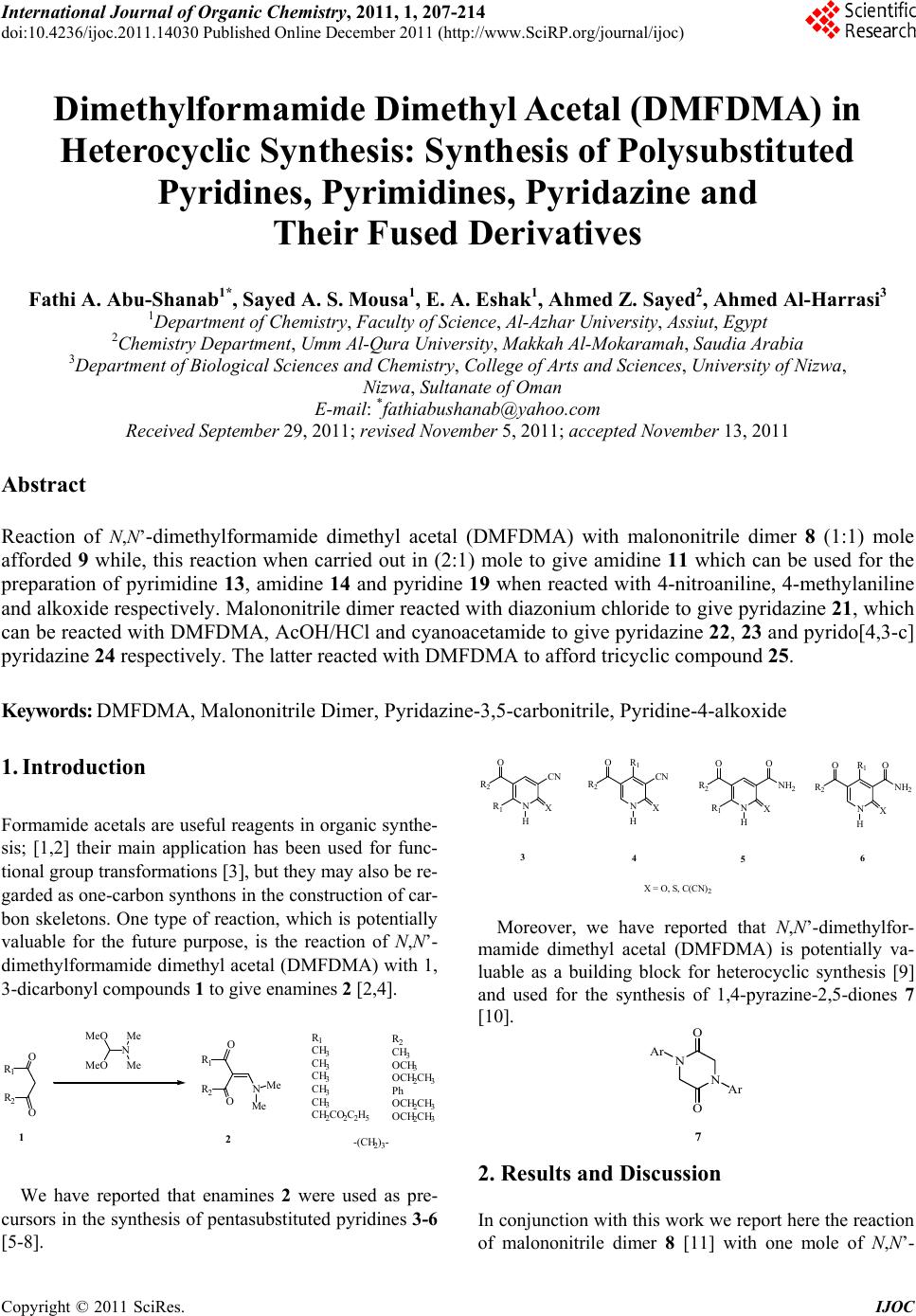
 International Journal of Organic Chemistry, 2011, 1, 207-214 doi:10.4236/ijoc.2011.14030 Published Online December 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC 207 Dimethylformamide Dimethyl Acetal (DMFDMA) in Heterocyclic Synthesis: Synthesis of Polysubstituted Pyridines, Pyrimidines, Pyridazine and Their Fused Derivatives Fathi A. Abu-Shanab1*, Sayed A. S. Mousa1, E. A. Eshak1, Ahmed Z. Sayed2, Ahmed Al-Harrasi3 1Department of Chemistry, Faculty of Science, Al-Azhar University, Assiut, Egypt 2Chemistry Department, Umm Al-Qura University, Makkah Al-Mokaramah, Saudia Arabia 3Department of Biol o gi cal Sciences and Chemis t ry , College of Arts and Sciences, University of Nizwa, Nizwa, Sultanate of Oman E-mail: *fathiabushanab@yahoo.com Received September 29, 2011; revised November 5, 2011; accepted November 13, 2011 Abstract Reaction of N,N’-dimethylformamide dimethyl acetal (DMFDMA) with malononitrile dimer 8 (1:1) mole afforded 9 while, this reaction when carried out in (2:1) mole to give amidine 11 which can be used for the preparation of pyrimidine 13, amidine 14 and pyridine 19 when reacted with 4-nitroaniline, 4-methylaniline and alkoxide respectively. Malononitrile dimer reacted with diazonium chloride to give pyridazine 21, which can be reacted with DMFDMA, AcOH/HCl and cyanoacetamide to give pyridazine 22, 23 and pyrido[4,3-c] pyridazine 24 respectively. The latter reacted with DMFDMA to afford tricyclic compound 25. Keywords: DMFDMA, Malononitrile Dimer, Pyridazine-3,5-carbonitrile, Pyridine-4-alkoxide 1. Introduction Formamide acetals are useful reagents in organic synthe- sis; [1,2] their main application has been used for func- tional group transformations [3], but they may also be re- garded as one-carbon synthons in the construction of car- bon skeletons. One type of reaction, which is potentially valuable for the future purpose, is the reaction of N,N’- dimethylformamide dimethyl acetal (DMFDMA) with 1, 3-dicarbonyl compounds 1 to give enamines 2 [2,4]. O O R2 R1 MeO MeO N Me Me O O R2 R1 NMe Me R1 CH 3 CH 3 CH 3 CH 3 CH 3 CH 2CO 2C2H5 R2 CH 3 OCH 3 OCH 2CH 3 Ph OCH 2CH 3 OCH 2CH 3 -(CH 2)3- 12 We have reported that enamines 2 were used as pre- cursors in the synthesis of pentasubstituted pyridines 3-6 [5-8]. N H NH2 R2 R1X O N H NH2 R2 X O N H CN R2 R1X O N H CN R2 X OR 1R1 OO 3456 X = O, S, C(CN)2 Moreover, we have reported that N,N’-dimethylfor- mamide dimethyl acetal (DMFDMA) is potentially va- luable as a building block for heterocyclic synthesis [9] and used for the synthesis of 1,4-pyrazine-2,5-diones 7 [10]. N N O O Ar Ar 7 2. Results and Discussion In conjunction with this work we report here the reaction of malononitrile dimer 8 [11] with one mole of N,N’-  F. A. ABU-SHANAB ET AL. 208 dimethylformamide dimethyl acetal (DMFDMA) in dry dioxane afforded only one product that could be formu- lated as 9 or 10 as result of condensation on either the amino or active methylene group. The structure of the isolated product was elucidated based on the spectral analysis. The 1H-NMR spectrum shows two singlet sig- nals at δH = 3.2 and 3.25 ppm corresponding to the two methyl groups of NMe2 moiety, singlet signal at δH= 7.59 ppm corresponding to methylene group or amino group and singlet signal at δH = 7.99 ppm corresponding to methine proton. While we could not differentiate be- tween 9 and 10 by 1H-NMR, DEPT-135 of 13C-NMR shows a methylene group at –66.78 ppm which indicates that the isolated product is 9 and not 10. This can be at- tributed to the fact that the nucleophilicity of the amino group is greater than that of methylene group. The treatment of malononitrile dimer 8 with two moles of N,N’-dimethylformamide dimethyl acetal (DM- FDMA) afforded amidine 11 in which N,N’-dimethyl- formamide dimethyl acetal (DMFDMA) reacted with bo- th the amino group and the active methylene. The mass spectrum of this compound shows molecular weight at m/z 242 which corresponds to structure 11. Amidine 11 can also be obtained by treatment of amidine 9 with an- other one mole of DMFDMA. The reaction of amidine 11 with one mole of aromatic amines (1:1) afforded the corresponding pyrimidine de- rivative 13. while the treatment of amidine 11 with two moles of aromatic amines (1:2) afforded formamidine 14 (Scheme 1). This suggests that the isolated pyrimidine 13 was formed through the intermediate 12. The struc- ture of these compounds was confirmed by elemental analysis as well as spectral analysis. The IR spectrum of compound 14 shows the appearance of two bands of υmax at 3286.3 cm–1, 3208.2 cm–1 corresponding to two (NH) groups, while the IR spectrum of compound 13 shows the disappearance of NH groups. The mass spectrum of compound 14 shows the molecular ion peak at m/z 366 which is in agreement with the proposed structure 14. We expected that the treatment of amidine 11 with so- dium alkoxide (sodium ethoxide, sodium methoxide, so- dium n-propoxide or sodium isopropoxide) in the corre- sponding alcohol would afford pyrido[4,3-d]pyrimidine derivatives 16 [12] through the cyclization of the inter- mediate 15 in which, two molecules of alcohol were added on the two cyano groups. However, the mass spec- tra of the isolated products shows a molecular weight whi- ch does not agree with the expected structure 16. Also the 1H-NMR spectra shows three exchangeable protons corresponding to NH and NH2 groups as well as only one aromatic proton. This means that the isolated product is not 16 and the reaction takes place by another pathway in which the intermediate 15 is attacked by the alkoxide to give intermediate 17 in which N,N’-dimethylformami- dine moiety is replaced by alkoxide group followed by hydrolysis and cyclization to give 4-alkoxy-5-cyano- pyridine-2(1H)-one-3-carboxylic acid amide 19. The str- ucture of the isolated product was confirmed by elemen- tal analysis as well as spectral data in which the IR spec- tra show the presence of NH, NH2 and cyano group. Also 1H-NMR spectra show two exchangeable protons for NH & NH2 and one aromatic proton. Sodium isopropoxide cannot react with amidine 11. This is due to the fact that the isopropoxide group is a bulkynucleophile. Since it does not replace the N,N’-dimethylamidine moiety because of the steric hindrance, we could not isolate pyridine isopropo- xide derivative (19d) (Scheme 2). The reaction of malononitrile dimer 8 with diazo- nium salts of aromatic amines 20a-e furnished the corre- sponding pyridazine derivatives 21a-e. The structure of the isolated products was confirmed by elemental analy- sis as well as spectral data. The IR spectra of these com- pounds show the appearance of amino and imino groups. Also the 1H-NMR spectra of these compounds 21a-e show the appearance of aromatic protons and two exchan- geable broad singlet signals corresponding to NH2 and NH groups. The pyridazine derivatives 21a-e were found to be a good intermediate for the formation of fused heterocyclic com- pounds. Reaction of pyridazine derivatives 21a-e with N,N’- dimethylformamide dimethyl acetal (DMFDMA) afforded H 2 N CNNC CN H 2 N CNNC CN NMe Me N CNNC CN NMe Me N Me Me N CNNC CN N Me Me N CNNC CN NH Ar HN Ar N CNNC CN NH N Me Me Ar N CNNC CN NMe Me HN Ar N N CN NC CN Ar ArNH 2 ArNH 2 DMFDMA DMFDMA or (9)(10) (11) (14) (12) or (1:1) (1:2) (8) (13) (1:1) (1:2) Ar = C 6 H 4 CH 3 -pAr = C 6 H 4 NO 2 -p DMFDMA (1:1) Scheme 1. The reaction and treatment of amidine 11. Copyright © 2011 SciRes. IJOC 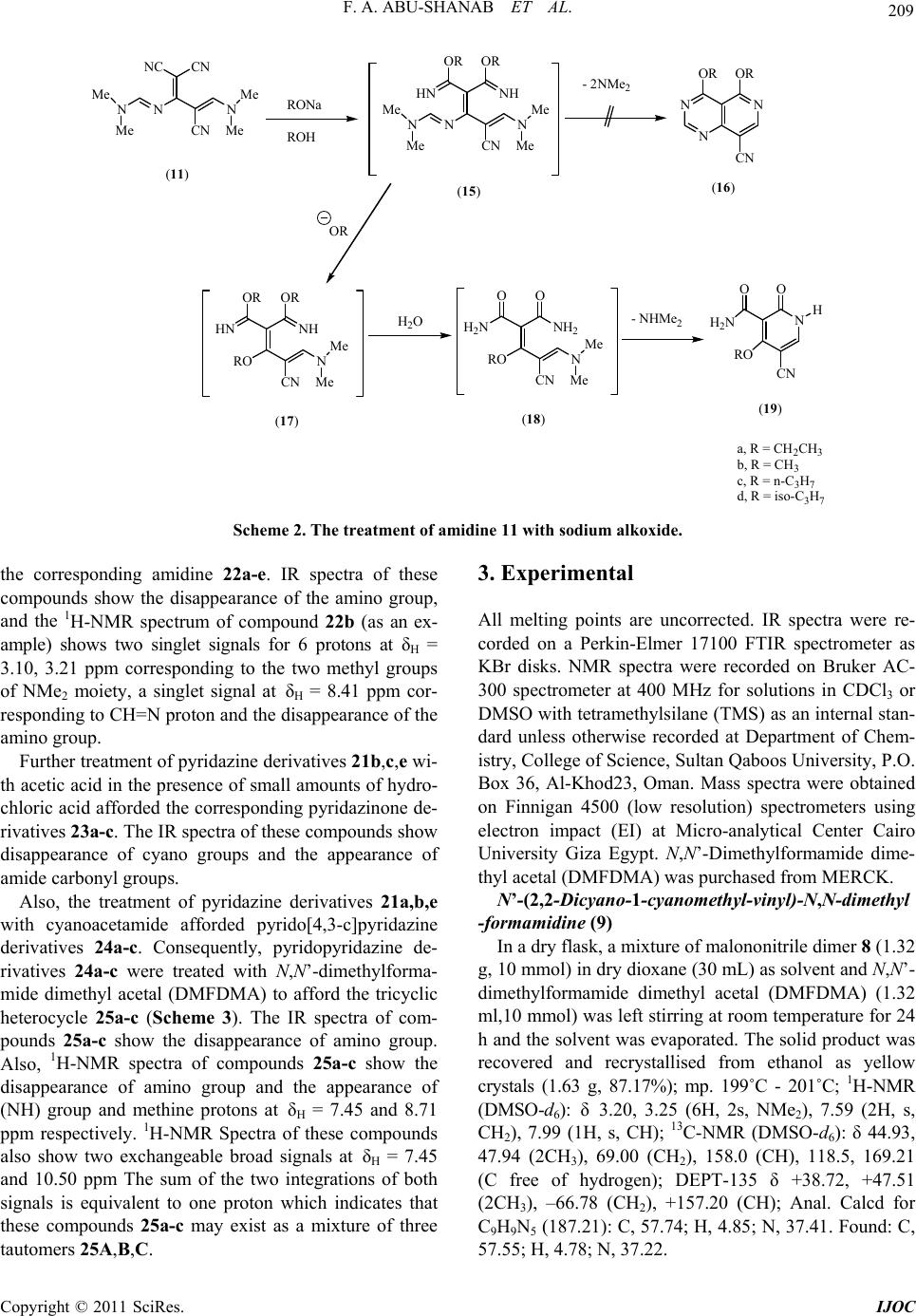 F. A. ABU-SHANAB ET AL. Copyright © 2011 SciRes. IJOC 209 N CNNC (11) CN NN Me Me Me Me N CN NN Me Me Me Me RONa ROH OROR NHHN - 2NMe2 NN N OROR CN OR RO CN N Me Me OROR NHHN H2O RO CN N Me Me OO NH2 H2N- NHMe2N O H CN O H2N RO (15)(16) (17)(18)(19) a, R = CH2CH3 b, R = CH3 c, R = n-C3H7 d, R = iso-C3H7 Scheme 2. The treatment of amidine 11 with sodium alkoxide. 