Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2010, 2, 13-18 doi:10.4236/msa.2010.11003 Published Online April 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA 13 Medpor® Acts on Stem Cells Derived from Peripheral Blood Vincenzo Sollazzo1, Annalisa Palmieri2, Ambra Girardi3, Francesca Farinella2, Giorgio Brunelli2, Giuseppe Spinelli4, Francesco Carinci3 1Orthopedic Clinic, University of Ferrara, Ferrara, Italy; 2Department of Maxillofacial Surgery, University of Ferrara, Ferrara, Italy; 3Department of Histology, Embryology and Applied Biology, University of Bologna, Bologna, Italy; 4Department of Maxillofacial Surgery, Careggi Hospital, Firenze, Italy. Email: crc@unife.it Received February 9th, 2010; revised March 11th, 2010; accepted March 15th, 2010. ABSTRACT Porous polyethylene (PP or Medpor®) is an alloplastic material used worldwide for craniofacial reconstruction. Al- though several clinical studies are available, how this material alters osteoblast activity to promote bone formation is poorly understood. To study how PP can induce osteoblast differentiation in mesenchymal stem cells, the expression levels of bone related genes and mesenchymal stem cells marker were analyzed, using real time Reverse Transcrip- tion-Polymerase Chain Reaction. PP causes induction of osteoblast transcriptional factor RUNX2 and of the bone re- lated genes osteocalcin (BGLAP) and alkaline phosphatase (ALPL). In contrast the expression of ENG was decreased in stem cells treated with PP respect to untreated cells, indicating the differentiation effect of this biomaterial on stem cells. The obtained results can be relevant to better understand the molecular mechanism of bone regeneration and as a model for comparing other materials with similar clinical effects. Keywords: Alloplastic Material, Porous Polyethylene, Gene Expression, Stem Cells 1. Introduction Craniofacial “skeletal” defects should be ideally correc- ted with autologous bone or cartilage. However, collect- ing an adequate amount of bone from other donor sites of the same patient is not always possible, it carries addi- tional morbidity and it determines prolonged operation time. On the contrary, homologue bank tissues and ani- mal derived products are not completely safe because unknown diseases can be potentially transferred. Conse- quently, alloplastic materials are used for craniofacial reconstruction. Among them, porous polyethylene (PP or Medpor®) has been extensively used since the nineties. Its properties make it an excellent choice for correcting cranial and facial defects. The implant is easy to shape, flexible, remarkably stable, and it exhibits rapid soft- tissue growth. PP has been used to repair cranial defects [1,2], to re- store facial deformities [3], to reconstruct both ear [4] and orbit [5], as spherical orbital prostheses [6], to cor- rect lower eyelid retraction [7] and to restore nasal func- tion and shape [8]. Reported complications are persistent pain and anesthesia, implant exposure, infection and sub- sequent graft removal. Although several studies have analyzed applications and risk factors associated with the use of this synthetic