Journal of Biomedical Science and Engineering
Vol. 5 No. 12A (2012) , Article ID: 26270 , 7 pages DOI:10.4236/jbise.2012.512A110
Non-rigid registration and KLT filter to improve SNR and CNR in GRE-EPI myocardial perfusion imaging
![]()
1Department of Internal Medicine, Cardiovascular Division, Dorothy M. Davis Heart and Lung Research Institute, Columbus, USA
2Imaging and Visualization, Siemens Corporate Research, Princeton, USA
3Shenzhen Institutes of Advanced Technology, Shenzhen University Town, Shenzhen, China
4Imaging & IT Division, Siemens AG, Healthcare Sector, Erlangen, Germany
Email: mihai.3@osu.edu
Received 18 October 2012; revised 21 November 2012; accepted 3 December 2012
Keywords: Cardiac First Pass Perfusion; Non-Rigid Registration; KLT Filter; CNR; Dynamic MRI
ABSTRACT
The purpose of the study was to evaluate the effect of motion compensation by non-rigid registration combined with the Karhunen-Loeve Transform (KLT) filter on the signal to noise (SNR) and contrast-tonoise ratio (CNR) of hybrid gradient-echo echoplanar (GRE-EPI) first-pass myocardial perfusion imaging. Twenty one consecutive first-pass adenosine stress perfusion MR data sets interpreted positive for ischemia or infarction were processed by non-rigid Registration followed by KLT filtering. SNR and CNR were measured in abnormal and normal myocardium in unfiltered and KLT filtered images following nonrigid registration to compensate for respiratory and other motions. Image artifacts introduced by filtering in registered and nonregistered images were evaluated by two observers. There was a statistically significant increase in both SNR and CNR between normal and abnormal myocardium with KLT filtering (mean SNR increased by 62.18% ± 21.05% and mean CNR increased by 58.84% ± 18.06%; p = 0.01). Motion correction prior to KLT filtering reduced significantly the occurrence of filter induced artifacts (KLT only-artifacts in 42 out of 55 image series vs. registered plus KLT-artifacts in 3 out of 55 image series). In conclusion the combination of nonrigid registration and KLT filtering was shown to increase the SNR and CNR of GRE-EPI perfusion images. Subjective evaluation of image artifacts revealed that prior motion compensation significantly reduced the artifacts introduced by the KLT filtering process.
1. INTRODUCTION
While cardiovascular magnetic resonance (CMR) plays an increasingly important role in the evaluation of patients with known or suspected coronary artery disease (CAD), the acceptance of first-pass perfusion CMR still depends on its ability to meet several conflicting technical requirements. The needs for high speed dynamic imaging, high in-plane resolution (<3 mm), multi-slice coverage of the heart, linearity (quantifiable relationship between signal intensity and contrast agent concentration) and sufficient contrast to distinguish normal and abnormally perfused myocardium [1,2] place high demands on MRI hardware and image acquisition techniques. The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) are very often limited in first pass perfusion imaging because high temporal resolution tracking of the contrast agent bolus and multi-slice coverage require ultra-fast image acquisition and the use of parallel imaging techniques.
Although spatial and/or temporal low pass filtering of dynamic first-pass perfusion images can improve SNR, simple Fourier filtering causes blurring that may interfere with the visual assessment of perfusion defects. To improve SNR without sacrificing the image quality, wavelet temporal [3] and spatial filtering [4] have been applied to denoise myocardial first-pass perfusion data. While improvement in quantitative results was significant, temporal filtering strictly relies on correct pixelby-pixel correspondences and its applicability is limited by significant myocardium motion. As a result, registering the perfusion images [5-8] to compensate for complex motion patterns introduced by breathing, irregular heart beat and imperfect cardiac gating becomes not only a prerequisite for quantification of the myocardial perfusion data, but a necessary preceding step for temporal filtering.