3. Experimental the corresponding amidine 22a-e. IR spectra of these compounds show the disappearance of the amino group, and the 1H-NMR spectrum of compound 22b (as an ex- ample) shows two singlet signals for 6 protons at δH = 3.10, 3.21 ppm corresponding to the two methyl groups of NMe2 moiety, a singlet signal at δH = 8.41 ppm cor- responding to CH=N proton and the disappearance of the amino group. All melting points are uncorrected. IR spectra were re- corded on a Perkin-Elmer 17100 FTIR spectrometer as KBr disks. NMR spectra were recorded on Bruker AC- 300 spectrometer at 400 MHz for solutions in CDCl3 or DMSO with tetramethylsilane (TMS) as an internal stan- dard unless otherwise recorded at Department of Chem- istry, College of Science, Sultan Qaboos University, P.O. Box 36, Al-Khod23, Oman. Mass spectra were obtained on Finnigan 4500 (low resolution) spectrometers using electron impact (EI) at Micro-analytical Center Cairo University Giza Egypt. N,N’-Dimethylformamide dime- thyl acetal (DMFDMA) was purchased from MERCK. Further treatment of pyridazine derivatives 21b,c,e wi- th acetic acid in the presence of small amounts of hydro- chloric acid afforded the corresponding pyridazinone de- rivatives 23a-c. The IR spectra of these compounds show disappearance of cyano groups and the appearance of amide carbonyl groups. N’-(2,2-Dicyano-1-cyanomethyl-vinyl)-N,N-dimethyl -formamidine (9) Also, the treatment of pyridazine derivatives 21a,b,e with cyanoacetamide afforded pyrido[4,3-c]pyridazine derivatives 24a-c. Consequently, pyridopyridazine de- rivatives 24a-c were treated with N,N’-dimethylforma- mide dimethyl acetal (DMFDMA) to afford the tricyclic heterocycle 25a-c (Scheme 3). The IR spectra of com- pounds 25a-c show the disappearance of amino group. Also, 1H-NMR spectra of compounds 25a-c show the disappearance of amino group and the appearance of (NH) group and methine protons at δH = 7.45 and 8.71 ppm respectively. 1H-NMR Spectra of these compounds also show two exchangeable broad signals at δH = 7.45 and 10.50 ppm The sum of the two integrations of both signals is equivalent to one proton which indicates that these compounds 25a-c may exist as a mixture of three tautomers 25A,B,C. In a dry flask, a mixture of malononitrile dimer 8 (1.32 g, 10 mmol) in dry dioxane (30 mL) as solvent and N,N’- dimethylformamide dimethyl acetal (DMFDMA) (1.32 ml,10 mmol) was left stirring at room temperature for 24 h and the solvent was evaporated. The solid product was recovered and recrystallised from ethanol as yellow crystals (1.63 g, 87.17%); mp. 199˚C - 201˚C; 1H-NMR (DMSO-d6): δ 3.20, 3.25 (6H, 2s, NMe2), 7.59 (2H, s, CH2), 7.99 (1H, s, CH); 13C-NMR (DMSO-d6): δ 44.93, 47.94 (2CH3), 69.00 (CH2), 158.0 (CH), 118.5, 169.21 (C free of hydrogen); DEPT-135 δ +38.72, +47.51 (2CH3), –66.78 (CH2), +157.20 (CH); Anal. Calcd for C9H9N5 (187.21): C, 57.74; H, 4.85; N, 37.41. Found: C, 57.55; H, 4.78; N, 37.22. 