Paper Menu >>
Journal Menu >>
 Vol.2, No.4, 321-331 (2010) Health doi:10.4236/health.2010.24049 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Study of the mechanisms regulating human umbilical artery contractility António José Santos-Silva1, Elisa Cairrão1,2, Ignacio Verde3 1Centro de Investigação em Ciências da Saúde, Universidade da Beira Interior, Avenida Infante Dom Henrique, Covilhã, Portugal 2Centro Hospitalar da Cova da Beira, Quinta do Alvito, Covilhã, Portugal 3Centro de Investigação em Ciências da Saúde, Universidade daBeira Interior, Avenida Infante Dom Henrique, Covilhã, Portugal; iverde@fcsaude.ubi.pt Received 10 November 2009; revised 27 December 2009; accepted 5 January 2010. ABSTRACT We studied the involvement of different types of Ca2+ channels, cyclic nucleotides and different kinases in the regulation of human umbilical artery (HUA) contractility. The elucidation of the precise mechanisms regulating the contractility of this artery could be very important to reveal potential therapeutic targets to treat HUA dis- orders such as preeclampsia. The relevancy of different types of Ca2+ channels on the regula- tion of HUA tonus was analyzed. Among the different Ca2+ channel inhibitors used, only the L-type calcium channels (LTCC) inhibition in- duced relaxation of HUA in Ca2+ containing me- dium. The inhibition of T-type calcium channels (TTCC) or TRP channels did not significantly affect HUA contractility. In presence of Ca2+, the intracellular increase of a cyclic nucleotide (cAMP or cGMP) induces relaxation of HUA, which was almost complete in histamine-con- tracted HUA, and lower effect was observed in arteries contracted by KCl and serotonin (5-HT). Inhibition of PKA and PKG weakly reduced the relaxations induced by the increase of cAMP and cGMP respectively, suggesting that the re- laxation induced by these nucleotides is not totally mediated by the activation of their re- spective kinases and that oth er mechani sms are involved. In calcium containing solution, PP2A inhibition produces relaxation of contracted HUA. In KCl contracted arteries, the OA and nifedipine relaxant effects are similar and not additive, suggesting that PP2A could activate LTCC. Besides, the increase of cyclic nucleo- tides significantly increased the OA effect, suggesting that the effect of PP2A inhibition is independent of the cyclic nucleotide pathways. The contractions induced by KCl, histamine and 5-HT in presence of Ca2+ were not significantly affected by ROCK, ERK1/2 or p38MAPK inhibi- tors. In absence of extracellular Ca2+, histamine and 5-HT elicited contractions of HUA charac- terized by two components, a rapid phasic con- tractile component followed by a decrease of the contraction until a tonic component. How- ever, KCl elicited sustained contractions of HUA in absence of extracellular Ca2+. As in presence of calcium, the ERK1/2 and p38MAPK inhibitors did not influence the contractions induced by KCl, histamine or 5-HT in absence of extracel- lular Ca2+. However, in these conditions, ROCK inhibition significantly relaxed the contractions induced by KCl and reduced the phasic and tonic components of the contraction elicited either by histamine or 5-HT. Our results show that calcium-dependent contractions of HUA depend on Ca2+ entry by LTCC, and these chan- nels seems to be positive regulated by PP2A. Cyclic nucleotides mediate HUA vasodilatation but their dependent kinases are not the unique responsible of this effect. HUA is able to con- tract independently of Ca2+ influx by activating the ROCK pathway and/or due to intracellular Ca2+ release. Keywords: Umbilical Artery; Smooth Muscle; L-type Ca2+ Channels; ROCK; Cyclic AMP; Cyclic GMP 1. INTRODUCTION The mechanisms regulating smooth muscle contractility in human umbilical artery (HUA) are very important for optimum gas and nutrient exchange between foetus and placenta. Since the umbilical blood vessels are not in- nervated, the con trol of umbilical blood flow depends of vasoactive substances either released locally or existing in the circulation [1,2]. In general, vascular smooth  A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 322 muscle (VSM) contractions are initiated by receptor or ion channel activation and involve Ca2+ dependent and/or independent mechanisms [3]. The increase of cytosolic free Ca2+ can be originated by a transient and rapid increase due to the Ca2+ release from the sar- coplasmic reticulum and/or by a sustained extracellular Ca2+ influx through Ca2+ channels [4,5]. This Ca2+ in- crease leads to myosin light chain kinase activation and contraction due to interaction between the thick and the thin myofilaments [6]. VSM relaxation can be mediated by activation of myosin light chain phosphatase which has opposite effects than myosin light kinase activation. Different types of Ca2+ channels have been involved in the control of VSM contractility, such as L-type Ca2+ channels (LTCC) [7,8], T-type Ca2+ channe ls (TTCC) [9], store-operated Ca2+ channels (SOCC) [10] or stretch- activated channels (SAC) [11,12]. Recently, the SOCC and SAC have been identified as being transient receptor potential (TRP) channels [13]. In VSM, different TRP channels were linked with Ca2+ store depletion, G-protein-coupled receptor activation, membrane stretch, pho- spholipid signals and other factors. However, the role of these channels was not clarified yet, probably due to the lack of specific pharmacological tools, such as specific activators and/or inhibitors [14]. The TTCC were mainly involved in the regulation of cell prolifera- tion [15]. Although these channels were involved in smooth muscle contraction by some authors [16], the information linking TTCC with VSM contractility is scant and their relative importance in this process needs further analysis. On the other hand, LTCC seems to be dominant in the regulation of VSM contractility because they have been appointed as the main pathway for Ca2+ entry. In HUA, some authors identified different LTCC and TTCC [17] and the LTCC blockers have appeared to be the most potent relaxants of this artery [18]. On the other hand, in smooth muscle cells, LTCC could be regulated by PP2A, although the number of studies is very low and the direction of this modulation has re- mained somewhat controversial. Some author have shown that PP2A does not modulate LTCC in tracheal smooth muscle [19], but in intestinal smooth muscle a dual effect of phosphatase inhibitors on LTCC has been reported [20]. In human umbilical vein, Groschner et al. have suggested that inhibition of PP2A activates LTCC [21]. Concerning the Ca2+ independent mechanism, it could involve Ca2+-sensitization mediated by the activation of the RhoA-kinase (ROCK) which causes inhibition of myosin light ch ain phosph a tase, leading to an increase of myosin light chain phosphorylation [5]. In this sense, some authors have reported that rhoA/ROCK pathway can contribute to agonist-induced contractions of HUA. However, this effect seems to be limited to intracellular Ca2+-induced contractions and may be more important in sustaining contractions rather than the initial phase of force development [22]. Other authors have suggested the existence, in VSM cells, of a Ca2+-dependent Rho stimulation mechanism linked to different receptor cou- pled to G proteins [23]. Also, it has been described that rabbit artery smooth muscle depolarization increases Ca2+ sensitization due to ROCK activation [24]. On the other hand, a role of some components of the MAPK cascade and its substrates (ERK1 and ERK2) in modu- lating the VSM contractility has been also suggested [25]. Some roles of these kinases in the regulation of cell proliferation and differentiation has been establish ed [26] but their role in VSM contraction and relaxation is al- most unknown. Some agonists inducing VSM contrac- tion can also activate ERKs [27,28]. In cerebral arteries, it was suggested that ERKs modulate Ca2+ sensitivity and contractility [25]. Other authors have observed that 5-HT induces rat aorta contractions involving p38 MAPK or Erk MAPK pathways [29-31]. The cyclic nucleotides, cAMP or cGMP, are the main second messenger involved in the regulation of vasodila- tion [32]. Intracellular accumulation of cAMP and cGMP can be achieved by stimulation of adenylate or guanylate cyclase, respectively, or by inhibition of phosphodiesterases (PDE) [33]. Concerning cAMP, in- tracellular increase of this nucleotide induces relaxation of different human arteries [34-36], including HUA [37]. Among the four families of PDE expressed in this smooth muscle (PDE1, PDE3, PDE4 and PDE5), PDE4 has been shown as the key enzyme involved in the regu- lation of HUA relaxation associated to cAMP [37]. Concerning cGMP, the increase of the intracellular level of this nucleotide also ind uces artery vasodilatation [38 ], including HUA vasodilatation [37,39]. PDE5 has been shown as the key enzyme involved in the regulation of HUA relaxation associated to cGMP [37]. Also, distinct authors have described that in different arteries [40,41], including HUA [39], the vasodilatation induced by cGMP is mediated by activation of potassium channels. Increases in cAMP and cGMP activate cAMP-dependent protein kinase (PKA) and cGMP-dependent protein kinase (PKG), respectively [42]. In VSM cells, the inhi- bition of LTCC by PKG has been reported [43]. How- ever, the cAMP pathway has been suggested to inhibit, to enhance, or to have no effect on smooth muscle LTCC [43]. Also, some authors have suggested that relaxation induced by cyclic nucleotides is not totally mediated by the activation of their respective kinases. In this sense, other mechanisms were involved, such as cross-activa- tion between cyclic nucleotide dependent kinases [44] or the regulation of other proteins having Epac (exchange protein directly activated by cAMP) which activates the small GTPbinding protein Rap1 [45]. Despite of the great importance of HUA before and during childbirth, the mechanisms involved in the regu-  A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 323 lation of the contractility of this artery have been weakly studied. Devoid of en ervation, the regulation of vascular tone of the HUA depends only by the local release of humoral factors, such as 5-HT and histamine [1,2]. In this work we have evaluated the relevancy of some mechanisms involved in the contraction and relaxation of this artery, such as extracellular Ca2+, cyclic nucleo- tides, Ca2+ channels and different kinase types. 2. METHODS 2.1. Tissue Preparation Umbilical cord pieces of 3-7 cm were obtained from normal term pregnancies with the consent of the donor mothers. All procedures carried out with these samples have been approved by the Ethics Committee of “Centro Hospitalar da Cova da Beira EPE”. The umbilical cord samples were collected in sterile physiological saline solution (composition, mM: NaCl 110; CaCl2 0.15; KCl 5; MgCl2 2; HEPES 10; NaHCO3 10; KH2PO4 0.5; NaH2PO4 0.5; Glucose 10; EDTA 0.49). In order to avoid contaminatio n and tissue degradatio n, penicillin (5 U/ml), streptomycin (5 µg/ml), amphotericin B (12.5 ng/ml) and antiproteases (leupeptine, 0.45 mg/l; ben- zamidine, 26 mg/l; and trypsin inhibitor, 10 mg/l) were added to the physiological saline solution. Umbilical artery rings of 3-5 mm were isolated from the surround- ing connective tissue. Vascular endothelium was me- chanically removed by gentle rubbing with a cotton bud introduced through the arterial lumen. These denuded HUA rings were used to perform contractility experi- ments. 2.2. Artery Tension Recording 2.2.1. Relaxation Studies in Ca2+ Cont aining Medium The HUA rings were placed in organ bath chambers (LE01.004, Letica) containing Krebs-bicarbonate solu- tion (composition, mM: NaCl 119, KCl 5.0, NaHCO3 25, KH2PO4 1.2, CaCl2 0.5, MgSO4 1.2, EDTA 0.03, glucose 11) at 37ºC and continuously gassed with car- bogen. The artery rings were suspended between two parallel stain- less steel wires and tension measurement was performed using isometric transducers (TRI201, Panlab SA, Spain), amplifier (ML118/D Quad Bridge, ADInstruments), interface PowerLab/4SP (ML750, ADInstruments) and a computerized system with Chart5 PowerLab software (ADInstruments). For analysis, the isometric tension measured has been ex- pressed in milligrams (mg) of force elicited by the ar- tery in presence of drugs. To analyze the relaxation data, we used the percentage of reduction on the maximal contraction induced by the contractile agents. During the resting periods, the organ bath solution was changed every 15 min. Initially, the rings were equili- brated for 60 min until a resting tension of 1000 mg was achieved. After this, the rings were challenged with 5-HT (1µM) to test their viability. Rings that in- duced a maximal contraction lower than 1 g when challenged with 5-HT were excluded from the study. Afterwards, the rings were contracted using KCl (60 mM), histamine (10 M) and 5-HT (1 M). To deter- mine the involvement of distinct types of Ca2+ channels, the LTCC blocker nifedipine (10 M), the TTCC blocker mibefradil (10 M) and the TRP blocker 2-aminoethoxydiphenyl borate (APB; 100 M) have been used. To analyze the involvement of the cAMP or cGMP pathways the following drugs were used in some cases: rolipram (1 M), a PDE4 selective inhibitor; forskolin (10 M), an adenylate cyclase activator; KT-5720 (KTa; 1 M), a PKA inhibitor; sodium nitroprusside (SNP; 10 M) a guanylate cyclase stimulator; dipyridamol (3 M), a PDE5 inhibitor; and KT-5823 (KTg; 1 M), a PKG inhibitor. To evaluate the possible involvement of PP2A, okadaic acid (OA; 5 nM) has been used in some experiments. Control experiments with ethanol, the vehi- cle used to dissolve some drugs, were alway s perform ed. 2.2.2. Relaxation S tudies in Ca2+-free Medium To analyze the HUA contractility in absence o f Ca2+, we used a Krebs solution without Ca2+ (composition, mM: NaCl 119, KCl 5.0, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, EDTA 0.03, EGTA 0.5, glucose 11). The rings were also contracted using KCl (60 mM), histamine (10 M) or 5-HT (1 M). To analyze the modulation of contractility by some kinases in Ca2+-free contractions, the following drugs were used in some experiments: Y-27632 (Y27; 10 M), a ROCK inhibitor; PD-98059 (PD; 50 M) an ERK1/2 inhibitor; and SB-203580 (SB; 25 M), a p38MAPK inhibitor. In some experiments, nifedipine (10 M) and OA were also used. 2.3. Drugs and Chemicals All drugs and chemicals have been purchased from Sigma-Aldrich Quimica (Sintra, Portugal), except for- skolin and rolipram, which were purchased from Biogen Cientifica (Madrid, Spain). Forskolin, rolipram and dipyridamol, were initially dissolved in ethanol and all the other drugs were initially disso lved in distilled water. Final solutions were obtained by dilution with Krebs solution. The final concentration of ethanol in the organ bath did never exceed 0.1%. 2.4. Statistical Analysis Statistical analysis of the data has been performed using the SigmaStat Statistical Analysis System, version 1.00 (1992). Results have been expressed as mean ± s.e.m. of 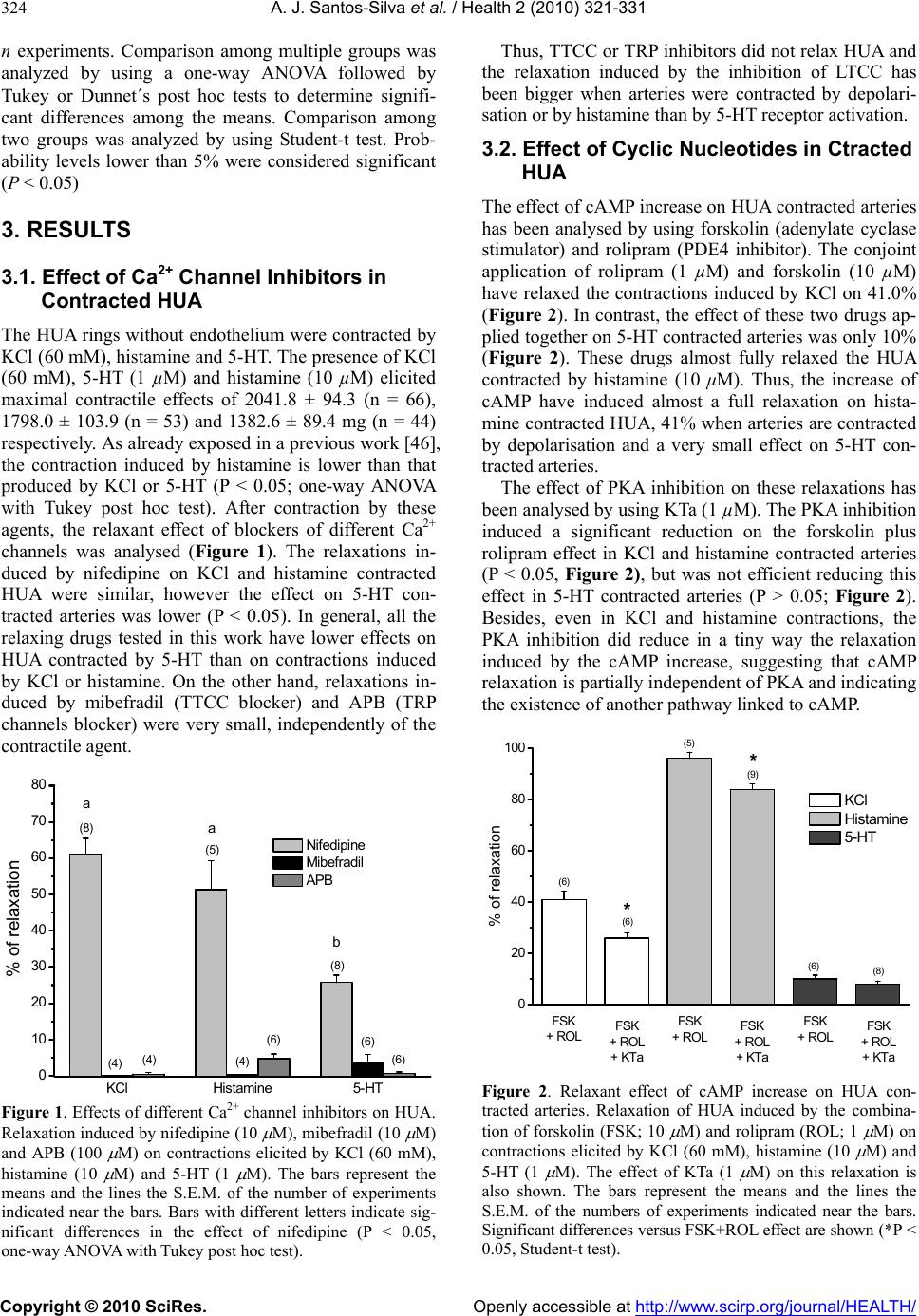 A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 324 n experiments. Comparison among multiple groups was analyzed by using a one-way ANOVA followed by Tukey or Dunnet´s post hoc tests to determine signifi- cant differences among the means. Comparison among two groups was analyzed by using Student-t test. Prob- ability levels lower than 5% were considered significant (P < 0.05) 3. RESULTS 3.1. Effect of Ca2+ Channel Inhibitors in Contracted HUA The HUA rings without endothelium were contracted by KCl (60 mM), histamine and 5-HT. The presence of KCl (60 mM), 5-HT (1 µM) and histamine (10 µM) elicited maximal contractile effects of 2041.8 ± 94.3 (n = 66), 1798.0 ± 103.9 (n = 53) and 1382.6 ± 89.4 mg (n = 44) respectively. As already exposed in a previous work [46], the contraction induced by histamine is lower than that produced by KCl or 5-HT (P < 0.05; one-way ANOVA with Tukey post hoc test). After contraction by these agents, the relaxant effect of blockers of different Ca2+ channels was analysed (Figure 1). The relaxations in- duced by nifedipine on KCl and histamine contracted HUA were similar, however the effect on 5-HT con- tracted arteries was lower (P < 0.05). In general, all the relaxing drugs tested in this work have lower effects on HUA contracted by 5-HT than on contractions induced by KCl or histamine. On the other hand, relaxations in- duced by mibefradil (TTCC blocker) and APB (TRP channels blocker) were very small, independently of the contractile agent. KClHistamine 5-HT 0 10 20 30 40 50 60 70 80 (6) (6) (4) (6) (4) (4) (5) (8) (8) a a % of relaxation Nifedipine Mibefradil APB b Figure 1. Effects of different Ca2+ channel inhibitors on HUA. Rela xati on ind uced by nife dipine (10 M), mibefradil (10 M) and APB (100 M) on contractions elicited by KCl (60 mM), histamine (10 M) and 5-HT (1 M). The bars represent the means and the lines the S.E.M. of the number of experiments indicated near the bars. Bars with different letters indicate sig- nificant differences in the effect of nifedipine (P < 0.05, one-way ANOVA with Tukey post hoc test). Thus, TTCC or TRP inhibitors did not relax HUA and the relaxation induced by the inhibition of LTCC has been bigger when arteries were contracted by depolari- sation or by histamine than by 5-HT receptor activation. 3.2. Effect of Cyclic Nucleotides in Ctracted HUA The effect of cAMP increase on HUA contracted arteries has been analysed by using forskolin (adenylate cyclase stimulator) and rolipram (PDE4 inhibitor). The conjoint application of rolipram (1 µM) and forskolin (10 µM) have relaxed the contractions induced by KCl on 41.0% (Figure 2). In contrast, the effect of these two drugs ap- plied together on 5-HT contracted arteries was only 10% (Figure 2). These drugs almost fully relaxed the HUA contracted by histamine (10 μM). Thus, the increase of cAMP have induced almost a full relaxation on hista- mine contracted HUA, 41% when arteries are contracted by depolarisation and a very small effect on 5-HT con- tracted arteries. The effect of PKA inhibition on these relaxations has been analysed by using KTa (1 µM). The PKA inhibition induced a significant reduction on the forskolin plus rolipram effect in KCl and histamine contracted arteries (P < 0.05, Figure 2), but was not efficient reducing this effect in 5-HT contracted arteries (P > 0.05; Figure 2). Besides, even in KCl and histamine contractions, the PKA inhibition did reduce in a tiny way the relaxation induced by the cAMP increase, suggesting that cAMP relaxation is partially indep enden t of PKA an d ind icating the existence of another pathway linked to cAMP. FSK + ROLFSK + ROL + KTaFSK + ROLFSK + ROL + KTaFSK + ROLFSK + ROL + KTa 0 20 40 60 80 100 FSK + ROL + KTa FSK + RO L FSK + ROL + KTa FSK + ROL FSK + ROLFSK + ROL + KTa * (8) KCl Histamine 5-HT * (9) (6) (5) (6) (6) % of relaxation Figure 2. Relaxant effect of cAMP increase on HUA con- tracted arteries. Relaxation of HUA induced by the combina- tion of forskolin (FSK; 10 M) and rolipram (ROL; 1 M) on contractions elicited by KCl (60 mM), histamine (10 M) and 5-HT (1 M). The effect of KTa (1 M) on this relaxation is also shown. The bars represent the means and the lines the S.E.M. of the numbers of experiments indicated near the bars. Significant differences versus FSK+ROL effect are shown (* P < 0.05, Student-t test). 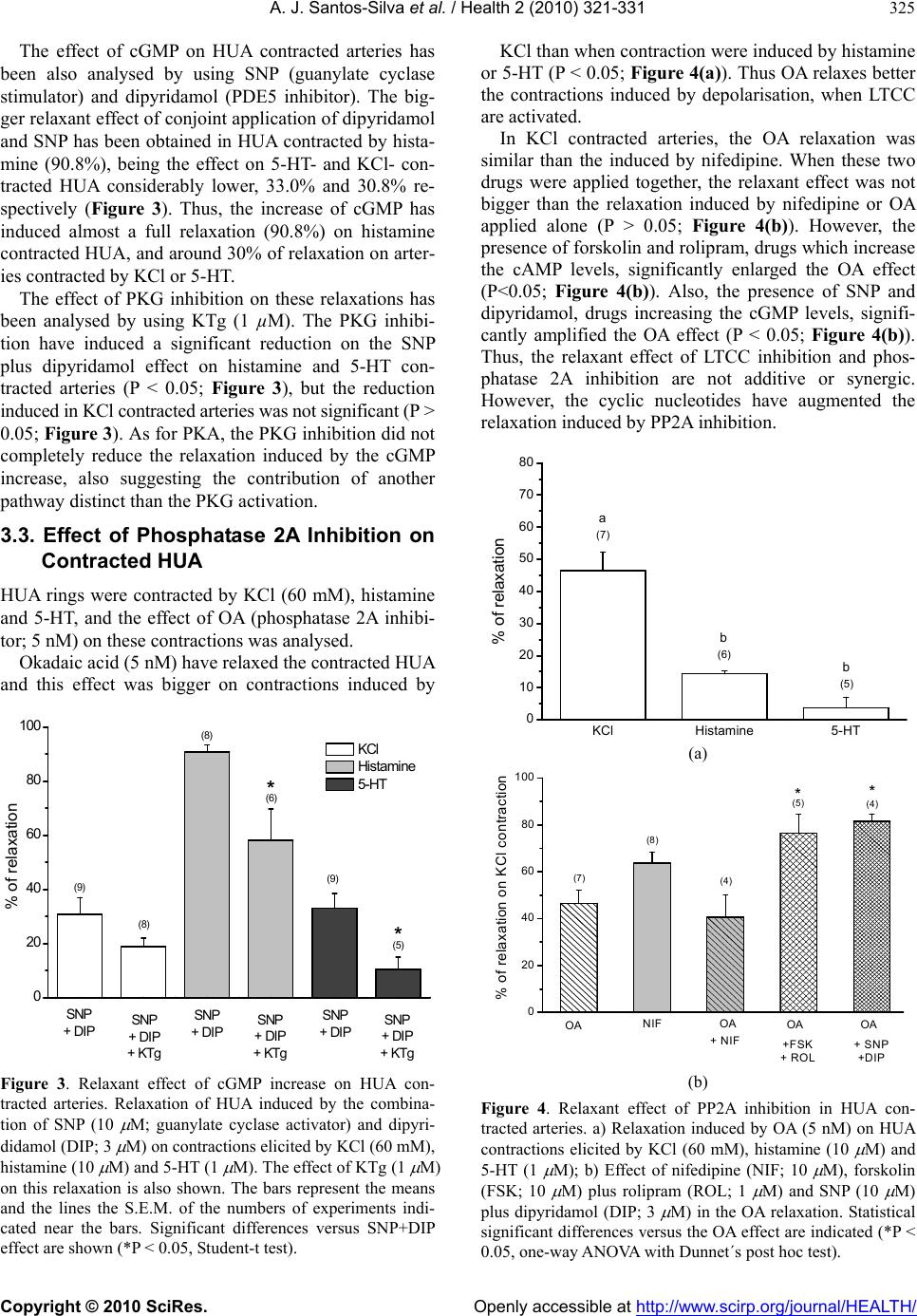 A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 325 The effect of cGMP on HUA contracted arteries has been also analysed by using SNP (guanylate cyclase stimulator) and dipyridamol (PDE5 inhibitor). The big- ger relaxant effect of conjoint application of dipyridamol and SNP has been obtained in HUA contracted by hista- mine (90.8%), being the effect on 5-HT- and KCl- con- tracted HUA considerably lower, 33.0% and 30.8% re- spectively (Figure 3). Thus, the increase of cGMP has induced almost a full relaxation (90.8%) on histamine contracted HUA, and around 30% of relaxation on arter- ies contracted by KCl or 5-HT. The effect of PKG inhibition on these relaxations has been analysed by using KTg (1 µM). The PKG inhibi- tion have induced a significant reduction on the SNP plus dipyridamol effect on histamine and 5-HT con- tracted arteries (P < 0.05; Figure 3), but the reduction induced in KCl contracted arteries was not significant (P > 0.05; Figure 3). As for PKA, the PKG inhibition did not completely reduce the relaxation induced by the cGMP increase, also suggesting the contribution of another pathway distinct than the PKG activation. 