 Open Journal of Vet eri nary M e dici ne , 2011, 1, 1-7 doi:10.4236/ojvm.2011.11001 Published Online December 2011 (http://www.SciRP.org/journal/ojvm) Copyright © 2011 SciRes. OJVM Comparison between Hypertonic Saline with Dextran and Mannitol on Vasodilatation of Encephalic Vessels Using a Magnetic Resonance Imaging in the Dogs Miki Akaishizawa1, Reiko Tabata1, Kazuyuki Suzuki1,2, Ryuji Asano1 1Department of Veterinary Medicine, College of Bioresourse Sciences, Nihon University, Kanagawa, Japan 2Department of Lar ge Animal Clinical Sciences, School of Veterinary Medicine, Rakuno Gakuen University, Hokkaido, Japan E-mail: kazuyuki@rakuno.ac.jp Received November 8, 2011; revised November 20, 2011; accepted December 13, 2011 Abstract This study aimed to investigate whether a small volume of 7.2% hypertonic saline solution with 6% dextran 70 (HSD) is superior to mannitol in vasodilatation of encephalic vessels in the dogs using magnetic resonance imaging (MRI). Fifteen healthy 2.4 ± 0.9 year-old purpose-bred male Beagle dogs were assigned to receive 5 mL/kg of isotonic saline solution (ISS) as control, 20% mannitol or HSD infusion at a flow rate of 20 mL/kg/hours via right cephalic vein.Venous blood samples were collected immediately before fluid infusion (pre) and ev ery 15 minutes until 120 min- utes after the initiation of fluid infusion. Immediately after collectio n of each blood sample, T1 and T2-weighted mag- netic resonance imaging recordings were undergone. Immediately after HSD infusion, the area of the cross-section of superior sagittal sinus was significantly greater than th at of beagles in the other groups (p < 0.001), reaching the 2.09 ± 0.25 times pre-value. During the 120 minutes period of observation after the initiation of fluid infusion, HSD infusion significantly reduced the area of the cross-section of CSF compared with the mannitol group (p < 0.001). Our results indicate that HSD induced a rapid and strong reduction in the area of the cross-section of CSF more than mannitol did. Therefore, it is suggested that 5 mL/kg of HSD might be superior to isovolume of mannitol in inducing vasodilatation in the dog. Keywords: Canine, Hypertonic Saline, Magnetic Resonance Imaging, Mannitol, Superior Sagittal Sinus 1. Introduction Hypertonic mannitol is commonly used to treat compro- mised encephalic circulation associated with neurologic disorders since mannitol is known to improve brain edema, intracranial hypertension, and encephalic blood flow [1,2]. Standard pre-hospital and initial room treat- ment of patients with clinical evidence of elevated in- tracranial pressure (ICP) includes intravenous admini- stration of a hypertonic mannitol [3-5]. This was pre- sumed to be secondary to a dehydrating effect or direct reduction of white matter water content [4]. However, it is associated with profound diuresis, itself a potential cause of morbidity, and also acute renal failure [6], and tending to decrease the blood pressure [7]. The effects of hypertonic saline solution (HSS) in the resuscitation of animals in hemorrhagic and endotoxic shock have been documented in a variety of laboratory and clinical studies [8-11]. Recently, investigative inter- est has focused on the intracranial effects of HSS resus- citation [12-15]. The administration of 7.5%-HSS with cryogenic encephalic regions causes a prompt and sub- stantial decrease in encephalic water content in rabbits as assessed by spine-echo T2-weighted magnetic resonance imaging (MRI) [16]. Therefore, HSS may prove to be more beneficial than osmotic diuretics because they augment intravascular volume and cardiovascular per- formance in addition to improving intracranial elastance [17]. The use of HSS alone, however, had a transient effect on encephalic circulation [18,19]. The addition of a hy- peroncotic agent, such as