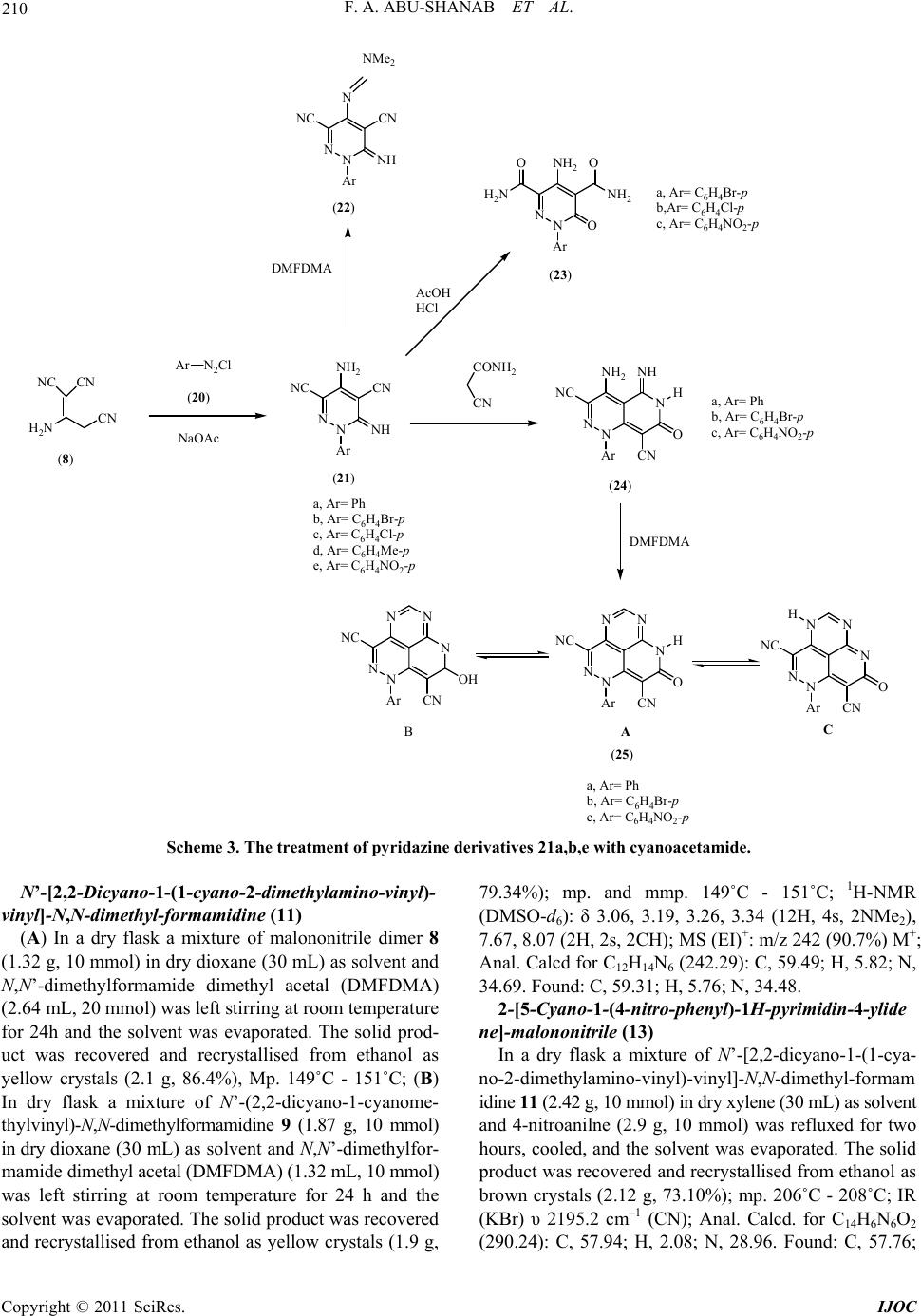 F. A. ABU-SHANAB ET AL. 210 CN H 2 N NC CN Ar N 2 Cl NaOAc NN Ar NH 2 NC CN NH Ar NN N NC CN NH NMe 2 DMFDMA NN Ar NH 2 O NH 2 H 2 N OO CONH 2 CN NN Ar NH 2 NC N NH H O CN NN Ar NC NH O CN NN DMFDMA Ar O CN N H NN NC N N NN Ar NC N CN NN OH A BC (24) a, Ar= Ph b, Ar= C 6 H 4 Br-p c, Ar= C 6 H 4 Cl-p d, Ar= C 6 H 4 Me-p e, Ar= C 6 H 4 NO 2 -p (8) (20) (21) (22) AcOH HCl (23) (25) a, Ar= C 6 H 4 Br-p b,Ar= C 6 H 4 Cl-p c, Ar= C 6 H 4 NO 2 -p a, Ar= Ph b, Ar= C 6 H 4 Br-p c, Ar= C 6 H 4 NO 2 -p a, Ar= Ph b, Ar= C 6 H 4 Br-p c, Ar= C 6 H 4 NO 2 -p Scheme 3. The treatment of pyridazine derivatives 21a,b,e with cyanoacetamide. N’-[2,2-Dicyano-1-(1-cyano-2-dimethylamino-vinyl)- vinyl]-N,N-dimethyl-formamidine (11) (A) In a dry flask a mixture of malononitrile dimer 8 (1.32 g, 10 mmol) in dry dioxane (30 mL) as solvent and N,N’-dimethylformamide dimethyl acetal (DMFDMA) (2.64 mL, 20 mmol) was left stirring at room temperature for 24h and the solvent was evaporated. The solid prod- uct was recovered and recrystallised from ethanol as yellow crystals (2.1 g, 86.4%), Mp. 149˚C - 151˚C; (B) In dry flask a mixture of N’-(2,2-dicyano-1-cyanome- thylvinyl)-N,N-dimethylformamidine 9 (1.87 g, 10 mmol) in dry dioxane (30 mL) as solvent and N,N’-dimethylfor- mamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol) was left stirring at room temperature for 24 h and the solvent was evaporated. The solid product was recovered and recrystallised from ethanol as yellow crystals (1.9 g, 79.34%); mp. and mmp. 149˚C - 151˚C; 1H-NMR (DMSO-d6): δ 3.06, 3.19, 3.26, 3.34 (12H, 4s, 2NMe2), 7.67, 8.07 (2H, 2s, 2CH); MS (EI)+: m/z 242 (90.7%) M+; Anal. Calcd for C12H14N6 (242.29): C, 59.49; H, 5.82; N, 34.69. Found: C, 59.31; H, 5.76; N, 34.48. 2-[5-Cyano-1-(4-nitro-phenyl)-1H-pyrimidin-4-ylide ne]-malononitrile (13) In a dry flask a mixture of N’-[2,2-dicyano-1-(1-cya- no-2-dimethylamino-vinyl)-vinyl]-N,N-dimethyl-formam idine 11 (2.42 g, 10 mmol) in dry xylene (30 mL) as solvent and 4-nitroanilne (2.9 g, 10 mmol) was refluxed for two hours, cooled, and the solvent was evaporated. The solid product was recovered and recrystallised from ethanol as brown crystals (2.12 g, 73.10%); mp. 206˚C - 208˚C; IR (KBr) υ 2195.2 cm–1 (CN); Anal. Calcd. for C14H6N6O2 (290.24): C, 57.94; H, 2.08; N, 28.96. Found: C, 57.76; Copyright © 2011 SciRes. IJOC  211 F. A. ABU-SHANAB ET AL. H, 2.02; N, 28.79. N-[2,2-Dicyano-1-(1-cyano-2-p-tolylamino-vinyl)-vin yl]-N’-p-tolyl-formamidine (14) In a dry flask a mixture of N’-[2,2-dicyano-1-(1-cya- no-2-dimethylamino-vinyl)-vinyl]-N,N-dimethyl-formam idine 11 (2.42 g, 10 mmol) in dry xylene (30 mL) as sol- vent and p-toluidine (2.14 g, 20 mmol) was refluxed for two hours, cooled and the solvent was evaporated. The solid product was recovered and recrystallised from eth- anol as dark brown crystals (2.61 g, 71.31%); mp. 289˚C - 291˚C; 1H-NMR (DMSO-d6): δ 2.12, 2.24 (6H, 2s, 2CH3), 6.42, 6.82 (8H, 2d, Ar-AB), 7.05, 7.09 (2H, 2s, 2NH), 7.54, 7.56 (2H, 2s, 2CH); IR (KBr) υ 3286.3, 3208.2 (2NH), 2225.2, 2204.3 cm–1 (3CN); MS (EI)+: m/z 366 (10.7%) M+; Anal. Calcd. for C22H18N6 (366.43): C, 72.11; H, 4.95; N, 22.93. Found: C, 71.92; H, 4.84; N, 22.70. Genera l proced ure for the prepara tion of comp ounds 19a-c A mixture of Compound 11 (10 mmol) and sodium al- koxide (10 mmol) in corresponding alcohol (30 mL) was refluxed for two hours. The mixture was left to cool then poured onto ice cold water. The solid product was re- covered by filtration and recrystallised from ethanol. 5-Cyano-4-ethoxy-2-oxo-1,2-dihydro-pyridine-3-carb oxylic acid amide (19a): Obtained from 11 (2.42 g, 10 mmol) with sodium ethoxide (Na 0.23 g, EtOH 30 mL, 10 mmol); mp. 219˚C - 221˚C as brown crystals (1.46 g, 70.53%); 1H-NMR (DMSO-d6): δ 1.28, 1.32, 1.35 (3H, t, CH3), 4.36, 4.39, 4.43, 4.47 (2H, q, CH2), 7.49 (1H, s, NH), 8.05 (2H, s, NH2, br), 8.47 (1H, s, ring-H); MS (EI)+: m/z 207 (39.9%) M+; Anal. Calcd. for C9H9N3O3 (207.19): C, 52.17; H, 4.38; N, 20.28. Found: C, 52.02; H, 4.25; N, 20.03. 