3.3. Effect of Phosphatase 2A Inhibition on Contracted HUA HUA rings were contracted by KCl (60 mM), histamine and 5-HT, and the effect of OA (phosphatase 2A inhibi- tor; 5 nM) on these contract i o ns was anal ys e d. Okadaic acid (5 nM) have relaxed the contracted HUA and this effect was bigger on contractions induced by SNP+DIPSNP + DIP + KTgSNP+DIPSNP + DIP + KTgSNP+DIPSNP + DIP + KTg 0 20 40 60 80 100 * (5) KCl H i s ta mine 5-HT * (9) (6) (8) (8) (9) % of relaxation SNP + DIPSNP + DIP + KTg SNP + DIPSNP + DIP + KTg SNP + DIPSNP + DIP + KTg Figure 3. Relaxant effect of cGMP increase on HUA con- tracted arteries. Relaxation of HUA induced by the combina- tion of SNP (10 M; guanylate cyclase activator) and dipyri- didamol (DIP; 3 M) on contractions elicited by KCl (60 mM), histamine (10 M) and 5-HT (1 M). The effect of KTg (1 M) on this relaxation is also shown. The bars represent the means and the lines the S.E.M. of the numbers of experiments indi- cated near the bars. Significant differences versus SNP+DIP effect are shown (*P < 0.05, Student-t test). KCl than when contraction were induced by histamine or 5-HT (P < 0.05; Figure 4(a)). Thus OA relaxes better the contractions induced by depolarisation, when LTCC are activated. In KCl contracted arteries, the OA relaxation was similar than the induced by nifedipine. When these two drugs were applied together, the relaxant effect was not bigger than the relaxation induced by nifedipine or OA applied alone (P > 0.05; Figure 4(b)). However, the presence of forsk olin and ro lipram, dr ugs which in crease the cAMP levels, significantly enlarged the OA effect (P<0.05; Figure 4(b)). Also, the presence of SNP and dipyridamol, drugs increasing the cGMP levels, signifi- cantly amplified the OA effect (P < 0.05; Figure 4(b)). Thus, the relaxant effect of LTCC inhibition and phos- phatase 2A inhibition are not additive or synergic. However, the cyclic nucleotides have augmented the relaxation induced by PP2A inhibition. KClHistamine 5-HT 0 10 20 30 40 50 60 70 80 % of relaxation a (7) b (6) b (5) (a) AONIFAO+NIFAO+FSK+ROL AO+SNP+DIP 0 20 40 60 80 100 (7) (8) % of relaxation on KCl contraction (4) * (5) * (4) AO NIF AO + NIFAO +FSK + ROL AO + SNP +DIP OA OA OA OA (b) Figure 4. Relaxant effect of PP2A inhibition in HUA con- tracted arteries. a) Relaxation induced by OA (5 nM) on HUA contractions elicited by KCl (60 mM), histamine (10 M) and 5-HT (1 M); b) Effect of nifedipine (NIF; 10 M), forskolin (FSK; 10 M) plus rolipram (ROL; 1 M) and SNP (10 M) plus dipyridamol (DIP; 3 M) in the OA relaxation. Statistical significant differences versus the OA effect are indicated (*P < 0.05, one-way ANOVA with Dunnet´s post hoc test).  A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 326 3.4. Effect of Rock Inhibition in HUA Con- tractility The role of different kinases in HUA contractility has been analysed by using the following inhibitors: Y27 (ROCK inhibitor; 10 M); PD (ERK1/2 inhibitor; 50 M); and SB (p38MAPK inhibitor; 25 M). Firstly, the effect of these inhibitors on HUA contrac- tility was analysed in Ca2+ containing medium. In these conditions, ROCK inhibition did not induce significant relaxation (P > 0.05) of HUA contracted by KCl (0.3± 0.2%, n=8), by histamine (0.1 ± 0.1%; n=5) or by 5-HT (0.8±0.8%; n=6). Neither, in the same conditions, ERK1/2 inhibition did not induce significant relaxation (P>0.05) of HUA contracted by KCl (3.0 ± 1.5%; n=9), by histamine (1.7 ± 1.7%; n=5) or by 5-HT (1.1±1.1%; n=4). Also, in presence of extracellular Ca2+, p38MAPK inhibition did no t induce significan t relax ation (P > 0.05) of HUA contracted by KCl (1.2 ± 1.0%; n=8), by hista- mine (1.1 ± 0.7%; n = 7) or by 5-HT (1.6 ± 1.0%; n=5). Thus, ROCK, ERK1/2 or p38MAPK inhibition did not affect the contraction induced either by KCl, histamine or 5-HT in presence of extracellular Ca2+. The effect of these inhibitors has been also analysed in absence of extracellular Ca2+ (0Ca medium). In these conditions KCl has induced sustained contractions (1013.6 ± 79.2 mg; n = 18) that were significantly lower than the induced in presence of Ca2+ (1859.8 ± 70.9 mg; n = 95)(P < 0.05; Student-t test). The ERK1/2 or p38 MAPK inhibition did not significantly influence the contractions induced by KCl in absence of Ca2+ (Figure 6(a)). Besides, the inhibition of PP2A or LTCC did not affect the contractions induced by KCl in absence of extracellular Ca2+ (Figure 6(a)). However, ROCK inhi- bition by Y27 relaxed on 81.6% the contractio n s indu ced by KCl in Ca2+ free medium (Figure 6(a)). Figure 5(a) shows a recor d of an experiment in which KCl (60 mM) induces contraction of HUA in Ca2+-free medium and Y27 relaxes this contraction. On the other hand, in Ca2+ free medium histamine and 5-HT have elicited two step contractions characterized by a rapid phasic component, 2-3 min after the receptor stimulation, followed by a decrease of the contraction until a tonic component, 15-20 min after. The ERK1/2 or p38MAPK inhibition did not affect the phasic or tonic components of the contraction elicited by histamine or 5-HT (P > 0.05; Figures 6(b) and 6(c)). However, ROCK inhibition significantly reduced the phasic and tonic components of the contraction elicited either by histamine or 5-HT (P < 0.05; Figures 6(b) and 6(c)). Figure 5(b) shows a record of an experiment in which, in Ca2+ free medium, 5-HT elicited two step contractions characterized by a phasic and tonic components which were reduced after ROCK inhibition. Thus, ROCK inhibition decreases the tension induced either by KCl, histamine or 5-HT in Ca2+ free medium. 0 1020304050607080 0.0 0.2 0.4 0.6 0.8 1.0 A Y27 10 M KCl 60mM TENSION (g) TIME (min) (a) 0510 15 20 2590 95100105110 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 5-HT 1 M C Y27 10 M 5-HT 1 M TENSION (g) TIME (min ) (b) Figure 5. Original records of two tension experiments with HUA rings in Ca2+ free medium. a) Ca2+-free me- dium contration of HUA induced by KCl (60 mM) which is relaxed by Y27 (10 M); b) Ca2+-free medium contrac- tion of HUA induced by 5-HT (1 M) in presence and in absence of Y27 (10 M). 