dextran, has been shown to prolong the cardiovascular resuscitative effect of HSS in the treatment of hemorrhag ic shock [7,19-21]. Therefore, there has been a clinical interest in HSS with 6% dextran 70 (HSD) for the treatment of cerebral hypertension and cardiovascular resuscitation [13,19]. Thus, Battison et al. [22] demonstrated that treatments reduced ICP with both  2 M. AKAISHIZAWA ET AL. mannitol (median decrease, 7.5 mm Hg) and HSD (me- dian decrease, 13 mm Hg). In addition, HSD caused a significantly greater decrease in intracranial pressure and had a longer duration of effect than equimolar dose of mannitol in human with brain injury [22]. Future HSD research needs to be performed in several areas with re- gard to cerebral edema, hypertension and brain injury in the dogs. Thus, studies comparing HSD and mannitol as primary agents to lower ICP are needed to explore their relative safety and efficacy. Therefore, this study aimed to investigate, using MRI, whether a small volume of HSD is superior to mannitol, when infused at the same volume, in the vasodilatation of encephalic vessels in the dogs. 2. Materials and Methods The work was carried out at the Veterinary teaching hos- pital, Department of Veterinary Medicine, College of Bioresourse Sciences, Nihon University, Kanagawa, Ja- pan. All procedures were in accordance with the College of Bioresource Sciences, Nihon University on the Code for the Care and Use of Laboratory Animals (approval number: 079) and the National Research Council on Guide for the Care and Use of Laboratory Animals [23]. The experiments were performed on 15 healthy 2.4 ± 0.9 year-old purpose-bred male Beagle dog s weigh ing 12.4 ± 1.4 kg (mean ± standard deviation). Dogs were deemed healthy on the basis of physical examination, biochemi- cal profile, electrocardiography, thoracic radiography and echocardiography analysis. A complete, balanced diet consisting of rationed concentrated pellet was pro- vided, and the dogs had unlimited access to fresh water. Food was removed 16 hours and water 1 hour prior to anesthesia. An isotonic saline solution (ISS, 300 mOsm/L., Nihon Zenyaku Kogyo, Fukushima, Japan) and 20% mannitol solution (1200 mOsm/L, Nikken Kagaku Co., Ltd., Tokyo, Japan) were purchased. A 7.2%-HSD (2400 mOsm/L) were kindly prepared and provided by Dr. S. Iwabuchi (Nihon Zenyaku Kogyo Co., Ltd). The cross-sections of superior sagittal sinus, which is represented by area of cerebral falx, in the axial trans- verse section of pituitary (Figure 1(A)), and facial vein at the axial transverse section of epencephalic limbic (Fig- ure 1(B)) were measured by spin-echo T1-weighted MRI using a 0.5 tesla-superconducting MRI system (Flexart MRT-50GP, Toshiba Medical Systems, Tokyo, Japan) set at TR/TE = 375/15 milliseconds, field of view = 14 × 14 cm, image matrix 192 × 256 pixels in T1- weighted image with 4-mm-thick slices. Changes in cross-section of superior sagittal sinus and facial vein were analyzed using image analysis software (Toshiba Med ical Systems). Figure 1. Measurement of cross-section of superior sagittal sinus and facial vein. A: sagittal transverse section views in the beagle and open rectangle indicates the location for the axial transverse section of pituitary; A-1: the axial T1 weighted images at the pituitary section. Arrowhead shows the area of the cerebral falx. The area of the superior sagit- tal sinus is represented by area of cerebral falx in the axial transverse section of pituitary; B: sagittal transverse section views in the beagle and open rectangle indicates the location for the axial transverse section of epencephalic limbic; B-1: the axial T1 weighted images at the epencephalic limbic. Arrowhead shows the cross-section of facial vein. Each region of interests (ROI), which traced by manually using an ROI tool kit (Toshiba