5-Cyano-4-methoxy-2-oxo-1,2-dihydro-pyridine-3-ca rboxylic acid amide (19b): Obtained from 11 (2.42 g, 10 mmol) with sodium me- thoxide (Na 0.23 g, MeOH 30 mL, 10 mmol); mp. 229˚C - 231˚C as brown crystals (1.43 g, 74.09%); IR (KBr) υ 3383.7, 3350.9 (NH2), 3237.5 (NH), 2230.1 (CN), 1670.5 cm–1 (C=O); Anal. Calcd. for C8H7N3O3 (193.16): C, 49.75; H, 3.65; N, 21.75. Found: C, 49.48; H, 3.56; N, 21.62. 5-Cyano-2-oxo-4-propoxy-1,2-dihydro-pyridine-3-car boxylic acid amide (1 9c): Obtained from 11 (2.42 g, 10mmol) with sodium n-propoxide (Na 0.23 g, n-propanol 30 mL, 10 mmol); mp. 210˚C - 212˚C as yellow crystals (1.91 g, 86.43 %); IR (KBr) υ 3325.64, 3202.22 (NH2) and (NH), 2214.84 (CN), 1659 cm–1 (C=O); MS (EI)+: m/z 219 (31.3%) [M-2]+; Anal. Calcd. for C10H11N3O3 (221.22): C, 54.30; H, 5.01; N, 18.99. Found: C, 54.14; H, 4.90; N, 18.76. Genera l proced ure for the prepara tion of comp ounds 21a-e A mixture of ice cold diazonium salts of aromatic ami- nes 20 [conc. HCl (20 mL) added to aromatic amine (10 mmol), cooled then added sodium nitrite (0.69 g, 10 mmol)] was added to malononitrile dimer 8 (1.32 g, 10 mmol) in ethanol (30 mL) as solvent in presence of so- dium acetate. The precipitate was collected by filtration and recrystallised from ethanol. 4-Amino-6-imino-1-phenyl-1,6-dihydro-pyridazine-3, 5-dicarbonitrile (21a): Obtained from malononitrile dimer 8 (1.32 g, 10 mmol) and aniline (0.93 g, 10 mmol); mp. > 300˚C as yellow crystals (2.20 g, 93.22%); 1H-NMR (DMSO-d6): δ 7.63 - 8.04 (5H, m, Ar), 8.85 (2H, s, NH2, br), 9.4 (1H, s, NH, br); IR (KBr) υ 3432.2, 3333.8 (NH2), 3306 (NH), 2207 (CN); Anal. Calcd for C12H8N6 (236.24): C, 61.01; H, 3.41; N, 35.57. Found: C, 60.83; H, 3.27; N, 35.35. 4-Amino-1-(4-bromo-phenyl)-6-imino-1,6-dihydro-py ridazine-3,5-dicarbonitrile (21b): Obtained from malo- nonitrile dimer 8 (1.32 g, 10 mmol) and 4-bromoaniline (1.725 g, 10 mmol); mp. > 300˚C as yellow crystals (2.91 g, 92.38%); 1H-NMR (DMSO-d6): δ7.41, 8.09 (4H, 2d, Ar-AB), 9.15 (2H, s, NH2, br), 9.94 (1H, s, NH, br); IR (KBr) υ 3423, 3337.21 (NH2), 3295.2 (NH), 2210.02 cm–1 (CN); Anal. Calcd. for C12H7BrN6 (315.13): C, 45.74; H, 2.24; N, 26.67. Found: C, 45.52; H, 2.10; N, 26.48. 4-Amino-1-(4-chloro-phenyl)-6-imino-1,6-dihydro-py ridazine-3,5-dicarbonitrile (21c): Obtained from malononitrile dimer 8 (1.32 g, 10 mmol) and 4-chloroaniline (1.275g, 10 mmol); mp. > 300˚C as yellow crystals (2.49 g, 92.22%); 1H-NMR (DMSO-d6): δ 7.52, 7.98 (4H, 2d, Ar-AB), 9.18 (2H, s, NH2, br), 9.88 (1H, s, NH, br); IR (KBr) υ 3415.6, 3326.61 (NH2), 3308.3 (NH), 2209.06 (CN); Anal. Calcd. for C12H7ClN6 (270.68): C, 53.25; H, 2.61; N, 31.05. Found: C, 53.07; H, 2.55; N, 30.89. 4-Amino-6-imino-1-p-tolyl-1,6-dihydro-pyridazine-3, 5-dicarbonitrile (21d): Obtained from malononitrile dimer 8 (1.32 g, 10 mmol) and 4-methylaniline (1.07 g, 10 mmol); mp. > 300˚C as yellow crystals (2.27 g, 90.8%); 1H-NMR (DMSO-d6): δ = 2.10 (3H, s, CH3), 7.57, 7.69 (4H, 2d, Ar-AB), 9.16 (2H, s, NH2, br), 9.88 (1H, s, NH, br); IR (KBr) υ 3413.25, 3318.8 (NH2), 3298.5 (NH), 2209.63 cm–1 (CN); Anal. Calcd. for C13H10N6 (250.26): C, 62.39; H, 4.03; N, 33.58. Found: C, 62.11; H, 3.88; N, 33.37. 4-Amino-6-imino-1-(4-nitro-phenyl)-1,6-dihydro-pyri dazine-3,5-dicarbonitrile (21e): Obtained from malononitrile dimer 8 (1.32 g, 10 mmol) and 4-nitroaniline (1.38 g, 10mmol); mp. > 300˚C as brown crystals (2.57 g, 91.46%); 1H-NMR (DMSO-d6): δ = 7.58, 7.72 (4H, 2d, Ar-AB), 9.23 (2H, s, NH2, br), Copyright © 2011 SciRes. IJOC  F. A. ABU-SHANAB ET AL. 212 9.89 (1H, s, NH, br); IR (KBr) υ 3433.2, 3340.25 (NH2), 3300.5 (NH), 2211.03 cm–1 (CN); Anal. Calcd. for C12H7N7O2 (281.24): C, 51.25; H, 2.51; N, 34.86. Found: C, 51.04; H, 2.43; N, 34.69. Genera l proced ure for the prepara tion of comp ounds 22a-e Compound 21 (10 mmol) and N,N’-dimethylforma- mide dimethyl acetal (DMFDMA) (10 mmol) in dry