graft, there is a lack as regards research into genetic ef- fects. In previous studies by using cDNA microarray contai- ning 19,200 genes, we identified in osteoblast-like cell lines (i.e. MG-63) cultured on PP, several genes where expression were differentially regulated. The differentia- lly expressed genes cover a broad range of functional activities: 1) signal transduction, 2) transcription, 3) translation, 4) cell cycle regulation, 5) vesicular transport, and 6) production of cytoskeletal elements, cell-adhesion molecules and extracellular matrix components [9]. We therefore investigated, by using microRNA microarray techniques, the translation regulation in osteoblasts expo- sed to PP. We identified miRNA that regulates the tra- nsduction of genes related to bone formation (OSTF1), skeletal development (CHRD, EN1, ADAMTS4, GHR- HR) and cartilage remodeling (MGP, NOG, LECT1, PTH) [10]. Since PP is always fixed onto bone and the mechanism by which PP acts on osteoblasts is incompletely known, we therefore attempted to get more inside by using hu- man stem cells isolated from peripheral blood.  Medpor® Acts on Stem Cells Derived from Peripheral Blood 14 Because no reports analyze the genetic effects of PP on stem cells, the expression of genes related to the os- teoblast differentiation were analyzed using cultures of human mesenchymal stem cells derived from peripheral blood (PB-hMSCs) treated with PP. To investigate the osteogenic differentiation of PB- hMSCs, the quantitative expression of the mRNA of spe- cific genes, like transcriptional factor (RUNX2), bone re- lated genes (SPP1, COL1A1, COL3A1, BGLAP, ALPL, and FOSL1) and mesenchymal stem cells marker (ENG) were examined by means of real time Reverse Transcrip- tion-Polymerase Chain Reaction (real time RT-PCR). 2. Materials and Methods 2.1 Stem Preparation PB-hMSCs were obtained for gradient centrifugation fr- om peripheral blood of healthy anonymous volunteers, using the Acuspin System-Histopaque 1077 (Sigma Al- drich, Inc., St Louis, Mo, USA). Firstly, 30 ml of heap- rinizated peripheral blood were added to the Acuspin Sy- stem-Histopaque 1077 tube and centrifugated at 1000 × g for 10 minutes. After centrifugation the interface contain- ing mononuclear cells was transferred in another tube, washed with PBS and centrifugated at 250 × g per 10 minutes. The enriched mononuclear pellets was resus- pended in 10 ml of Alphamem medium (Sigma Aldrich, Inc., St Louis, Mo, USA) supplemented with antibiotics (Penicillin 100 U/ml and Streptomycin 100 micro- grams/ml - Sigma, Chemical Co., St Louis, Mo, USA) and amminoacids (L-Glutamine - Sigma, Chemical Co., St Louis, Mo, USA). The cells were maintained in a 5% CO2 humidified atmosphere at 37℃. Medium was changed after 24 hours. PB-hMSC were selected for ade- sivity and characterized for staminality by immunoflo- rescence. 