The Karhunen-Loeve Transform (KLT) filter has been shown to significantly improve SNR in dynamic MRI [9]. The KLT filter can be viewed as a “smart averaging” approach and its performance is highly dependent on the temporal correlation between images in a dynamic series [10]. Using the KL transform, the information content of a highly correlated time series of images can be represented in a relatively small set of eigenimages; other modes representing the noise are dropped during the KLT reconstruction, making the KLT filter very effective at preserving information and reducing noise. Unfortunately, the complex motion patterns introduced by breathing, irregular cardiac rhythm and imperfect cardiac gating often disturbs the temporal correlation of first-pass perfusion images, and thereby spreads information over a larger number of eigenimages, leading to less efficient KLT reconstruction and discernible artifacts between perfusion frames with significant motion. In order to successfully apply the KLT filter to clinical perfusion datasets with significant myocardial motion, we hereby propose to apply non-rigid registration to compensate for respiratory motion and myocardial deformation between image frames. This novel combined approach of nonrigid registration prior to filtering is expected to improve the effectiveness of KLT filtering by concentrating the information content into a smaller number of eigenimages [11].
The purpose of this study was to evaluate the effect of applying a non-rigid registration algorithm for motion correction prior to KLT filtering to reduce image noise and improve SNR and CNR in GRE-EPI first-pass perfusion images.
2. MATERIALS AND METHODS
2.1. Magnetic Resonance Imaging
Twenty-one, first-pass adenosine stress perfusion datasets clinically interpreted as positive for ischemia or infarction (diagnosis confirmed by a previous X-ray angiography exam in eight cases) were selected, processed and analyzed retrospectively with institutional review board (IRB) approval. Each dataset consisting of four 10 mm slices (one horizontal long-axis and 3 shortaxis views with approximately 10 mm gap) was acquired using an average field-of-view of 400 mm × 320 mm and a matrix size of 160 × 120. Images were acquired every heart beat during bolus injection of gadopentetate dimeglumine (Gd-DTPA) at a dose of 0.1 mmol/Kg with an injection rate of 4 mL/s. Perfusion images were acquired using a fat suppressed saturation-recovery T1-weighted GRE-EPI sequence with TSENSE acceleration rate 2 on a 1.5 T MR System (MAGNETOM Avanto, Siemens Healthcare, Germany). Acquisition parameters were as follows: TR/TE = 5.8 ms/1.09 ms, TI = 100 ms, bandwidth = 1935 Hz/pixel, EPI factor = 4, flip angle = 25˚, center-out k-space reordering and 60 measurements (cardiac cycles). Patients were instructed to hold their breath as long as possible, and afterwards they were allowed to breathe freely. For each subject, only those slices (either short axis or horizontal long axis views) that showed one or more clinically interpreted perfusion defects were included in the analysis. This resulted in a total of 55 image series (out of the 84 available) that were processed as described in the following sections.
2.2. Non-Rigid Registration
The applied motion correction is based on a variational non-rigid registration algorithm [12,13]. This approach can be considered as an extension of the classic optical flow method. In this framework, a dense deformation field is estimated as the solution to a calculus of variation problem and the cost function is defined as the sum of image similarity measure and regularization terms. The classic gradient descent is used to solve the corresponding Euler equation. To speed-up the convergence and avoid local optima, a multi-scale image pyramid is created. We selected the local cross correlation ratio as the image similarity measure, as its explicit derivative can be more efficiently calculated than mutual information and still general enough to cope with intensity fluctuation and imaging noise between two adjacent perfusion frames.
The registration of perfusion frames is more robust if the two slices to be aligned have similar contrast. A consecutive motion compensation strategy was therefore developed to improve the performance of registration. The first step of proposed registration workflow aims to detect a key-frame for the perfusion series. This keyframe will be defined as the reference image and relative motion between other phases and this reference will be corrected. To improve the motion compensation, this key-frame should be a frame in which the myocardium has good contrast against the blood pool and surrounding tissues. We propose a key-frame selection approach which is based on the observation that during the contrast uptake the image intensity in regions where the contrast bolus enters will have higher standard deviation (SD) along the time dimension. As the first step, the standard deviation image for the perfusion series is computed.