di- oxane (30 mL) was refluxed for two hours, cooled and evaporated. The precipitate was collected by filtration and recrystallised from ethanol. N’-(3,5-Dicyano-6-imino-1-phenyl-1,6-dihydro-pyrid azin-4-yl)-N,N-dimethyl-formamidine (22a): Obtained from Compound 21a (2.36 g, 10 mmol) with N,N’-dimethylformamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol); mp. 203˚C - 205˚C as yellow crys- tals (2.24g, 76.98%); 1H-NMR (DMSO-d6): δ 3.29, 3.42 (6H, 2s, NMe2), 6.85 (1H, s, NH, br), 7.34 - 7.94 (5H, m, Ar), 8.27 (1H, s, CH); IR (KBr) υ 3306.9 (NH), 2208.9 cm–1 (CN); Anal. Calcd for C15H13N7 (291.32): C, 61.85; H, 4.50; N, 33.66. Found: C, 61.63; H, 4.37; N, 33.49. N’-[1-(4-Bromo-phenyl)-3,5-dicyano-6-imino-1,6-dih ydro-pyridazin-4-yl]-N,N-dimethyl-formamidine (22b): Obtained from Compound 21b (3.15 g, 10 mmol) with N,N’-dimethylformamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol); mp. 209˚C - 211˚C as deep brown crystals (2.85 g, 77.03%); 1H-NMR (DMSO-d6): δ 3.10, 3.21 (6H, 2s, NMe2), 6.98 (1H, s, NH, br), 7.46, 7.72 (4H, 2d, Ar-AB), 8.41 (1H, s, CH); IR (KBr) υ 3302.8 (NH), 2202 cm–1 (CN); Anal. Calcd. for C15H12BrN7 (370.21): C, 48.67; H, 3.27; N, 26.48. Found: C, 48.44; H, 3.12; N, 26.22. N’-[1-(4-Chloro-phenyl)-3,5-dicyano-6-imino-1,6-dih ydro-pyridazin-4-yl]-N,N-dimethyl-formamidine (22c): Obtained from Compound 21c (2.7 g, 10 mmol) with N,N’-dimethylformamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol) mp. 197˚C - 199˚C as brown crys- tals (2.40 g, 73.85%); 1H-NMR (DMSO-d6): δ 3.22, 3.31 (6H, 2s, NMe2), 6.86 (1H, s, NH, br), 7.34, 7.68 (4H, 2d, Ar-AB), 8.46 (1H, s, CH); IR (KBr) υ 3312.8 (NH), 2213.8 cm–1 (CN); Anal. Calcd. for C15H12ClN7 (325.76): C, 55.31; H, 3.71; N, 30.10. Found: C, 55.15; H, 3.60; N, 29.93. N’-(3,5-Dicyano-6-imino-1-p-tolyl-1,6-dihydro-pyrid azin-4-yl)-N,N-dimethyl-formamidine (22d): Obtained from Compound 21d (2.5 g, 10 mmol) with N,N’-dimethylformamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol); mp. 221˚C - 223˚C as yellow crys- tals (2.34 g, 76.72%); 1H-NMR (DMSO-d6): δ 2.35 (3H, s, CH3), 3.08, 3.20 (6H, 2s, NMe2), 6.65 (1H, s, NH, br), 7.40, 7.82 (4H, 2d, Ar-AB), 8.45 (1H, s, CH); IR (KBr) υ 3301.8 (NH), 2209.7 cm–1 (CN); Anal. Calcd. for C16H15N7 (305.34): C, 62.94; H, 4.95; N, 32.11. Found: C, 62.75; H, 4.84; N, 31.90. N’-[3,5-Dicyano-6-imino-1-(4-nitro-phenyl)-1,6-dihy dro-pyridazin-4-yl]-N,N-dimethyl-formamidine (22e) : Obtained from Compound 21e (2.81 g, 10 mmol) with N,N’-dimethylformamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol); mp. 223˚C - 225˚C as deep brown crystals (2.82 g, 83.93%); 1H-NMR (DMSO-d6): δ 3.12, 3.28 (6H, 2s, NMe2), 7.40 (1H, s, NH, br), 7.88, 8.39 (4H, 2d, Ar-AB), 8.50 (1H, s, CH); IR (KBr) υ 3290.8 (NH), 2207.6 cm–1 (CN); Anal. Calcd. for C15H12N8O2 (336.32): C, 53.57; H, 3.60; N, 33.32. Found: C, 53.35; H, 3.48; N, 33.15. Genera l proced ure for the prepara tion of comp ounds 23a-c Compound 21 (10 mol) in acetic acid (20 mL) and hy- drochloric acid (3 mL) was refluxed for four hours, cooled, and poured onto ice cold water.. The precipitate which formed was recovered by filtration and recrystal- lised from ethanol. 4-Amino-1-(4-bromo-phenyl)-6-oxo-1,6-dihydro-pyri dazine-3,5-dicarboxylic acid diamide (23a): Obtained from Compound 21b (3.15 g, 10 mmol); mp. > 300˚C as deep brown crystals (2.65 g, 75.28%); 1H-NMR (DMSO-d6): δ 7.82; 8.64 (4H, 2d, Ar-AB), 7.98 (2H, s, NH2), 9.79 (2H, s, NH2, br); IR (KBr) υ 3376.3, 3314.6 (NH2), 1701, 1663.9 cm–1 (C=O); Anal. Calcd. for C12H10BrN5O3 (352.15): C, 40.93; H, 2.86; N, 19.89. Found: C, 40.71; H, 2.74; N, 19.60. 