Medical Systems), un- derwent triplicate measurement and then used the aver- age. A detailed description of the procedure to measure the area of superior sagittal sinus and facial vein using MRI are described elsewhere [24]. The dogs were anesthetized with intravenous infusion of thiopental sodium (Ravonal for Injection, Tanabe Seiyaku Co., Osaka, Japan) at a dose of 18 mg/kg before being orotracheally intubated with a cuffed endotracheal tube and positioned in supine position. After tracheal intubations, each dog was put under general anesthesia and maintained in oxygen throughout the experiment using a 1.5 - 2.0 times the minimum alveolar concentra- tion of isoflurane (Forane, Abbott Japan Co, Osaka, Ja- pan). The end-tidal con centration of isoflurane and en d-tidal partial pressure of carbon dioxide were continuously monitored using an airway gas monitor (Datex Instru- ment Co., Helsinki, Finland). The animals were allowed to breathe spontaneously throughout the experiments. Fifteen dogs were randomly allocated one of three groups as follows: ISS, mannitol and HSD groups (n = 5 Copyright © 2011 SciRes. OJVM 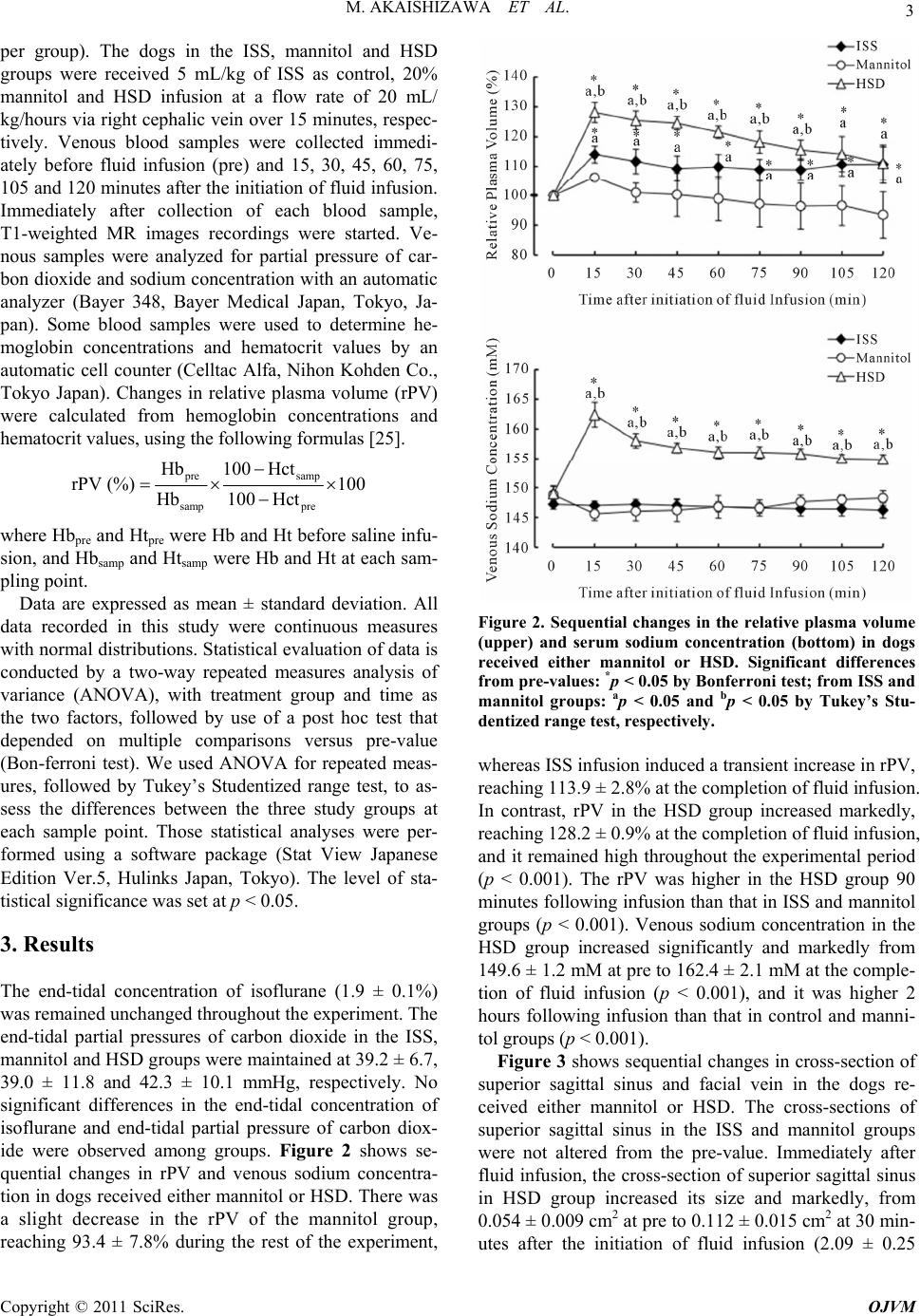 M. AKAISHIZAWA ET AL. 3 per group). The dogs in the ISS, mannitol and HSD groups were received 5 mL/kg of ISS as control, 20% mannitol and HSD infusion at a flow rate of 20 mL/ kg/hours via right cephalic vein over 15 minutes, respec- tively. Venous blood samples were collected immedi- ately before fluid infusion (pre) and 15, 30, 45, 60, 75, 105 and 120 minutes after the in itiation of fluid infusion. Immediately after collection of each blood sample, T1-weighted MR images recordings were started. Ve- nous samples were analyzed for partial pressure of car- bon dioxide and sodium concentration with an automatic analyzer (Bayer 348, Bayer Medical Japan, Tokyo, Ja- pan). Some blood samples were used to determine he- moglobin concentrations and hematocrit values by an automatic cell counter (Celltac Alfa, Nihon Kohden Co., Tokyo Japan). Changes in relative plasma volume (rPV) were calculated from hemoglobin concentrations and hematocrit values, using the following formulas [25]. pre samp samp pre Hb100 Hct rPV (%)100 Hb100 Hct where Hbpre and Htpre were Hb and Ht befo re saline infu- sion, and Hbsamp and Htsamp were Hb and Ht at each sam- pling point. Data are expressed as mean ± standard deviation. All data recorded in this study were continuous measures with normal distributions. Statistical evaluatio n of data is conducted by a two-way repeated measures analysis of variance (ANOVA), with treatment group and time as the two factors, followed by use of a post hoc test that depended on multiple comparisons versus pre-value (Bon-ferroni test). We used ANOVA for repeated meas- ures, followed by Tukey’s Studentized range test, to as- sess the differences between the three study groups at each sample point. Those statistical analyses were per- formed using a software package (Stat View Japanese Edition Ver.5, Hulinks Japan, Tokyo). The level of sta- tistical significance was set at p < 0.05. 3. Results The end-tidal concentration of isoflurane (1.9 ± 0.1%) was remained unchanged throughout the experiment. The end-tidal partial pressures of carbon dioxide in the ISS, mannitol and HSD groups were maintained at 39.2 ± 6.7, 39.0 ± 11.8 and 42.3 ± 10.1 mmHg, respectively. No significant differences in the end-tidal concentration of isoflurane and end-tidal partial pressure of carbon diox- ide were observed among groups. Figure 2 shows se- quential changes in rPV and venous sodium concentra- tion in dogs received either mannitol or HSD. There was a slight decrease in the rPV of the mannitol group, reaching 93.4 ± 7.8% during the rest of the experiment, Figure 2. Sequential changes in the relative plasma volume (upper) and serum sodium concentration (bottom) in dogs received either mannitol or HSD. Significant differences from pre-values: *p < 0.05 by Bonferroni test; from ISS and mannitol groups: ap < 0.05 and bp < 0.05 by Tukey’s Stu- dentized range test , res pectively. whereas ISS infusion induced a transient increase in rPV, reaching 113.9 ± 2.8% at the completion of fluid infusion. In contrast, rPV in the HSD group increased markedly, reaching 128.2 ± 0.9% at the completion of fluid infusion, and it remained high throughout the experimental period (p < 0.001). The rPV was higher in the HSD group 90 minutes following infu sion than that in ISS and mannitol groups (p < 0.001). Venous sodium concentration in the HSD group increased significantly and markedly from 149.6 ± 1.2 mM at pre to 162.4 ± 2.1 mM at the comple- tion of fluid infusion (p < 0.001), and it was higher 2 hours following infusion than that in control and manni- tol groups (p < 0.001). Figure 3 shows sequential changes in cross-section of superior sagittal sinus and facial vein in the dogs re- ceived either mannitol or HSD. The cross-sections of superior sagittal sinus in the ISS and mannitol groups were not altered from the pre-value. Immediately after fluid infusion, the cross-section of superior sagittal sinus in HSD group increased its size and markedly, from 0.054 ± 0.009 cm2 at pre to 0.112 ± 0.015 cm2 at 30 min- utes after the initiation of fluid infusion (2.09 ± 0.25 Copyright © 2011 SciRes. OJVM  4 M. AKAISHIZAWA ET AL. Figure 3. Sequential changes in areas of superior sagittal sinus (upper) and facial vain (bottom) in dogs received ei- ther mannitol or HSD. See Figure 2 for remainder of sym- bols. times pre-value), and remained constant throughout the experimental period (p < 0.001). The size of the cross- section of superior sagittal sinus was significantly reach- ing 128.2 ± 0.9% at the co mpletion of fluid in fusion, and it remained high throughout th e experimental period (p < 0.001). The changes in cross-section of facial vein in the HSD group were induced significantly, from 0.559 ± 0.101 cm2 at pre to 0.923 ± 0.111 cm2 at 30 minutes after the initiation of fluid infusion (1.68 ± 0.27 times pre-value), and it was significantly higher than that in the ISS and mannitol groups (p < 0.001). 4. Discussion In the present study, the superior efficacy of 5 mL/kg of HSD over equal volumes of 20% mannitol was implied by the fact that vasodilatation of encephalic vessels such as superior sagittal sinus was more rapid and stronger in the HSD group than in the mannitol group. In addition, HSD had a longer duration of eff ect than mannitol Aggressive ICP management is considered a corner- stone in the treatment of dogs with severe head injures. Hypertonic mannitol is commo nly used to treat critically ill patients with compromised encephalic circulation as- sociated with various neurologic disorders, although its mechanism is not fully understood. Two differential mechanisms by which mannitol reduce ICP have been established. Improvement of blood rheology leading to an increased encephalic blood flow and compensatory encephalic vasoconstriction may cause an early drop in ICP, which was observed shortly after infusion [3,26]. A delayed decrease in ICP following 30 minutes to 6 hours after infusion is attributed to an osmotic gradient pro- duced between plasma and parenchyma cells [21]. The osmotic effect of mannitol is delayed for 15 - 30 minutes until gradients are established between plasma and cells. Its effect persists for a variable period ranging from 90 minutes to 6 hours or more, depending on the clinical conditions [27]. However, Arai et al. [1] demonstrated that the administration of hypertonic mannitol failed to improve and even worsened encephalic blood flow, but it did decrease ICP for several hours, most likely due to the excessive urine losses it