Although the inconsistent myocardial motion can degrade the sharpness of myocardium, the contrast between myocardium and surrounding tissues in the SD image is found to be consistently noticeable. This observation holds true for the described perfusion MR pulse sequences. The next step is to select a frame having similar contrast as the SD image. For this purpose, the cross correlation ratios (CC) between every phase in the perfusion series and the SD image are computed. During the passing of contrast bolus, the CC ratio will keep increasing and reaches its peak around the time point where the myocardium blood perfusion is maximized. We therefore pick the phase corresponding to the maximal CC ratio as the key-frame.
As shown in Figure 1, motion compensation starts from the key-frame and its direct neighbors (previous and next). After the first registration is finished, the next frame is registered to its warped neighbor that has been transformed into the key-frame coordinate system. The complete series is corrected by consecutively performing multiple 2D-2D registrations between temporally adjacent slices.
2.3. Karhunen-Loeve Transform (KLT) Filter
The applied denoising filter is based on the Karhunen -Loeve Transform (KLT, a.k.a. Principal Component Analysis) in the temporal dimension. The KLT filter takes advantage of temporal redundancy in the dynamic image series. It can be considered as a “smart averaging” method to enhance SNR because any KLT filtered image is a linear combination of the original series of images. The KLT filtering removes eigenimages associated with low variance (or small eigenvalues), therefore the
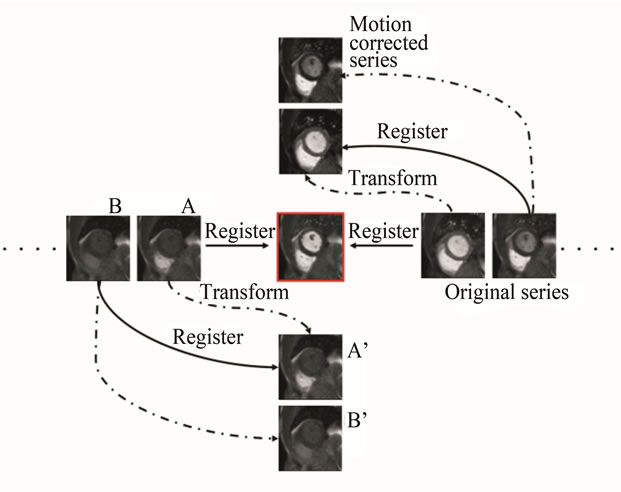
Figure 1. An illustration of the consecutive motion correction strategy. Motion compensation starts from the keyframe (central image) and its direct neighbors. Every image is aligned to its transformed previous neighbor. In this scheme, registration is performed between two perfusion phases with similar contrast, and every slice is registered to the transformed version of its predecessor. Specifically, in Step 1 a direct neighboring frame A is registered to the key frame; in step 2 the frame A is transformed (wrapped) to the coordinate of the key frame, and A’ frame is obtained. During step 3 frame B, successor of frame A is registered to the transformed slice A’. In step 4 frame B is transformed (wrapped) to the coordinate of the key frame and B’ is obtained. As a result the motion corrected series consist of slices A’, B’.
filtering operation is a low-rank approximation of the original data matrix and is optimal in the 2-norm or Frobenius norm sense [14].
Similar to a traditional low-pass filter, the KLT filter has three steps. First, the temporal KLT is applied to the dynamic image series to generate a series of eigenimages [15]. Unlike the original images, eigenimages form an orthogonal basis set. The total variance of each eigenimage is the corresponding eigenvalue. Second, the noisedominated eigenimages are identified and eliminated. Only eigenimages that contain significant spatially coherent structures are kept. It has been shown previously that the noise only eigenimages can be automatically selected based on the width of the central peak of the 2D autocorrelation function of each eigenimage—a measure of the spatially coherent structure in the image [9]. The width of the central peak can be described by its full width at half maximum (FWHM). We choose FWHM = 2.0 pixels as the cutoff criterion; thereby all eigenimages with autocorrelation FWHM less than this are considered to contain only noise. Third, after identifying those eigenimages dominated by noise, filtered images are reconstructed by applying the inverse KLT to the remaining eigenimages. By removing the noise-dominated eigenimages, the noise level is lowered and the SNR is improved. In other words, the KLT eigenvalues represent the distribution of the source data’s energy (variance) among each of the eigenimages. When m eigenimages are retained in the data set, the percentage energy contained in the KLT filtered images is the sum of the first m eigenvalues scaled by the total energy (sum of all p eigenvalues). The root-mean-square (RMS) relative noise level (RNL) of the filtered images is defined as the ratio of the noise standard deviation after to before KLT filtering:
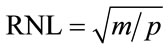 (1)
(1)
when m out of p eigenimages pass through the filter. The KLT filter takes advantage of the temporal redundancy in the image series; the registration process increases temporal redundancy such that energy is concentrated into fewer eigenimages, thereby improving the efficiency of the filtering. Less energy loss through the filtering process translates into fewer artifacts in the filtered images. However, not every frame has the same temporal redundancy as its neighbors and as a result the SNR gain is expected to vary across the image series after KLT filtering.