4. DISCUSSION The present study investigated the involvement of some mechanism, such as different types of Ca2+ channels, cyclic nucleotides and different kinases, in th e regulation of the HUA contractility. The elucidation of the precise mechanism regulating the contractility of this artery could be very important to detect potential therapeutic targets to treat HUA disorders such as preeclampsia. We firstly investigated th e relevanc y of different types of Ca2+ channels on the regulation of HUA tonus. Our results show that the inhibition of LTCC relaxes con- tracted HUA, even if this effect does not reach 100% of relaxation. Other authors have previously reported that, among different Ca2+ channel blockers, nifedipine is the most potent umbilical vasodilator [18]. At the vascular level, it has been shown that nifedipine does not fully relax contractions induced by KCl. The existence of in- tracellular Ca2+ release when the arteries are contracted  A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 327 Y27 AOPDSBNIF 0 20 40 60 80 100 % of relaxation on KCl contraction in 0Ca m edium OA (a) HISHIS + Y27HIS + PDHIS + SB 0 200 400 600 800 1000 1200 (6) (8) (18) (6) (8) (18) Contraction induced by HIS (mg) in 0Ca medium Phasic contraction Tonic contraction * (4) # (4) (b) 5-H T 5-HT + Y2 7 5-HT + PD5-HT + SB 0 200 400 600 800 1000 120 0 (5) (5) (5) (5) (14) (14) Contraction induced by 5-HT (mg) in 0Ca medium Phasic contraction Tonic con traction * (4) # (4) (c) Figure 6. Rel a xan t e ffect of different inhibitors of kinases on HUA cont racted in Ca2+-free medium. a) Effect of Y27 (10 M), OA (5 nM), PD (50 M), SB (25 M) and nifedipine (NIF; 10 M) on KCl induced contraction in Ca2+-free medium; b) Effect of Y27 (10 M), PD (50 M) and SB (25 M) on the phasic and tonic contraction in- duced by histamine (10 M) in Ca2+-free medium; c) Ef- fect of Y27 (10 M), PD (50 M) and SB (25 M) on the phasic and tonic contraction induced by 5-HT (1 M) in Ca2+-free medium. The bars represent the means and the lines the S.E.M. of the numbers of experiments indicated near the bars. Statistical significant differences versus the effect in absence of inhibitor are indicated (*P < 0.05, one-way ANOVA with Dunnet´s post hoc test). by KCl has been suggested as responsible of the lack of a full relaxant effect of LTCC inhibitors [7]. In this sense, and as we will discuss later, the ability of KCl to in duce HUA contractions in Ca2+-free medium could be due to stimulation of intracellular Ca2+ release. The relaxant effect of nifedipine was stronger when HUA were con- tracted by depolarization or by histamine than in arteries contracted by 5-HT. Mikkelsen et al. also have observed that, in human pulmonary arteries, the contraction in- duced by 5-HT is more resistant to nifedipine than the contraction mediated by depolarization [8]. On the other hand, several studies demonstrated that TTCC and TRP channels are involved in VSM contraction in different arteries [5,9,10,47]. However, our results show the ab- sence of effect of mibefradil and APB, suggesting that Ca2+ entry through TTCC and TRP channels does not contribute to HUA contraction. The precise regulation of the intracellular levels of cAMP and cGMP plays an important role in many phy- siological processes, including vascular smooth muscle contractility [32]. However, the mechanisms by which increases in cAMP and cGMP concentration lead to ar- tery relaxation are still unclear. Some authors have re- ported that forskolin, a direct stimulator of adenylate cyclase, induced relaxation of different human vessels such as dorsal artery [34], pulmonary artery [35], pla- cental vessels [36] and umbilical artery [37]. On the other hand, among the four families of PDE expressed in this smooth muscle (PDE1, PDE3, PDE4 and PDE5), PDE4 has been shown as the key enzyme involved in the regulation of HUA relaxation associated to cAMP [37]. In this sense, we used forskolin (adenylate cyclase stimulator) and rolipram (PDE4 inhibitor) as drugs that can elicit the maximal increase in cAMP levels in HUA smooth muscle cells. The conjoint application of both drugs elicited different degree of relaxation depending on the contractile agent used. When applied together, these drugs almost fully relaxed histamine contracted HUA, but only 41% and 10% of relaxation was obtained on HUA contracted by KCl and 5-HT, respectively. We have previously shown that 5-HT2A, 5-HT1B/1D and 5-HT7 receptors are present in HUA smooth muscle cells. The HUA contraction induced by 5-HT are mainly me- diated by the activation of 5-HT2A, which activation in- creases IP3 levels, and 5-HT1B/1D receptors, which acti- vation inhibits adenylate cyclase [48]. Concerning the histamine receptors, H1 receptor activation induces con- traction and H2 and H3 receptors activation mediates HUA relaxation through the increase of cAMP intracel- lular levels [48]. According with th ese previo u s findings, the activation of different 5-HT receptors in HUA inh ib- its adenylate cyclase and the activation of different his- tamine receptors induces contraction, but also induces a small increase of cAMP levels due to H2 and H3. Thus, histamine contraction s are more susceptible to activato rs  A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 328 of adenylate cyclase or to PDE4 inhibitors than 5-HT contractions. On the other hand, apparently KCl does not affect adenylate cyclase activity and the effect of forskolin and rolipram has been lower than in histamine-contracted arteries and bigger than HUA contracted by 5-HT. Is well known that NO activates soluble guanylate cy- clase increasing cGMP levels, which induces vasodilata- tion [38], including HUA vasodilatation [37,39]. On the other hand, PDE5 has been shown as being the key en- zyme involved in the regu lation of HUA relaxatio n asso- ciated to cGMP [37]. In this sense, we used SNP (guanylate cyclase stimulator) and rolipram (PDE5 in- hibitor) as drugs that can elicit the maximal increase in cGMP levels in HUA smooth muscle cells. As for cAMP, the conjoint application of these drugs has elicited dif- ferent degree of relaxation depending on the contractile agent used. The biggest relaxant effect was observed in contraction induced by histamine, followed by contrac- tions produced by 5-HT and by KCl depolarization. Numerous authors have described that cGMP induced vasodilatation is mediated by activation of potassium channels in different arteries [40,41], including HUA [39]. The regulation of voltage-dependent potassium channels (KV) by KCl and 5-HT could be responsible of these differences. It has been described that vascular contraction induced by KCl is mainly due to the influx of extracellular Ca2+ via voltage-dependent Ca2+ chan- nels [1], but Kv channel