caused. In our study, mannitol induced a slight decrease in the systemic circulation volume, reaching 93.4 ± 10.0%, whereas the rPV re- mained high in the HSD group 2 hours following infu- sion. This result supports the report by Arai et al [1]. One of the primary mechanisms by which HSD exerts its action on the encephalic circulation is via its osmotic effects [13]. By dehydrating tissues, HSD can simulta- neously improve perfusion by pulling fluid into the in- travascular compartment and decrease edema in critical areas such as the brain [13,28]. Several animal models of traumatic brain injury have demonstrated a decrease in encephalic water content with use of HSD, often through dehydration of the uninjured hemisphere [16,28,29]. The cranial vault is a fixed space consisting of 3 compart- ments such as parenchyma, cerebrospinal fluid (CSF) and encephalic blood within calvaria by Monroe-Kellie doctrine. Therefore, increases in encephalic blood results in a compensatory decrease in CSF in order to maintain ICP in normovolemic dogs. In this study, HSD infusion induced a greater and faster vasodilatation of superior sagittal sinus than mannitol did. Therefore, this result suggests that HSD might be superior to mannitol in ini- tial treatment of cerebral hypertension in dogs with ele- vated ICP. The principal physiological mechanism underlying the efficacy of HSD, mannitol, and other osmotically active agents in decreasing ICP is believed to be an osmotic gradient-induced shift of extra- to intra-vascular water across the blood-brain-barrier (BBB) [30]. The BBB consists of tight junctions between encephalic endothe- lial cells that allow free passage to water but are not permeable to osmotically active substances such as so- dium and low molecular weight compounds [31]. The reason why the HSD is superior to mannitol on im- provement of encephalic circulation is that the BBB is Copyright © 2011 SciRes. OJVM  M. AKAISHIZAWA ET AL. 5 better able to exclude HSD because of tight gap junctions and its higher polarity, resulting in a reflection coeffi- cient of 1.0 for NaCl as compared with 0.9 for mannitol [13,14]. In the short term, hypertonic solutions expand plasma volume and reduce brain edema. It is known that the mannitol turn toward filtratio n and in time result in a net increase tissue volume [32]. However, HSS is the only hypertonic solution that did not induce a rebound effect. One explanation may be that sodium ion pumps prevent intracellular accumulation of sodium and chlo- ride, whereas mannitol shows transient accumulation in the intracellular space. This hypothesis is consistent that fluid microvascular permeability was increased by HSD but was not influenced by mannitol [32]. There are more than 300 studies published in which a small-volume solution of 7.2% to 7.5% NaCl is com- bined with 6% to 10% dextran. Interestingly, the original HSD choice of 7.2% NaCl with 6% dextran 70 by Smith et al. [5] was entirely arbitrary [9]. A few studies have evaluated variations in concentration or total dose [9]. These studies show that it is not a specific concentration of the crystalloid or the colloid component that contrib- utes to a particular efficacy. Clinical studies support its efficacy, and to our knowledge there has not been a sin- gle adverse event reported with HSD in more than 1,000 patients treated to date in published clinical trials [9]. HSS and HSD are given as a rapid IV bolus (1 mL/kg per minutes) at a dose of 4 to 6 mL/kg in the veterinary practice [33]. In the sev eral literatures, however, in fusion period for HSD of 10 to 20 minutes is recommended for the initial resuscitation of animals with hypotensive trauma [8,9,34]. A continuous infusion of 7.5%-HSS with splenectomy resulted in minimal blood loss and improved survival in rat [34]. Based on new data that show