2.4. Quantitative Measurements
Non-rigid registration was first performed in each series to allow for semi-quantitative analysis of signal enhancement and to improve the temporal correlation among dynamic images prior to KLT filtering. The registered images were checked to assess the registration success by visually comparing the similarity of the left ventricular shape between the unregistered and registered images. Subsequently the KLT filter was applied to all image series. The mean energy (image information) concentrated in the preserved eigenvalues after registration and KLT filtering was evaluated and compared, using a Student t-test, to the mean energy concentrated in the eigenimages after KLT filtering in images that were not registered prior to filtering. Three consecutive image frames showing peak contrast enhancement in the normal myocardium were selected and regions of interest (ROI) were manually drawn in abnormal and normal myocardium for CNR calculation according to the equation CNR = (Snormal – Sabnormal)/σn, where Snormal, Sabnormal are the mean signal intensities of normal and abnormal perfused myocardium and σn is the standard deviation of the noise from a region outside the body. While parallel acquisition techniques like TSENSE are known to cause spatial variability in image noise, we only compare relative changes in SNR and CNR measured in the same ROIs in identical images processed by different methods. In this context, ROI-based measurements of signal and noise are appropriate. The three consecutive image frames at peak contrast enhancement were chosen for signal evaluation to account for the variable reduction of the temporal noise on each image frame introduced by the application of the KLT filter. Identical ROIs were drawn simultaneously in registered unfiltered images and registered filtered images using a Leonardo workstation (Siemens Healthcare, Germany).
In order to asses blurring or other image artifacts induced by the filtering process, KLT filtered images with and without prior registration were evaluated by two experienced observers by comparing them to the registered non-filtered images. All images series were scored by each of the observers according to a binary scoring system (artifact = 1, no change = 0). A score of 1 from either one of the two observers resulted in an overall artifact score for the image series.
3. RESULTS
All 55 image series were successfully registered and no artifacts due to registration were noted. The comparison of energy preservation between non-registered and registered KLT filtered images showed that a higher percentage of energy (hence image information) is preserved when the same eigenimage cutoff is applied (Figure 2). Motion correction by non-rigid registration resulted in a statistically significant increase (t-test, p < 0.001) in the mean energy concentrated in the preserved eigenimages with KLT filtering (1.46% image information loss without motion correction). SNR and CNR were significantly improved by the combination of motion correction and KLT filtering. Figure 3 demonstrates the SNR improvement that non-rigid registration plus KLT filtering had on the short-axis perfusion images of one patient. The expected SNR gain due to KLT filtering calculated for each of the 55 dynamic image series was estimated using Eq.1 to be 95.95% ± 26.02% increase over the baseline value (minimum SNR gain over the baseline value: 50.76%, maximum SNR gain: 144.95%). The variability in SNR gain was due to variability in the automatically calculated filter cutoff from series to series. The regionally measured mean increase over baseline in SNR with KLT filtering for the three evaluated peak enhancement frames was 62.18% (minimum SNR gain over baseline value: 21.48%, maximum SNR gain over baseline value: 106.81%). This SNR increase translated into a statistically significant increase in CNR between normal and abnormally perfused myocardium (mean increase over baseline value 58.84% ± 18.06%; 2 sample t-test, p = 0.01); the increase in CNR over baseline value ranged from 10.62% to 111% after registration and filtering (Figure 4).