inactivation at high potassium concentrations (60 mM) was also demonstrated in some blood vessels [49]. Recently, using patch clamp tech- niques, some authors have demonstrated that 5-HT de- creases Kv channel activity in cells of rat pulmonary [50] and mesenteric [51] arteries. Thus, as the cGMP relaxant effect seems to be mediated by potassium channels acti- vation, this effect is lower when HUA are contracted by KCl and 5-HT because these agents inhibit these chan- nels. Our results have shown that the PKA inhibition in- duced a significant reduction on the forskolin plus rolip- ram effect in KCl and histamine contracted arteries, even if this effect is small. The relaxant effect of cAMP in- crease in 5-HT contracted arteries was very low (around 10%) and KTa have failed to significantly decrease the relaxations induced by forskolin plus rolipram. Also, the PKG inhibition induced a significant reduction on the SNP plus dipyridamol effect in histamine and 5-HT con- tracted arteries. The analysis of these results suggests that both PKA and PKG are involved in HUA relaxa tion mediated by cAMP and cGMP respectively. However, the contribution of these kinases is very small and in some cases was not significant. These results suggest that the relaxation induced by cyclic nucleotides is not totally mediated by the activation of their respective kinases and other mechanisms can be involved as de- scribed by other authors [44,45] As we mentioned before, LTCC are critically impor- tant for regulating HUA contraction. It has been de- scribed that LTCC can be dephosphoralyted by PP2A, and inhibition of PP2A was found to result in ch anges in functional properties of the LTCC [21]. However, there is small number of studies on this matter in smooth mus- cle and the direction of this modulation has remained somewhat controversial. Some authors have shown that in tracheal smooth muscle PP2A does not modulate LTCC [19]. Also, it has been shown a dual effect of phosphatase inhibitors on Ca2+ channels from intestinal smooth muscle cells [20]. At the vascular level, Gro- schner et al. have demonstrated that, in human umbilical vein, when PP2A is inhibited by OA there is increase of LTCC activity [21]. Our results show that PP2A inhibi- tion produces relaxation of HUA, namely when this ar- tery is contracted by KCl and histamine. This relaxant effect is bigger in arteries contracted by KCl. Our results also show that in KCl contracted arteries, the OA effect is similar to the nifedipine effect. When applied together, the relaxant effect is not bigger than the relaxation in- duced by nifedipine or OA applied alone. These results suggest that PP2A could activate LTCC in HUA. On the other hand, the increase of cAMP or cGMP induced by the conjoint application of cyclase activators and PDE inhibitors significantly increased the OA effect. These results suggest that the relaxation induced by PP2A inhi- bition is independent of the cyclic nucleotide pathway. We also have analyzed the effect of some kinases on HUA contractility. Our results show that ROCK, ERK1/2 or p38MAPK inhibition does not affect the contraction induced by KCl, histamine or 5-HT in Ca2+- containing extracellular solution. Other authors have obtained significant relaxation of HUA contracted by 5-HT after ROCK inhibition, but using higher concen- trations of Y27 (100 µM) which can inhibit also myosin light chain kinase directly [22]. Also, Tasaki et al. ob- served that SB significantly inhibits 5-HT-induced con- tractions in rat aorta [30]. Some authors indicated that 5-HT induces contractions of rat aortic smooth muscle by activating the MAPK pathway [29]. Also, activation of p38 MAPK [30] and Erk MAPK [31] by 5-HT have been shown in rat aorta. Our results suggest that in the contractions induced by KCl, histamine and 5-HT in presence of Ca2+ there is not involvement of pathways concerning ROCK, ERK1/2 or p38MAPK. Sakurada et al. have shown the existence of a Ca2+-dependent Rho stimulation mechanism in VSM from rabbit aorta which is activated b y excitatory recep- tor agonists [23]. However, in HUA our results exclude this possibility, because in presence of extracellular Ca2+ ROCK does not seem to be activated by KCl, histamine or 5-HT. On the other hand, in absence of extracellular Ca2+, histamine and 5-HT elicited contractions of HUA char- acterized by two components, a rapid phasic contractile  A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 329 component, 2-3 min after stimulation by the agonist, followed by a decrease of the contraction until a tonic component, 15-20 min after. The initial transient com- ponent has been associated with Ca2+ release from the sarcoplasmic reticulum, whereas the tonic component seems to be dependent on the increase on extracellular Ca2+. Both contractile responses are dependent on the Ca2+ sensitization and two main pathways have been implicated in this phenomenon: the inhibition of myosin light chain phosphatase (MLCP) by ROCK; and the phosphorylation of the thin filament proteins by p38MAPK and ERK1/2 [5 ,52]. In contrasts, KCl elicited sustained contractions of HUA in absence of extracellu- lar Ca2+. This effect could be induced by a progressive Ca2+ release from intracellular Ca2+ stores and/or an in- crease of Ca2+ sensitization. These data agree with the obtained by Tufan et al. in HUA, which have suggested that Ca2+ independent isozymes of protein kinase C may also be involved in the contractions produced by KCl in absence of extracellular Ca2+ [1]. Some authors have also suggested that depolarization by KCl induces intra- cellular Ca2+ release in human arteries [7]. Besides, it has been described that KCl depolarization increases Ca2+ sensitization by ROCK activation in smooth muscle cells from rabbit arteries [24]. As expected, in absence of extracellular Ca2+, nifedipine did not relax the contrac- tions elicited by KCl. Also in these conditions, OA did not relax the contractions elicited by KCl. Once more, these data suggest a functional relationship between the PP2A and LTCC in the regulation of HUA contractility. Concerning the kinases, the ERK1/2 and p38MAPK inhibitors did not reduced or relax the contractions in- duced by KCl, histamine or 5-HT in absence of ex- tracellular Ca2+. Thus, these results demonstrate that KCl-induced contractions are not linked to the activation of ERK1/2 and p38MAPK. However, in Ca2+-free me- dium, the ROCK inhibition significantly relaxed the contractions induced by KCl and reduced the phasic and tonic components of the contraction elicited either by histamine or 5-HT. Similar results were obtained by Ark et al. when HUA were contracted by 5-HT in Ca2+ free medium [22]. Our data demonstrate that