the possibility of vasodilatation and/or cardiac ar- rhythmia, and concerns regarding increased bleeding, several researchers suggest that a 10- to 20-miniute in fu- sion of 4- to 5-mL/kg of HSD be recommende d for criti- cal care [8,9,34]. Mannitol has become popular as “a small volume resuscitation fluid” and has often been compared with HSS and HSD. Several literatures rec- ommended that a 20% or 25% mannitol at a dose from 0.25 to 1.0 g/kg be given intravenously over 10 to 15 minutes to treat head injury in human [2,30,35]. There- fore, 15 minutes continuous 20% mannitol and HSD at a dose of 5 mL/kg was adopted in this study. MRI offers an opportunity for repeated, noninvasive in vivo deter- minations of the cross-section of encephalic vessel and encephalic water content [16,36]. Therefore, we com- pared HSD with mannitol on CSF in anesthetized dog using the noninvasive and serial spin-echo T1-weighted MRI from ethical consideration. The present study demonstrated that 5 mL/kg of HSD induced a rapid and strong vasodilatation of superior sagittal sinus more than isovolu me of 20% mannitol did. Based on these results, the question may be raised whether HSD is a better choice to improve an encephalic circulation and to treat brain edema than mannitol. Clinical research is necessary before definitive recom- mendations of HSD can be made regarding the optimal fluid for use in the treatment of cerebral hypertension in the dog with elevated ICP. 5. Acknowledgements This study was supported by a grant-in-aid for Science Research from the Ministry of Education, Culture and Sciences of Japan (18580319 and 21580393) to Suzuki K. 6. References [1] T. Arai, I. Tsukahara, K. Nitta and T. Watanabe, “Effects of Mannitol on Cerebral Circulation after Transient Com- plete Cerebral Ischemia in Dog,” Critical Care Medicine, Vol. 14, No. 7, 1986, pp. 634-637. doi:10.1097/00003246-198607000-00010 [2] A. D. Mendelow, G. M. Teasdale, T. Russell, J. Flood, J. Patterson and G. D. Murray, “Effect of Mannitol on Cere- bral Blood Flow and Cerebral Perfusion Pressure in Hu- man Head Injury,” Journal of Neurosurgery, Vol. 63, No. 1, 1985, pp. 43-48. doi:10.3171/jns.1985.63.1.0043 [3] S. P. Freshman, F. D. Battistella, M. Matteucci and D. H. Wisner, “Hypertonic Saline (7.5%) Versus Mannitol: A Comparison for Treatment of Acute Head Injuries,” Journal of Trauma, Vol. 35, No. 3, 1993, pp. 344-348. doi:10.1097/00005373-199309000-00003 [4] F. Nath and S. Galbraith, “The Effect of Mannitol on Cerebral White Matter Water Content,” Journal of Neu- rosurgery, Vol. 65, No. 1, 1986, pp. 41-43. doi:10.3171/jns.1986.65.1.0041 [5] H. P. Smith, D. L. Kelly, J. M. McWhorter, D. Armstrong, R. Johnson, C. Transou and G. Howard, “Comparison of Mannitol Regimens in Patients with Severe Head Injury Undergoing Intracranial Monitoring,” Journal of Neuro- surgery, Vol. 65, No. 6, 1986, pp. 820-824. doi:10.3171/jns.1986.65.6.0820 [6] A. M. Kaufmann and E. R. Cardoso, “Aggravation of Vasogenic Edema by Multiple-Dose Mannitol,” Journal of Neurosurgery, Vol. 72, No. 4, 1992, pp. 584-589. doi:10.3171/jns.1992.77.4.0584 [7] S. Berger, L. Schurer, R. Hartl, K. Messmer and A. Baethmann, “Reduction of Post-Traumatic Intracranial Hypertension by Hypertonic/Hyperoncotic Saline/Dextr- an and Hypertonic Mannitol,” Neurosurgery, Vol. 37, No. 1, 1995, pp. 98-108. doi:10.1227/00006123-199507000-00015 [8] O. Chiara, P. Pelosi, L. Brazzi, N. Bottino, P. Taccone, S. Cimbanassi, M. Segala, L. Gattinoni and T. Scalea. “Re- Copyright © 2011 SciRes. OJVM  6 M. AKAISHIZAWA ET AL. suscitation from Hemorrhagic Shock: Experimental Model Comparing Normal Saline, Dextran, and Hyper- tonic Saline Solutions,” Critical Care Medicine, Vol. 31, No. 7, 2003, pp. 1915-1922. doi:10.1097/01.CCM.0000074725.62991.42 [9] G. C. Kramer, “Hypertonic Resuscitation: Physiologic