2.2 Immunofluorescence Cells were washed with PBS for three times and fixed with cold methanol for 5 min at room temperature. After washing with PBS, cells were blocked with bovine albu- min 3% (Sigma Aldrich, Inc., St Louis, Mo, USA) for 30 min at room temperature. The cells were incubated over- night sequentially at 4℃ with primary antibodies raised against CD105 1:200, mouse (BD Biosciences, San Jose, CA, USA), CD73 1:200, mouse (Santa Cruz Biotecnol- ogy, Inc., Santa Cruz, CA, USA), CD90 1:200, mouse (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA), CD34 1:200, mouse (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA). They were washed with PBS and incubated for 1 h at room temperature with secondary antibody conjugated-Rodamine goat anti-mouse 1:200 (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA). Subsequently, cells were mounted with the Vectashield Mounting Medium with DAPI (Vector Laboratories, Inc., Burlingame, CA, USA) and observed under a fluores- cence microscope (Eclipse TE 2000-E, Nikon Instru- ments S.p.a., Florence, Italy). 2.3 Cell Culture PB-hMSCs at fourth passage were cultured in Alphamem medium (Sigma Aldrich, Inc., St Louis, Mo, USA) sup- plemented with 10% fetal calf serum, antibiotics (Peni- cillin 100 U/ml and Streptomycin 100 micrograms/ml - Sigma Aldrich, Inc., St Louis, Mo, USA) and ammino- acids (L-Glutamine - Sigma Aldrich, Inc., St Louis, Mo, USA). The cells were maintained in a 5% CO2 humidi- fied atmosphere at 37℃. For the assay, cells were coll- ected and seeded at a density of 1 × 105 cells/ml into 9 cm2 (3 ml) wells by using 0.1% trypsin, 0.02% EDTA in Ca++ – and Mg – free Eagle’s buffer for cell release. In one set of wells a sheet of Medpor® (Porex Corpo- ration, Fairburn, Georgia, USA) was placed on the bot- tom leaving cells growing on it for 7 days. Another set of wells containing untreated cells was used as control. The medium was changed every 3 days. After seven days, when cultures were sub-confluent, cells were processed for RNA extraction. 2.4 RNA Processing Reverse transcription to cDNA was performed directly from cultured cell lysate using the TaqMAn Gene Ex- pression Cells-to-Ct Kit (Ambion Inc., Austin, TX, USA), following manufacturer's instructions. Briefly, cultured cells were lysed with lysis buffer and RNA released in this solution. Cell lysate were reverse transcribed to cDNA using the RT Enzyme Mix and appropriate RT buffer (Ambion Inc., Austin, TX, USA). Finally the cDNA was amplified by real-time PCR usi- ng the included TaqMan Gene Expression Master Mix and the specific assay designed for the investigated genes. 2.5 Real Time PCR Expression was quantified using real time RT-PCR. The gene expression levels were normalized to the expression of the housekeeping gene RPL13A and were expressed as fold changes relative to the expression of the untreated PB-hMSCs. Quantification was done with the delta/ delta calculation method [11]. Forward and reverse primers and probes for the selected genes were designed using Primer Express Software (Ap- plied Biosystems, Foster City, CA, USA) and are listed in Table 1. All PCR reactions were performed in a 20 µl volume using the ABI PRISM 7500 (Applied Biosystems, Foster City, CA, USA). Each reaction contained 10 µl 2X TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA, USA), 400 nM concentration of each primer and 200 nM of the probe, and cDNA. The ampli- fication profile was initiated by 10-minute incubation at Copyright © 2010 SciRes. MSA 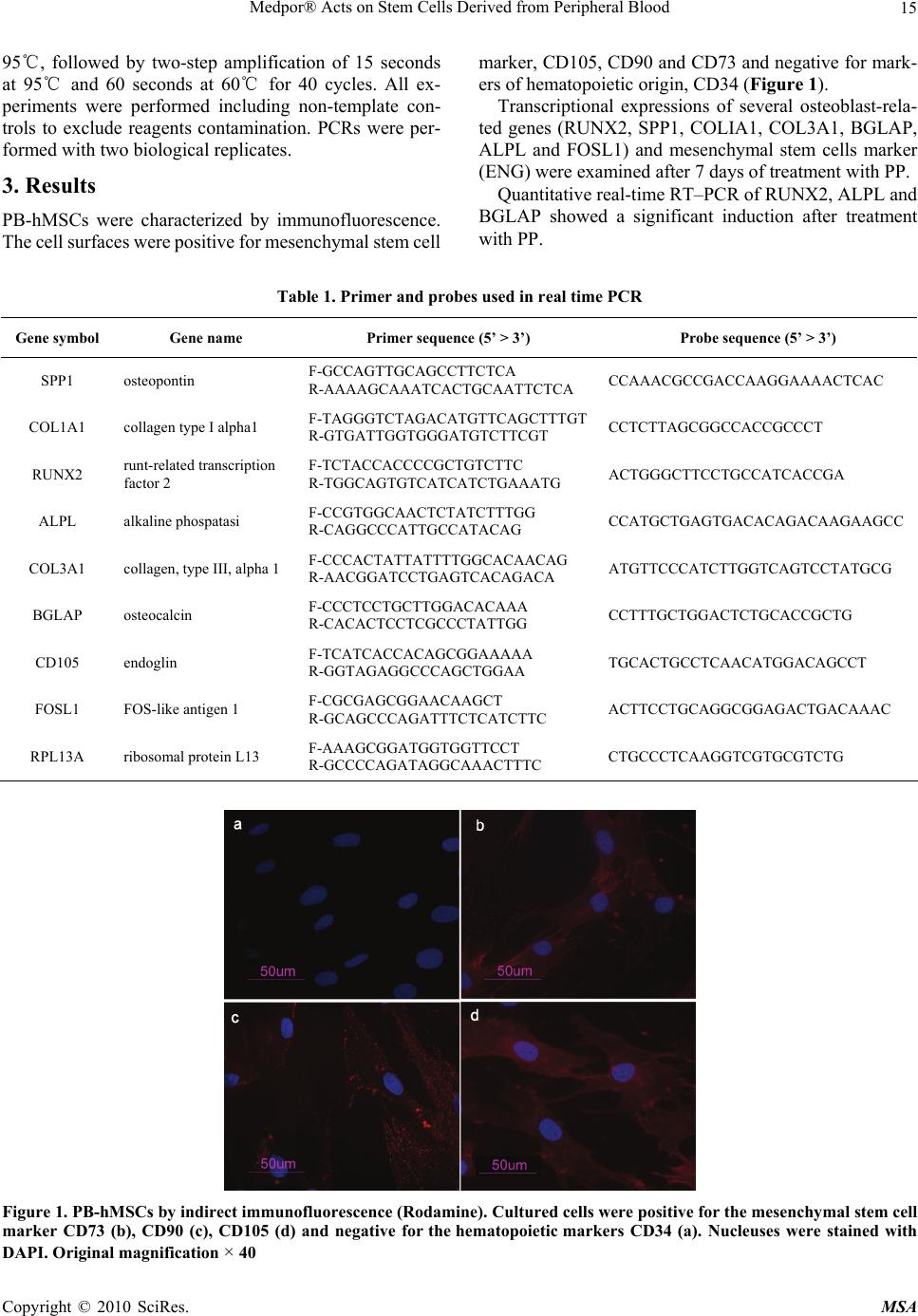 Medpor® Acts on Stem Cells Derived from Peripheral Blood Copyright © 2010 SciRes. MSA 15 95℃, followed by two-step amplification of 15 seconds at 95℃ and 60 seconds at 60℃ for 40 cycles. All ex- periments were performed including non-template con- trols to exclude reagents contamination. PCRs were per- formed with two biological replicates. 