4-Aamino-1-(4-chloro-phenyl)-6-oxo-1,6-dihydro-pyr idazine-3,5-dicarboxylic acid diamide (23b): Obtained from Compound 21c (2.7 g, 10 mmol); mp. > 300˚C as brown crystals (2.23 g, 72.64%); 1H-NMR (DMSO-d6): δ 7.78, 8.40 (4H, 2d, Ar-AB), 8.01 (2H, s, NH2), 9.85 (2H, s, NH2, br); IR (KBr) υ 3314.5, 3197.7 (NH2), 1697.7, 1630 cm–1 (C=O); Anal. Calcd. for C12H10ClN5O3 (307.70): C, 46.84; H, 3.28; N, 22.76. Found: C, 46.59; H, 3.13; N, 22.61. 4-Amino-1-(4-nitro-phenyl)-6-oxo-1,6-dihydro-pyrid azine-3,5-dicarboxylic acid diamide (23c): Obtained from Compound 21e (2.81 g, 10 mmol); mp. > 300˚C as brownish crystals (2.42 g, 76.10%); 1H- NMR (DMSO-d6): δ 7.90, 8.55 (4H, 2d, Ar-AB), 8.30 (2H, s, NH2), 9.60 (2H, s, NH2, br); IR (KBr) υ 3381.0, 3272.0 (NH2), 1691.9, 1654.7 cm–1 (C=O); Anal. Calcd. for C12H10N6O5 (318.25): C, 45.29; H, 3.17; N, 26.41. Found: C, 45.03; H, 3.06; N, 26.24. Genera l proced ure for the prepara tion of comp ounds 24a-c A mixture of Compound 21 (10 mmol) and cyanoa- cetamide (10 mmol) in ethanol (30 mL) and 3-5 drops of piperidine as a base was refluxed for two hours, cooled, and poured onto ice cold water. The precipitate was re- Copyright © 2011 SciRes. IJOC  213 F. A. ABU-SHANAB ET AL. covered by filtration and recrystallised from ethanol. 4-Amino-5-imino-7-oxo-1-phenyl-1,5,6,7-tetrahydro- pyrido[4,3-c]pyridazine-3,8-dicarbonitrile (24a): Obtained from Compound 21a (2.36 g, 10 mmol) with cyanoacetamide (0.84 g, 10 mmol); mp. > 300˚C as brown crystals (2.22 g, 73.27%); 1H-NMR (DMSO-d6): δ 7.23 (2H, s, NH2), 7.61, 10.52 (2H, 2s, 2NH, br), 7.64 - 8.12 (5H, m, Ar); IR (KBr) υ 3420.6, 3382.7 (NH2), 3343 cm-1 (NH), 2210.5 cm-1 (CN), 1683.2 cm–1 (C=O); Anal. Calcd. for C15H9N7O (303.29): C, 59.41; H, 2.99; N, 32.33. Found: C, 59.22; H, 2.87; N, 32.19. 4-Amino-1-(4-bromo-phenyl)-5-imino-7-oxo-1,5,6,7-t etrahydro-pyrido[4,3-c]pyridazine-3,8-dicarbonitrile (24b): Obtained from Compound 21b (3.15 g, 10 mmol) with cyanoacetamide (0.84 g, 10 mmol); mp. 179˚C - 181˚C as brown crystals (2.83 g, 74.08%); 1H-NMR (DMSO- d6): δ 6.70 (2H, s, NH2), 7.68, 10.20 (2H, 2s, 2NH, br), 7.50, 8.20 (4H, 2d, Ar-AB); IR (KBr) υ 3402.0, 3325.1 (NH2), 3175.1 (NH), 2204.8 (CN), 1617.5 cm–1 (C=O); Anal. Calcd. for C15H8BrN7O (382.18): C, 47.14; H, 2.11; N, 25.65. Found: C, 46.91; H, 2.02; N, 25.41. 4-Amino-5-imino-1-(4-nitro-phenyl)-7-oxo-1,5,6,7-tet rahydro-pyrido[4,3-c]pyridazine-3,8-dicarbonitrile (24c): Obtained from Compound 21e (2.81 g, 10 mmol) with cyanoacetamide (0.84 g, 10 mmol) mp. 239˚C - 241˚C as brown crystals (2.39 g, 68.68%); 1H-NMR (DMSO-d6): δ 7.19 (2H, s, NH2), 7.55, 10.60 (2H, 2s, 2NH, br), 7.78, 8.34 (4H, 2d, Ar-AB); IR (KBr) υ 3462.0, 3352.3 (NH2), 3228.2 (NH), 2192 (CN), 1630.0 cm–1 (C=O); Anal. Calcd. for C15H8N8O3 (348.28): C, 51.73; H, 2.32; N, 32.17. Found: C, 51.50; H, 2.19; N, 32.13. Genera l proced ure for the prepara tion of comp ounds 25a-c: Compound 24 (10 mmol) and N,N’-dimethylforma- mide dimethyl acetal (DMFDMA) (10 mmol) in dry di- oxane (30 mL) was refluxed for two hours, cooled, and evaporated. The precipitate was collected by filtration and recrystallised from ethanol. 8-Oxo-1-phenyl-7,8-dihydro-1H-1,2,4,6,7-pentaaza-p henalene-3,9-dicarbonitrile (25a): Obtained from Compound 24a (3.03 g, 10 mmol) with N,N’-dimethylformamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol); mp. > 300˚C as brown crystals (2.29 g, 73.16%); 1H-NMR (DMSO-d6): δ 7.37 (1H, s, NH, br), 7.81-8.36 (5H, m, Ar), 8.86 (1H, s, CH), 10.30 (1H, s, OH, br); IR (KBr) υ 3333.7 (NH), 2205.7 (CN), 1629 cm–1 (C=O); Anal. Calcd. for C16H7N7O (313.28): C, 61.34; H, 2.25; N, 31.30. Found: C, 61.18; H, 2.08; N, 31.16. 