Mechanisms and Recommendations for Trauma Care,” Journal of Trauma, Vol. 54, No. 5, 2003, pp. S89-S99. [10] L. M. Liu, J. A. Ward and M. A. Dubick, “Effects of Crystalloid and Colloid Resuscitation on Hemorrhage- Induced Vascular Hyporesponsiveness to Norepinephrine in the Rat,” Journal of Trauma, Vol. 54, No. 5, 2003, pp. S159-S168. [11] K. Suzuki, T. Ajito and S. Iwabuchi. “Effect of Infusion of Hypertonic Saline Solution on Conscious Heifer with Hypoxemia Caused by Endotoxin Infusion,” American Journal of Veterinary Research, Vol. 59, No. 4, 1997, pp. 452-457. [12] C. H. Chen, T. J. Toung, A. Sapirstein and A. Bhardwaj, “Effect of Duration of Osmotherapy on Blood-Brain Bar- rier Disruption and Regional Cerebral Edema after Ex- perimental Stroke,” Journal of Cerebral Blood Flow & Metabolism, Vol. 26, 2006, pp. 951-958. doi:10.1038/sj.jcbfm.9600248 [13] J. A. Doyle, D. P. Davis and D. B. Hoyt, “The Use of Hypertonic Saline in the Treatment of Traumatic Brain Injury,” Journal of Trauma, Vol. 50, No. 2, 2001, pp. 367-383. doi:10.1097/00005373-200102000-00030 [14] K. Nagasawa, H. Chiba, H. Fujita, T. Kojima, T. Saito, T. Endo and N. Sawada, “Possible Involvement of Gap Junctions in the Barrier Functions of Tight Junctions of Brain and Lung Endothelial Cells,” Journal of Cellular Physiology, Vol. 208, No. 1, 2006, pp. 123-132. doi:10.1002/jcp.20647 [15] R. Vialet, J. Albanese, L. Thomachot, F. Antonini, A. Bourgouin, B. Alliez and C. Martin, “Isovolume Hyper- tonic Solutes (Sodium Chloride or Mannitol) in the Treatment of Refractory Posttraumatic Intracranial Hy- pertension: 2 mL/kg 7.5% Saline is More Effective Than 2 mL/kg 20% Mannitol,” Critical Care Medicine, Vol. 31, No. 6, 2003, pp. 1683-1687. doi:10.1097/01.CCM.0000063268.91710.DF [16] A. Bacher, J. Wei, M. R. Grafe, M. J. Quast and M. H. Zornow, “Serial Determinations of Cerebral Water Con- tent by Magnetic Resonance Imaging after an Infusion of Hypertonic Saline,” Critical Care Medicine, Vol. 26, No. 1, 1998, pp. 108-114. doi:10.1097/00003246-199801000-00024 [17] J. I. Suarez, A. I. Qureshi, A. Bhardwaj, M. A. Williams, M. S. Schnitzer, M. Mirski, D. F. Hanley and J. A. Ula- towski, “Treatment of Refractory Intracranial Hyperten- sion with 23.4% Saline,” Critical Care Medicine, Vol. 26, No. 6, 1998, pp. 1118-1122. doi:10.1097/00003246-199806000-00038 [18] N. Ito, K. Suzuki, H. Koie, S. Tsumagari, K. Kanayama, M. Miyahara and R. Asano, “The Effect of 7.2% Hyper- tonic Saline Solution on the Duration of Sodium Gradient between the Cerebrospinal Fluid and the Venous Circula- tion in the Dog,” Journal of Veterinary Medical Science, Vol. 68, No. 2, 2006, pp. 183-185. doi:10.1292/jvms.68.183 [19] A. Somell, A. Sollevi, A. Suneson, L. Riddez and H. Hjelmqvist, “Beneficial Effects of Hypertonic Sa- line/Dextran on Early Survival in Porcine Endotoxin Shock,” Acta Anaesthesiologica Scandinavica, Vol. 49, No. 8, 2005, pp. 1124-1134. doi:10.1111/j.1399-6576.2005.00807.x [20] J. Kristensen and J. Modig, “Ringer’s Acetate and Dex- tran-70 with or without Hypertonic Saline in En- dotoxin-Induced Shock in Pig,” Critical Care Medicine, Vol. 18, No. 11, 1990, pp. 1261-1268. doi:10.1097/00003246-199011000-00016 [21] J. M. Pascual, J. C. Watson, A. E. Runyon, C. E. Wade and G. C. Kramer, “Resuscitation of Intraoperative Hy- povolemia: A Comparison of Normal Saline and Hy- perosmotic/Hyperoncotic Solutions in Swine,” Critical Care Medicine, Vol. 20, No. 2, 1992, pp. 200-210. doi:10.1097/00003246-199202000-00009 [22] C. Battison, P. J. Andrews, C. Graham and T. Petty, “Randomized, Controlled Trial on the Effect of a 20% Mannitol Solution and a 7.5% Saline/6% Dextran Solu- tion on Increased Intracranial Pressure after Brain In- jury,” Critical Care Medicine, Vol. 33, No. 1, 2005, pp. 196-202. doi:10.1097/01.CCM.0000150269.65485.A6 [23] National