3. Results PB-hMSCs were characterized by immunofluorescence. The cell surfaces were positive for mesenchymal stem cell marker, CD105, CD90 and CD73 and negative for mark- ers of hematopoietic origin, CD34 (Figure 1). Transcriptional expressions of several osteoblast-rela- ted genes (RUNX2, SPP1, COLIA1, COL3A1, BGLAP, ALPL and FOSL1) and mesenchymal stem cells marker (ENG) were examined after 7 days of treatment with PP. Quantitative real-time RT–PCR of RUNX2, ALPL and BGLAP showed a significant induction after treatment with PP. Table 1. Primer and probes used in real time PCR Gene symbol Gene name Primer sequence (5’ > 3’) Probe sequence (5’ > 3’) SPP1 osteopontin F-GCCAGTTGCAGCCTTCTCA R-AAAAGCAAATCACTGCAATTCTCA CCAAACGCCGACCAAGGAAAACTCAC COL1A1 collagen type I alpha1 F-TAGGGTCTAGACATGTTC AGCTTTGT R-GTGATTGGTGGGATGTCTTCGT CCTCTTAGCGGCCACCGCCCT RUNX2 runt-related transcription factor 2 F-TCTACCACCCCGCTGTCTTC R-TGGCAGTGTCATCATCTGAAATG ACTGGGCTTCCTGCCATCACCGA ALPL alkaline phospatasi F-CCGTGGCAACTCTATCTTTGG R-CAGGCCCATTGCCATACAG CCATGCTGAGTGACACAGACAAGAAGCC COL3A1 collagen, type III, alpha 1 F-CCCACTATTATTTTGGCACAACAG R-AACGGATCCTGAGTCACAGACA ATGTTCCCATCTTGGTCAGTCCTATGCG BGLAP osteocalcin F-CCCTCCTGCTTGGACACAAA R-CACACTCCTCGCCCTATTGG CCTTTGCTGGACTCTGCACCGCTG CD105 endoglin F-TCATCACCACAGCGGAAAAA R-GGTAGAGGCCCAGCTGGAA TGCACTGCCTCAACATGGACAGCCT FOSL1 FOS-like antigen 1 F-CGCGAGCGGAACAAGCT R-GCAGCCCAGATTTCTCATCTTC ACTTCCTGCAGGCGGAGACTGACAAAC RPL13A ribosomal protein L13 F-AAAGCGGATGGTGGTTCCT R-GCCCCAGATAGGCAAACTTTC CTGCCCTCAAGGTCGTGCGTCTG Figure 1. PB-hMSCs by indirect immunofluorescence (Rodamine). Cultured cells were positive for the mesenchymal stem cell marker CD73 (b), CD90 (c), CD105 (d) and negative for the hematopoietic markers CD34 (a). Nucleuses were stained with DAPI. Original magnification × 40 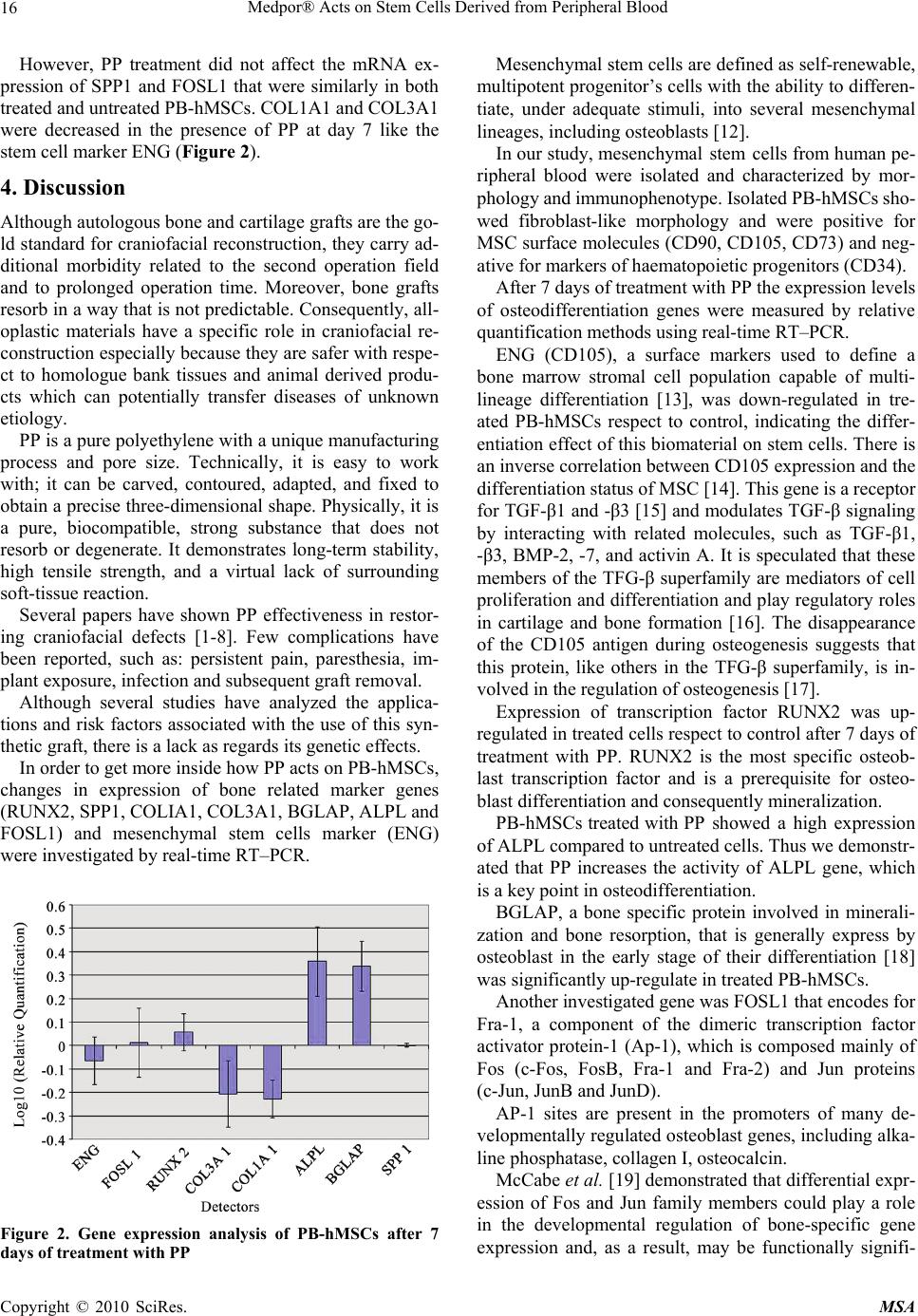 Medpor® Acts on Stem Cells Derived from Peripheral Blood 16 However, PP treatment did not affect the mRNA ex- pression of SPP1 and FOSL1 that were similarly in both treated and untreated PB-hMSCs. COL1A1 and COL3A1 were decreased in the presence of PP at day 7 like the stem cell marker ENG (Figure 2). 4. Discussion Although autologous bone and cartilage grafts are the go- ld standard for craniofacial reconstruction, they carry ad- ditional morbidity related to the second operation field and to prolonged operation time. Moreover, bone grafts resorb in a way that is not predictable. Consequently, all- oplastic materials have a specific role in craniofacial re- construction especially because they are safer with respe- ct to homologue bank tissues and animal derived produ- cts which can potentially transfer diseases of unknown