 2011. http://balwois.com/balwois/administration/full_paper/ffp- 1422.pdf [109] T. Pérez-Ruiz, C. Martínez-Lozano, V. Tomás and J. Martín, “Chemiluminescence Determination of Carbofuran and Promecarb by Flow Injection Analysis Using Two Photochemical Reactions,” Analyst, Vol. 127, No. 11, 2002, pp. 1526-1530. doi:10.1039/b207460p [110] A. Waseem, M. Yaqoob and A. Nabi, “Photodegradation and Flow-Injection Determination of Simetryn Herbicide by Luminol Chemiluminescence Detection,” Analytical Sciences, Vol. 24, No. 8, 2008, pp. 979-983. doi:10.2116/analsci.24.979 [111] D. J. Beale, N. A. Porter and F. A. Roddick, “A Fast Screening Method for the Presence of Atrazine and Other Triazines in Water Using Flow Injection with Chemilu- minescent Detection,” Talanta, Vol. 78, No. 2, 2009, pp. 342-347. [112] S. C. Nanita, A. M. Pentz and F. Q. Bramble, “High- Throughput Pesticide Residue Quantitative Analysis Achi- eved by Tandem Mass Spectrometry with Automated Flow Injection,” Analytical Chemistry, Vol. 81, No. 8, 2009, pp. 3134-3142. doi:10.1021/ac900226w [113] X.-Q. Li, A. Ng, R. King and D. G. Durnford, “A Rapid and Simple Bioassay Method for Herbicide Detection,” Biomarker Insights, Vol. 3, 2008, pp. 287-291. [114] M. Amutha, J. G. Banu, T. Surulivelu and N. Gopala- krishnan, “Effect of Commonly Used Insecticides on the Growth of White Muscardine Fungus, Beauveria bassi- ana under Laboratory Conditions,” Journal of Biopesti- cides, Vol. 3, No. 1, 2010, pp. 143-146. [115] A. W. Garrison, J. K. Avants and R. D. Miller, “Loss of Propiconazole and Its Four Stereoisomers from the Water Phase of Two Soil-Water Slurries as Measured by Capil- lary Electrophoresis,” 2011. http://www.mdpi.com/16604601/8/8/3453/ [116] Z. L. Xu, D. P. Zeng, J. Y. Yang, Y. D. Shen, R. C. Beier, H. T. Lei, H. Wang and Y. M. Sun, “Monoclonal Anti- body-Based Broad-Specificity Immunoassay for Moni- toring Organophosphorus Pesticides in Environmental Water Samples,” Journal of Environmental Monitoring, Vol. 13, No. 11, 2011, pp. 3040-3048.
|