1-(4-Bromo-phenyl)-8-oxo-7,8-dihydro-1H-1,2,4,6,7- pentaaza-phenalene-3,9-dicarbonitrile (25b): Obtained from Compound 24b (3.82 g, 10 mmol) with N,N’-dimethylformamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol); mp. > 300˚C as deep brown crys- tals (2.76 g, 70.41%); 1H-NMR (DMSO-d6): δ 7.51 (1H, s, NH, br), 7.69, 8.28 (4H, 2d, Ar-AB), 8.84 (1H, s, CH), 10.02 (1H, s, OH, br); IR (KBr) υ 3326.9 (NH), 2206.1 (CN), 1623 cm–1 (C=O); Anal. Calcd. for C16H6BrN7O (392.18) C, 49.00; H, 1.54; N, 25.00. Found: C, 48.79; H, 1.42; N, 24.85. 1-(4-Nitro-phenyl)-8-oxo-7,8-dihydro-1H-1,2,4,6,7-p entaaza-phenalene-3,9-dicarbonitrile (25c): Obtained from Compound 24c (3.48 g, 10 mmol) with N,N’-dimethylformamide dimethyl acetal (DMFDMA) (1.32 mL, 10 mmol); mp. > 300˚C as brown crystals (2.52 g, 70.39%); 1H-NMR (DMSO-d6): δ 7.45 (1H, s, NH, br), 7.78, 8.32 (4H, 2d, Ar-AB), 8.71 (1H, s, CH), 10.50 (1H, s, OH, br); IR (KBr) υ 3338.4 (NH), 2202.5 (CN), 1618 cm–1 (C=O); Anal. Calcd. for C16H6N8O3 (358.28): C, 53.64; H, 1.69; N, 31.28. Found: C, 53.41; H, 1.58; N, 31.05. 4. References [1] V. G. Granik, A. M. Zhidkova and R. G. Glushkov, “Ad- vances in the Chemistry of the Acetals of Acid Amides and Lactams,” Russian Chemical Reviews, Vol. 46, No. 4, 1977, pp. 361-366. doi:10.1070/RC1977v046n04ABEH002137 [2] R. F. Abdulla and R. S. Brinkmeyer, “The Chemistry of Formamide Acetals,” Tetrahedron, Vol. 35, No. 14, 1979, pp. 1675-1735. doi:10.1016/0040-4020(79)88001-1 [3] P. L. Anelli, M. Brocchetta, D. Palano and M. Visigalli, “Mild Conversion of Primary Carboxamides into Car- boxylic Esters,” Tetrahedron Letters, Vol. 38, No. 13, 1997, pp. 2367-2368. doi:10.1016/S0040-4039(97)00350-X [4] M. Malesic, A. Krbavcic, A. Golobic, L. Golic and B. Stanovenik, “The Synthesis and Transformation of Ethyl 2-(2-Acetyl-2-benzoyl-1-ethenyl)amino-3-dimethylamino propenoate. A New Synthesis of 2,3,4-Trisubstituted Pyr- roles,” Journal of Heterocyclic Chemistry, Vol. 34, No. 6, 1997, pp. 1757-1762. doi:10.1002/jhet.5570340619 [5] F. A. Abu-Shanab, A. D. Redhouse, J. R. Thompson and B. J. Wakefield, “Synthesis of 2,3,5,6-Tetrasubstituted Pyridines from Enamines Derived from N,N-Dimethyl- formamide Dimethyl Acetal,” Synthesis, Vol. 5, 1995, pp. 557-560. doi:10.1055/s-1995-3954 [6] F. A. Abu-Shanab, F. M. Aly and B. J. Wakefield, “Syn- thesis of Substituted Nicotinamides from Enamines De- rived from N,N-Dimethylformamide Dimethyl Acetal,” Synthesis, Vol. 8, 1995, pp. 923-925. doi:10.1055/s-1995-4039 [7] F. A. Abu-Shanab, A. M. Hessen and S. A. S. Mousa, “Dimethylformamide Dimethyl Acetal in Heterocyclic Synthesis: Synthesis of Polyfunctionally Substituted Pyri- dine Derivatives as Precursors to Bicycles and Polycy- Copyright © 2011 SciRes. IJOC  F. A. ABU-SHANAB ET AL. Copyright © 2011 SciRes. IJOC 214 cles,” Journal of Heterocyclic Chemistry, Vol. 44, No. 4, 2007, pp. 787-791. doi:10.1002/jhet.5570440406 [8] F. A. Abu-Shanab, M. H. Elnagdi, F. M. Aly and B. J. Wakefield, Journal of the Chemical Society, Perkin Tran- sactions 1, Vol. 1, 1994, pp. 1449-1452. [9] F. A. Abu-Shanab, S. A. S. Mousa, et al., “Dimethylfor- mamide Dimethyl Acetal as a Building Block in Hetero- cyclic Synthesis,” Journal of Heterocyclic Chemistry, Vol. 46, No. 5, 2009, pp. 801-827. doi:10.1002/jhet.69 [10] F. A. Abu-Shanab, A. El-Harrasi and S. A. S. Mousa, “Synthesis of 1,4-Diaryl-piperazine-2,5-diones: New Be- havior of N,N-Dimethylformamide Dimethyl Acetal (DMF DMA),” Synthetic Communications, Vol. 38, No. 3, 2008, pp. 376-382. doi:10.1080/00397910701767098 [11] R. A. Carboni, D. D. Conffman and E. G. Howard, “Cyanocarbon Chemistry. XI.1 Malononitrile Dimer,” Journal of the American Chemical Society, Vol. 80, No. 11, 1958, pp. 2838-2840. [12] F. A. Abu-Shanab, Y. M. Elkholy and M. H. Elnagdi, “Enaminones as Building Blocks in Organic Synthesis: Synthesis of New Polyfunctional Pyridines, Condensed Pyridines, and Penta Substituted Benzene,” Synthetic Communications, Vol. 32, No. 22, 2002, pp. 3493-3502.
|