etiology. PP is a pure polyethylene with a unique manufacturing process and pore size. Technically, it is easy to work with; it can be carved, contoured, adapted, and fixed to obtain a precise three-dimensional shape. Physically, it is a pure, biocompatible, strong substance that does not resorb or degenerate. It demonstrates long-term stability, high tensile strength, and a virtual lack of surrounding soft-tissue reaction. Several papers have shown PP effectiveness in restor- ing craniofacial defects [1-8]. Few complications have been reported, such as: persistent pain, paresthesia, im- plant exposure, infection and subsequent graft removal. Although several studies have analyzed the applica- tions and risk factors associated with the use of this syn- thetic graft, there is a lack as regards its genetic effects. In order to get more inside how PP acts on PB-hMSCs, changes in expression of bone related marker genes (RUNX2, SPP1, COLIA1, COL3A1, BGLAP, ALPL and FOSL1) and mesenchymal stem cells marker (ENG) were investigated by real-time RT–PCR. Figure 2. Gene expression analysis of PB-hMSCs after 7 days of treatment with PP Mesenchymal stem cells are defined as self-renewable, multipotent progenitor’s cells with the ability to differen- tiate, under adequate stimuli, into several mesenchymal lineages, including osteoblasts [12]. In our study, mesenchymal stem cells from human pe- ripheral blood were isolated and characterized by mor- phology and immunophenotype. Isolated PB-hMSCs sho- wed fibroblast-like morphology and were positive for MSC surface molecules (CD90, CD105, CD73) and neg- ative for markers of haematopoietic progenitors (CD34). After 7 days of treatment with PP the expression levels of osteodifferentiation genes were measured by relative quantification methods using real-time RT–PCR. ENG (CD105), a surface markers used to define a bone marrow stromal cell population capable of multi- lineage differentiation [13], was down-regulated in tre- ated PB-hMSCs respect to control, indicating the differ- entiation effect of this biomaterial on stem cells. There is an inverse correlation between CD105 expression and the differentiation status of MSC [14]. This gene is a receptor for TGF-β1 and -β3 [15] and modulates TGF-β signaling by interacting with related molecules, such as TGF-β1, -β3, BMP-2, -7, and activin A. It is speculated that these members of the TFG-β superfamily are mediators of cell proliferation and differentiation and play regulatory roles in cartilage and bone formation [16]. The disappearance of the CD105 antigen during osteogenesis suggests that this protein, like others in the TFG-β superfamily, is in- volved in the regulation of osteogenesis [17]. Expression of transcription factor RUNX2 was up- regulated in treated cells respect to control after 7 days of treatment with PP. RUNX2 is the most specific osteob- last transcription factor and is a prerequisite for osteo- blast differentiation and consequently mineralization. PB-hMSCs treated with PP showed a high expression of ALPL compared to untreated cells. Thus we demonstr- ated that PP increases the activity of ALPL gene, which is a key point