Research Concil, “Guide for the Care and Use of Laboratory Animals,” National Academy Press, Washington DC, 1996, pp. 1-70. [24] K. Suzuki, H. Koie, T. Matsumoto and R. Asano, “The Effect of Hypertonic Saline Solution on Vasodilatation of the Superior Sagittal Sinus Using Magnetic Resonance Imaging,” Research in Veterinary Science, Vol. 84, No. 3, 2008, pp. 465-470. doi:10.1016/j.rvsc.2007.05.016 [25] J. E. Greenleaf, V. A. Convertino and G. R. Mangeseth. “Plasma Volume during Stress in Man: Osmolality and Red cell Volume,” Journal of Applied Physiology, Vol. 47, No. 5, 1979, pp. 1031-1038. [26] R. Härtl, T. F. Bardt, K. L. Kiening, A. S. Sarrafzadeh, G. H. Schneider and A. W. Unterberg, “Mannitol Decreases ICP But Does Not Improve Brain-Tissue PO2 in Severely Head-Injured Patients with Intracranial Hypertension,” Acta Neurochirurgica Supplementum, Vol. 70, 1997, pp. 40-42. [27] Brain Trauma Foundation, “Guidelines for the Manage- ment of Severe Traumatic Brain Injury. II. Hyperosmolar Therapy,” Journal of Neurotrauma, Vol. 24, No. 1, 2007, pp. S14-S20. [28] A. Sheikh, T. Matsuoka and D. Wisner, “Cerebral Effects of Resuscitation with Hypertonic Resuscitation of Shock and Brain Injury: Short- and Long-Term Effects,” Jour- nal of Trauma, Vol. 24, No. 4, 1996, pp. 1226-1232. [29] A. I. Qureshi, J. I. Suarez, A. Bhardwaj, M. Mirski, M. S. Schnitzer, D. F. Hanley and J. A. Ulatowski, “Use of Hypertonic (3%) Saline/Acetate Infusion in the Treat- ment of Cerebral Edema: Effect on Intracranial Pressure and Lateral Displacement of the Brain,” Critical Care Medicine, Vol. 26, No. 3, 1998, pp. 440-446. doi:10.1097/00003246-199803000-00011 Copyright © 2011 SciRes. OJVM  M. AKAISHIZAWA ET AL. Copyright © 2011 SciRes. OJVM 7 [30] A. M. Mirski, I. D. Denchev, S. M. Schnitzer and F. D. Hanley, “Comparison between Hypertonic Saline and Mannitol in the Reduction of Elevated Intracranial Pres- sure in a Rodent Model of Acute Cerebral Injury,” Jour- nal of Neurosurgical Anesthesiology, Vol. 12, No. 4, 2000, pp. 334-344. doi:10.1097/00008506-200010000-00006 [31] D. Wisner, L. Schuster and C. Quinn, “Hypertonic Saline Resuscitation of Head Injury: Effects on Cerebral Water Content,” Journal of Trauma, Vol. 30, No. 1, 1990, pp. 75-78. doi:10.1097/00005373-199001000-00011 [32] S. Holbeck, P. Bentzer and P. O. Grande, “Effects of Hypertonic Saline, Mannitol, and Urea with Regard to Absorption and Rebound Filtration in Cat Skeletal Mus- cle,” Critical Care Medicine, Vol. 30, No. 1, 2002, pp. 212-217. doi:10.1097/00003246-200201000-00030 [33] E. Rozanski and M. Rondeau, “Choosing Fluids in Trau- matic Hypovolemic Shock: The Role of Crystalloids, Colloids, and Hypertonic Saline,” Journal of American Animal Hospital Association, Vol. 38, No. 6, 2002, pp. 499-501. [34] M. M. Krausz and M. Hirsh, “Bolus Versus Continuous Fluid Resuscitation and Splenectomy for Treatment of Uncontrolled Hemorrhagic Shock after Massive Splenic Injury,” Journal of Trauma, Vol. 55, No. 1, 2003, pp. 62- 68. doi:10.1097/01.TA.0000074110.77122.46 [35] P. Horn, E. Munch, P. Vajkoc zy , P. Herrma n, M. Quinte l, L. Schilling, P. Schmiedek and L. Schrer, “Hypertonic Saline Solution for Control of Elevated Intracranial Pres- sure in Patients with Exhausted Response to Mannitol and Barbiturates,” Neurological Research, Vol. 21, No. 8, 1999, pp. 758-764. [36] A. V. Deliganis, D. J. Fisher, A. M. Lam and K. R. Maravilla, “Cerebrospinal Fluid Signal Intensity Increase on FLAIR MR Images in Patients under General Anes- thesia: The Role of Supplemental O2,” Radiology, Vol. 218, No. 1, 2001, pp. 152-156. [37] A. V. Deliganis, D. J. Fisher, A. M. Lam and K. R. Maravilla, “Cerebrospinal Fluid Signal Intensity Increase on FLAIR MR Images in Patients under General Anes- thesia: The Role of Supplemental O2,” Radiology, Vol. 218, No. 1, 2001, pp. 152-156.
|