ROCK is capa- ble of mediating the contractile response after stimula- tion of a receptor agonist or by depolarization. Thus, in absence of Ca2+, the sustained contractions elicited by KCl and the phasic and tonic components of the contrac- tion induced by histamine or 5-HT depend on ROCK activation. Consequently, these contractions depend of Ca2+ sensitization by the inhibition of MLCP by ROCK. Our data show that the relevancy of the Ca2+ dependent or independent mechanism in HUA varies in function of the extracellular Ca2+ level, and further experiments are necessary to deeply study this dependence. On the other hand, our results suggest that HUA is a good sample to study the Ca2+ sensitization mechanism induced by ROCK. In conclusion, our results demonstrate that the LTCC are the main way for Ca2+ entry and is involved in HUA contractions induced by depolarization or by agonists. A positive regulation of LTCC by PP2A seems to occur in HUA smooth muscle cells. Cyclic nucleotides are in- volved in HUA vasodilatation. The relaxant effect of cyclic nucleotides is partially due to the activation of their respective kinases, but other pathways are also in- volved. HUA is able to contract independently of Ca2+ influx by activating the ROCK pathway and/or due to intracellular Ca2+ release. 5. ACKNOWLEDGEMENTS We thank the donor mothers and the Gynaecoogy-Obste -trics Depart- ment staff of “Centro Hospitalar da Cova da Beira” (Covilhã, Portugal) for their disinteested collabration and the “Fundação para a Ciência e a Tecnologia” (Portugal) for supporting the.grant SFRH/BDE/15532/ 2004. REFERENCES [1] Tufan, H., Ayan-Polat, B, Tecder-Unal, M, Polat, G, Kayhan, Z and Ogus, E. (2003) Contractile responses of the human umbilical artery to KCl and serotonin in Ca-free medium and the effects of levcromakalim. Life Sciences, 72, 1321-1329. [2] Leung, S.W., Quan, A., Lao, T.T. and Man, R.Y. (2006) Efficacy of different vasodilators on human umbilical ar- terial smooth muscle under normal and reduced oxygen conditions. Early Human Development, 82, 457-462. [3] Michael, S.K., Surks, H.K., Wang, Y., Zhu, Y., Blanton, R., Jamnongjit, M., Aronovitz, M., Baur, W., Ohtani, K., Wilkerson, M.K., Bonev, A.D., Nelson, M.T., Karas, R.H. and Mendelsohn, M.E. (2008) High blood pressure aris- ing from a defect in vascular function. Proceedings of the National Academy of Sciences of the United States of America, 105, 6702-6707. [4] McCarron, J.G., Craig, J.W., Bradley, K. N. and Muir, T.C. (2002) Agonist-induced phasic and tonic responses in smooth muscle are mediated by InsP(3). Journal of Cell Science, 115, 2207-2218. [5] Hilgers, R.H. and Webb, R.C. (2005) Molecular aspects of arterial smooth muscle contraction: Focus on Rho. Experimental Biology and Medicine (Maywood), 230, 829-835. [6] Somara, S. and Bitar, K.N. (2006) Phosphorylated HSP27 modulates the association of phosphorylated cal- desmon with tropomyosin in colonic smooth muscle. American Journal of Physiology - Gastrointestinal and Liver Physiology, 291, G630-639. [7] Hall, J., Jones, T.H., Channer, K.S. and Jones, R.D. (2006) Mechanisms of agonist-induced constriction in isolated human mesenteric arteries. Vascular Pharmacology, 44, 427-433. [8] Mikkelsen, E.O., Sakr, A.M. and Jespersen, L.T. (1983) Effects of nifedipine on contractile responses to potas- sium, histamine, and 5-hydroxytryptamine in isolated human pulmonary vessels. Journal of Cardiovascular  A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 330 Pharmacology, 5, 317-320. [9] Potocnik, S.J., Murphy, T.V., Kotecha, N. and Hill, M.A. (2000) Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca(2+). Brit- ish Journal of Pharmacology, 131, 1065-1072. [10] Liu, M., Large, W.A. and Albert, A.P. (2005) Stimulation of beta-adrenoceptors inhibits store-operated channel currents via a cAMP-dependent protein kinase mecha- nism in rabbit portal vein myocytes. Journal of Physiol- ogy, 562, 395-406. [11] Muraki, K., Iwata, Y., Katanosaka, Y., Ito, T., Ohya, S., Shigekawa, M. and Imaizumi, Y. (2003) TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circulation Research, 93, 829- 838. [12] Park, K.S., Lee, H.A., Earm, K.H., Ko, J.H., Earm, Y.E. and Kim, S.J. (2006) Differential distribution of mech- anosensitive nonselective cation channels in systemic and pulmonary arterial myocytes of rabbits. Journal of Vascular Research, 43, 347-354. [13] Dietrich, A., Chubanov, V., Kalwa, H., Rost, B.R. and Gudermann, T. (2006) Cation channels of the transient receptor potential superfamily: Their role in physiologi- cal and pathophysiological processes of smooth muscle cells. Pharmacology & Therapeutics. [14] Beech, D.J., Muraki, K. and Flemming, R. (2004) Non- selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol, 559, 685-706. Li, S., Gosling, M. and Poll, C. (2005) Determining the functional role of TRPC channels in primary cells. Pflugers Archiv (European Journal of Physiology), 451, 43-52. [15] Cribbs, L.L. (2006) T-type Ca2+ channels in vascular smooth muscle: Multiple functions. Cell Calcium, 40, 221-230. [16] Akaike, N., Kanaide, H., Kuga, T., Nakamura, M., Sa- doshima, J. and Tomoike, H. (1989) Low-voltage-acti- vated calcium current in rat aorta smooth muscle cells in primary culture. Journal of Physiology, 416, 141-160. [17] Salemme, S., Rebolledo, A., Speroni, F., Petruccelli, S. and Milesi, V. (2007) L, P-/Q- and T-type Ca2+ channels in smooth muscle cells from human umbilical artery. Cellular Physiology and Biochemistry, 20, 55-64. [18] Belfort, M.A., Saade, G.R., Suresh, M., Johnson, D. and Vedernikov, Y.P. (1995) Human umbilical vessels: re- sponses to agents frequently used in obstetric patients. American Journal of Obstetrics and Gynecology, 172, 1395-1403. [19] Obara, K., Takai, A., Ruegg, J.C. and de Lanerolle, P. (1989) Okadaic acid, a phosphatase inhibitor, produces a Ca2+ and calmodulin-independent contraction of smooth muscle. Pflugers Archiv (European Journal of Physiol- ogy), 414, 134-138. [20] Obara, K. and Yabu, H. (1993) Dual effect of phos- phatase inhibitors on calcium channels in intestinal smooth muscle cells. American Journal of Physiology, 264, C296-301. [21] Groschner, K., Schuhmann, K., Mieskes, G., Baumgart- ner, W. and Romanin, C. (1996) A type 2A phosphatase- sensitive phosphorylation site controls modal gating of L-type Ca2+ channels in human vascular smooth-muscle cells. Biochemical Journal, 318, 513-517. [22] Ark, M., Ozveren, E., Yazici, G., Korkmaz, B., Buyukaf- sar, K., Arikan, O., Kubat, H. and Songu-Mize, E. (2004) Effects of HA-1077 and Y-27632, two rho-kinase inhibi- tors, in the human umbilical artery. Cell Biochemistry and Biophysics, 41, 331-342 [23] Sakurada, S., Takuwa, N., Sugimoto, N., Wang, Y., Seto, M., Sasaki, Y. and Takuwa, Y. (2003) Ca2+-dependent ac- tivation of Rho and Rho kinase in membrane depolariza- tion-induced and receptor stimulation-induced vascular smooth muscle contraction. Circulation Research, 93, 548-556. [24] Ratz, P.H. and Miner, A.S. (2009) Role of protein kinase Czeta and calcium entry in KCl-induced vascular smooth muscle calcium sensitization and feedback control of cellular calcium levels. Journal of Pharmacology and Experimental Therapeutics, 328, 399-408. [25] Zhao, Y., Long, W., Zhang, L. and Longo, L.D. (2003) Extracellular signal-regulated kinases and contractile re- sponses in ovine adult and fetal cerebral arteries. Journal of Physiology, 551, 691-703. [26] Muslin, A.J. (2008) MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeu- tic targets. Clinical Science (London), 115, 203-218. [27] Somlyo, A.P. and Somlyo, A.V. (1994) Signal transduc- tion and regulation in smooth muscle. Nature, 372, 231-236. [28] Watts, S.W. (1996) Serotonin activates the mito- gen-activated protein kinase pathway in vascular smooth muscle: Use of the mitogen-activated protein kinase in- hibitor PD098059. Journal of Pharmacology and Ex- perimental Therapeutics, 279, 1541-1550. [29] Kim, B., Kim, J., Bae, Y.M., Cho, S.I., Kwon, S.C., Jung, J.Y., Park, J.C. and Ahn, H.Y. (2004) p38 mito- gen-activated protein kinase contributes to the dimin- ished aortic contraction by endothelin-1 in DOCA-salt hypertensive rats. Hypertension, 43, 1086-1091. [30] Tasaki, K., Hori, M., Ozaki, H., Karaki, H. and Waka- bayashi, I. (200 3) Difference in si gnal transduction mecha- nisms involved in 5-hydroxytryptamine- and U46619-in- duced vasoconstrictions. Journal of Smooth Muscle Re- search, 39, 107-117. [31] Banes, A., Florian, J.A. and Watts, S.W. (1999) Mecha- nisms of 5-hydroxytryptamine(2A) receptor activation of the mitogen-activated protein kinase pathway in vascular smooth muscle. Journal of Pharmacology and Experi- mental Therapeutics, 291, 1179-1187. [32] Matsumoto, T., Kobayashi, T. and Kamata, K. (2003) Phosphodiesterases in the vascular system. Journal of Smooth Muscle Research, 39, 67-86. [33] Bentley, J.K. and Beavo, J.A. (1992) Regulation and function of cyclic nucleotides. Current Opinion in Cell Biology, 4, 233-240. [34] Segarra, G., Medina, P., Vila, J.M., Martinez-Leon, J.B., Domenech, C., Prieto, F. and Lluch, S. (2002) Relaxation induced by milrinone and rolipram in human penile ar- teries and veins. European Journal of Pharmacology, 444, 103-106. [35] Rabe, K.F., Tenor, H., Dent, G., Schudt, C., Nakashima, M. and Magnussen, H. (1994) Identification of PDE isozymes in human pulmonary artery and effect of selec- tive PDE inhibitors. American Journal of Physiology, 266, L536-543.  A. J. Santos-Silva et al. / Health 2 (2010) 321-331 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 331 [36] Figueroa, R., Martinez, E., Fayngersh, R.P., Tejani, N., Mohazzab, H.K. and Wolin, M.S. (2000) Alterations in relaxation to lactate and H2O2 in human placental vessels from gestational diabetic pregnancies. American Journal of Physiology—Heart and Circulatory Physiology, 278, H706-713. [37] Santos-Silva, A.J., Cairrão, E., Morgado, M., Álvarez, E. and Verde, I. (2008) PDE4 and PDE5 regulate cyclic nu- cleotides relaxing effects in human umbilical arteries. European Journal of Pharmacology, 582, 102-109. [38] Kristek, F., Koprdova, R. and Cebova, M. (2007) Long- term effects of early administered sildenafil and NO do- nor on the cardiovascular system of SHR. Journal of Physiology and Pharmacology, 58, 33-43. [39] Lovren, F. and Triggle, C. (2000) Nitric oxide and so- dium nitroprusside-induced relaxation of the human um- bilical artery. British Journal of Pharmacology, 131, 521-529. [40] Wu, C.C., Chen, S.J. and Yen, M.H. (1999) Cyclic GMP regulates cromakalim-induced relaxation in the rat aortic smooth muscle: role of cyclic GMP in KATP-channels. Life Sciences, 64, 2471-2478. [41] Li, P.L., Jin, M.W. and Campbell, W.B. (1998) Effect of selective inhibition of soluble guanylyl cyclase on the KCa channel activity in coronary artery smooth muscle. Hypertension, 31, 303-308. [42] Lincoln, T.M., Cornwell, T.L. and Taylor, A.E. (1990) cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. American Journal of Physiology, 258, C399-407; Murray, K.J. (1990) Cyclic AMP and mechanisms of vasodilation. Pharmacology & Therapeutics, 47, 329-345. [43] Keef, K.D., Hume, J.R. and Zhong, J. (2001) Regulation of cardiac and smooth muscle Ca2+ channels (Ca(V) 1.2a,b) by protein kinases. American Journal of Physiol- ogy, 281, C1743-1756. [44] Sausbier, M., Schubert, R., Voigt, V., Hirneiss, C., Pfeifer, A., Korth, M., Kleppisch, T., Ruth, P. and Hofmann, F. (2000) Mechanisms of NO/cGMP-dependent vasorelaxa- tion. Circulation Research, 87, 825-830. [45] Jensen, J. (2007) More PKA independent beta-adrenergic signalling via cAMP: Is Rap1-mediated glucose uptake in vascular smooth cells physiologically important? Brit- ish Journal of Pharmacology, 151, 423-425. [46] Cairrao, E., Alvarez, E., Santos-Silva, A.J. and Verde, I. (2008) Potassium channels are involved in testoster- one-induced vasorelaxation of human umbilical artery. Naunyn-Schmiedeber’s Archives of Pharmacology, 376, 375-383. [47] Tosun, M., Paul, R.J. and Rapoport, R.M. (1998) Cou- pling of store-operated Ca2+ entry to contraction in rat aorta. Journal of Pharmacology and Experimental Therapeutics, 285, 759-766. [48] Santos-Silva, A.J., Cairrao, E., Marques, B. and Verde, I. (2009) Regulation of human umbilical artery contractility by different serotonin and histamine receptors. Repro- ductive Sciences, 16, 1175-1185. [49] Jarajapu, Y.P., Oomen, C., Uteshev, V.V. and Knot, H.J. (2006) Histamine decreases myogenic tone in rat cerebral arteries by H2-receptor-mediated KV channel activation, independent of endothelium and cyclic AMP. European Journal of Pharmacology, 547, 116-124. [50] Cogolludo, A., Moreno, L., Lodi, F., Frazziano, G., Co- beno, L., Tamargo, J. and Perez-Vizcaino, F. (2006) Se- rotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: Role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circula- tion Research, 98, 931-938. [51] Bae, Y. M., Kim, A., Kim, J., Park, S.W., Kim, T.K., Lee, Y.R., Kim, B. and Cho, S.I. (2006) Serotonin depolarizes the membrane pot entia l in rat mese nteric a rtery myocyte s by decreasing voltage-gated K+ currents. Biochemical and Biophysical Research Communications, 347, 468- 476. [52] Hirano, K., Derkach, D.N., Hirano, M., Nishimura , J. and Kanaide, H. (2003) Protein kinase network in the regula- tion of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Molecular and Cellular Bio- chemistry, 248, 105-114. |