in osteodifferentiation. BGLAP, a bone specific protein involved in minerali- zation and bone resorption, that is generally express by osteoblast in the early stage of their differentiation [18] was significantly up-regulate in treated PB-hMSCs. Another investigated gene was FOSL1 that encodes for Fra-1, a component of the dimeric transcription factor activator protein-1 (Ap-1), which is composed mainly of Fos (c-Fos, FosB, Fra-1 and Fra-2) and Jun proteins (c-Jun, JunB and JunD). AP-1 sites are present in the promoters of many de- velopmentally regulated osteoblast genes, including alka- line phosphatase, collagen I, osteocalcin. McCabe et al. [19] demonstrated that differential expr- ession of Fos and Jun family members could play a role in the developmental regulation of bone-specific gene expression and, as a result, may be functionally signifi- Copyright © 2010 SciRes. MSA  Medpor® Acts on Stem Cells Derived from Peripheral Blood17 cant for osteoblast differentiation. In our study FOSL1 was down-regulated, probably because cells were at early stage of differentiation. Kim et al. [20] studying the effect of a new anabolic agents that stimulate bone formation, find that this gene is acti- vated in the late stage of differentiation, during the cal- cium deposition. SSP1 encodes osteopontin, which is a phosphoglyco- protein of bone matrix and it is the most representative non collagenic component of extracellular bone matrix [21]. Osteopontin is actively involved in bone resorbitive processes directly by ostoclasts [22]. Osteopontin pro- duced by osteoblasts, show high affinity to the molecules of hydroxylapatite in extracellular matrix and it is che- mo-attractant to osteoclasts [23]. However, in our expe- rimental model, SPP1 expression during osteogenic in- duction was similar in both treated and control because this gene regulates the later stages of osteoblast differen- tiation and bone development [22]. This result is not in contrast with those previously reported as we investi- gated stem cells treated for 7 days. PP also modulates the expression of genes encoding for collagenic extracellular matrix proteins like collagen type 1α1 (COL1A1). Collagen type1 is the most abun- dant in the human organism [24]. COL1A1 and COL3A1 were significantly down ex- pressed as compared to the control when exposed to PP probably because these genes are activated in the late stage of differentiation and are related to extracellular matrix synthesis. COL3A1 encodes the pro-alpha1 chains of type III col- lagen, a fibrillar collagen that is found in extensible con- nective tissues [25]. The present study shows the effect of PP on PB-hM- SCs in the early differentiation stages: PP is an inducer of osteogenesis on human stem cells, as demonstrated by the expression of bone related genes like RUNX2, ALPL and BGLAP. Moreover, we have chosen to perform the experiment after 7 days in order to get information on the early stages of stimulation. It is our understanding, there- fore, that more investigations with different time points are needed in order to get a global comprehension of the molecular events related to PP action. The reported mo- del is useful to investigate the effects of different sub- stances on stem cells. 5. Acknowledgments This work was supported by FAR from the University of Ferrara (FC), Ferrara, Italy, and from Regione Emilia Romagna, Programma di Ricerca Regione Università, 2007-2009, Area 1B: Patologia osteoarticolare: ricerca pre-clinica e applicazioni cliniche della medicina rigen- erativa, Unità Operativa n. 14. REFERENCES [1] T. Wellisz, W. Dougherty and J. Gross, “Craniofacial Applications for the Medpor Porous Polyethylene Flex- block Implant,” Journal of Craniofacial Surgery, Vol. 3, 1992, pp. 101-107. [2] J. Park and M. Guthikonda, “The Medpor Sheet as a Sel- lar Buttress after Endonasal Transsphenoidal Surgery: Technical Note,” Surgical Neurology, Vol. 61, 2004, pp. 488-492; discussion 493. [3] T. Wellisz, “Clinical Experience with the Medpor Porous Polyethylene Implant,” Aesthetic Plastic Surgery, Vol. 17, 1993, pp. 339-344. [4] T. Wellisz, “Reconstruction of the Burned External Ear Using a Medpor Porous Polyethylene Pivoting Helix Framework,” Plastic and Reconstructive Surgery, Vol. 91, 1993, pp. 811-818. [5] J. J. Romano, N. T. Iliff and P. N. Manson, “Use of Med- por Porous Polyethylene Implants in 140 patients with Facial Fractures,” Journal of Craniofacial Surgery, Vol. 4, 1993, pp. 142-147. [6] J. W. Karesh and S. C. Dresner, “High-Density Porous Polyethylene (Medpor) as a Successful Anophthalmic Socket Implant,” Ophthalmology, Vol. 101, 1994, pp. 1688-1695; discussion 1695-1686. [7] J. F. Wong, C. N. Soparkar and J. R. Patrinely, “Correc- tion of Lower Eyelid Retraction with High Density Po- rous Polyethylene: The Medpor((R)) Lower Eyelid Spacer,” Orbit, Amsterdam, Vol. 20, 2001, pp. 217-225. [8] T. Romo, 3rd, A. P. Sclafani, P. Sabini, “Use of Porous High-Density Polyethylene in Revision Rhinoplasty and in the Platyrrhine Nose,” Aesthetic Plastic Surgery, Vol. 22, 1998, pp. 211-221. [9] F. Carinci, A. Palmieri, V. Perrotti, A. Piattelli, R. Cenzi, G. Brunell, M. Martinelli, M. Arlotti and F. Pezzetti, “Ge- netic Effects of Medpor on Osteoblast-Like Cells,” Jour- nal of Craniofacial Surgery, Vol. 17, 2006, pp. 1243- 1250. [10] A. Palmieri, F. Pezzetti, G. Brunelli, M. Martinelli, L. Scapoli, M. Arlotti, E. Masiero and F. Carinci, “Medpor Regulates Osteoblast's MicroRNAs,” Bio-Medical Mate- rials and Engineering, Vol. 18, 2008, pp. 91-97. [11] K. J. Livak and T. D. Schmittgen, “Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method,” Methods, San Diego, Vol. 25, 2001, pp. 402-408. [12] A. Alhadlaq and J. J. Mao, “Mesenchymal Stem Cells: Isolation and Therapeutics,” Stem Cells and Development, Vol. 13, 2004, pp. 436-448. [13] M. F. Pittenger, A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simon- etti, S. Craig and D. R. Marshak, “Multilineage Potential of Adult Human Mesenchymal Stem Cells,” Science, New York, Vol. 284, 1999, pp. 143-147. [14] H. J. Jin, S. K. Park, W. Oh, Y. S. Yang, S. W. Kim and S. J. Choi, “Down-Regulation of CD105 is Associated with Multi-Lineage Differentiation in Human Umbilical Cord Copyright © 2010 SciRes. MSA  Medpor® Acts on Stem Cells Derived from Peripheral Blood Copyright © 2010 SciRes. MSA 18 Blood-Derived Mesenchymal Stem Cells,” Biochemical and Biophysical Research Communications, Vol. 381, 2009, pp. 676-681. [15] F. P. Barry, R. E. Boynton, S. Haynesworth, J. M. Mur- phy and J. Zaia, “The Monoclonal Antibody SH-2, Raised against Human Mesenchymal Stem Cells, Recognizes an Epitope on Endoglin (CD105),” Biochemical and Bio- Physical Research Communications, Vol. 265, 1999, pp. 134-139. [16] M. Jakob, O. Demarteau, D. Schafer, B. Hintermann, W. Dick, M. Heberer and I. Martin, “Specific Growth Factors during the Expansion and Redifferentiation of Adult Hu- man Articular Chondrocytes Enhance Chondrogenesis and Cartilaginous Tissue Formation in Vitro,” Journal of Cellular Biochemistry, Vol. 81, 2001, pp. 368-377. [17] S. E. Haynesworth, M. A. Baber and A. I. Caplan, “Cell Surface Antigens on Human Marrow-Derived Mesen- chymal Cells are Detected by Monoclonal Antibodies,” Bone, Vol. 13, 1992, pp. 69-80. [18] E. Aubin, J. B. Lian and G. S. Stein, “Bone Formation: Maturation and Functional Activities of Osteoblast Line- age cells,” In: M. J. Favus, Ed., Primer on the Metabolic Bone Diseases and Disorders on Mineral Metabolism, The American Society for Bone and Mineral Research, Washington, D. C., 2003, pp. 13-28. [19] L. R. McCabe, C. Banerjee, R. Kundu, R. J. Harrison, P. R. Dobner, J. L. Stein, J. B. Lian and G. S. Stein, “De- velopmental Expression and Activities of Specific Fos and Jun Proteins are Functionally Related to Osteoblast Maturation: Role of Fra-2 and Jun D during differentia- tion,” Endocrinology, Vol. 137, 1996, pp. 4398-4408. [20] J. M. Kim, S. U. Lee, Y. S. Kim, Y. K. Min and S. H. Kim, “Baicalein Stimulates Osteoblast Differentiation via Coordinating Activation of MAP Kinases and Transcrip- tion Factors,” Journal of Cellular Biochemistry, Vol. 104, 2008, pp. 1906-1917. [21] M. D. McKee, M. C. Farach-Carson, W. T. Butler, P. V. Hauschka and A. Nanci, “Ultrastructural Immunolocali- zation of Noncollagenous (Osteopontin and Osteocalcin) and Plasma (Albumin and Alpha 2HS-Glycoprotein) Pro- teins in Rat Bone,” Journal of Bone and Mineral Re- search, Vol. 8, 1993, pp. 485-496. [22] R. A. Dodds, J. R. Connor, I. E. James, E. L. Rykac- zewski, E. Appelbaum, E. Dul and M. Gowen, “Human Osteoclasts, not Osteoblasts, Deposit Osteopontin onto Resorption Surfaces: An in Vitro and ex Vivo Study of Remodeling Bone,” Journal of Bone and Mineral Re- search, Vol. 10, 1995, pp. 1666-1680. [23] C. Ohtsuki, M. Kamitakahara and T. Miyazaki, “Bioac- tive Ceramic-Based Materials with Designed Reactivity for Bone Tissue Regeneration,” Journal of the Royal So- ciety, Interface / the Royal Society, Vol. 6, Supplement 3, 2009, pp. S349-360. [24] M. Suuriniemi, V. Kovanen, A. Mahonen, M. Alen, Q. Wang, A. Lyytikainen and S. Cheng, “COL1A1 Sp1 Polymorphism Associates with Bone Density in Early Puberty,” Bone, Vol. 39, 2006, pp. 591-597. [25] T. F. Chan, A. Poon, A. Basu, N. R. Addleman, J. Chen, A. Phong, P. H. Byers, T. E. Klein and P. Y. Kwok, “Natural Variation in Four Human Collagen Genes across an Ethnically Diverse Population,” Genomics, Vol. 91, 2008, pp. 307-314. |