The subjective evaluation of perfusion images by two independent observers showed that non-rigid registration reduced the occurrence of filter induced artifacts from 42 out of the 55 image series to only 3 out of 55 series. Figure 5 shows a representative example of how the registration process eliminates filtering induced artifacts; this artifact could have been mistakenly classified as a perfusion defect if only the KLT filtering would have been used to improve the overall SNR of the GRE-EPI perfusion image.
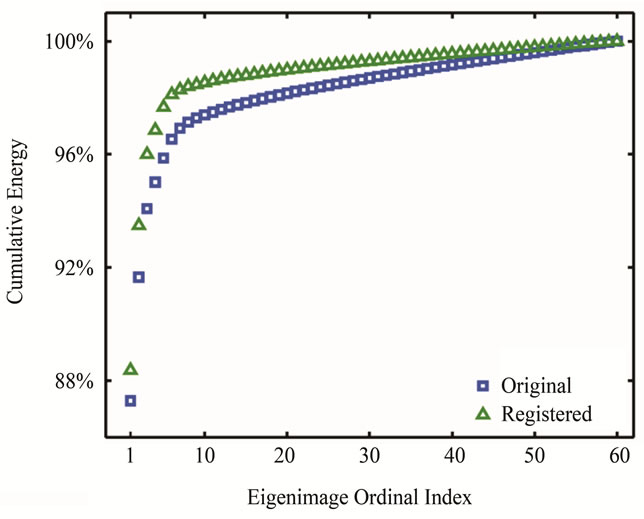
Figure 2. An example of percentage cumulative energy (variance) in the retained eigenmodes. After image registration, more energy is preserved when the same eigenimage cutoff is applied in KLT filtering.
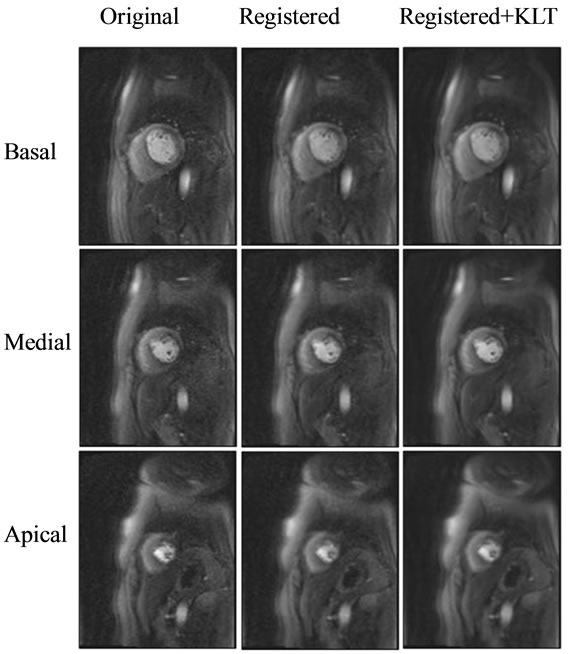
Figure 3. The effect of registration and KLT filtering in improving the image quality of the short axis first pass perfusion MR images in a patient. There is clear SNR/CNR improvement with registration and KLT filtering (middle and right column) over the original images (left column).
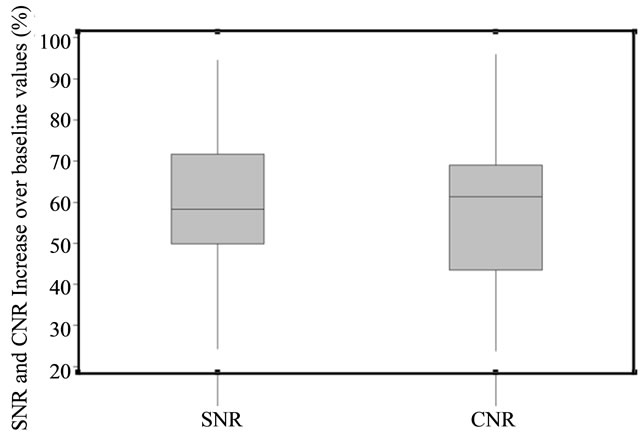
Figure 4. Boxplots reflecting the SNR and CNR increase due to KLT filtering after registration (as compared with only registered images).
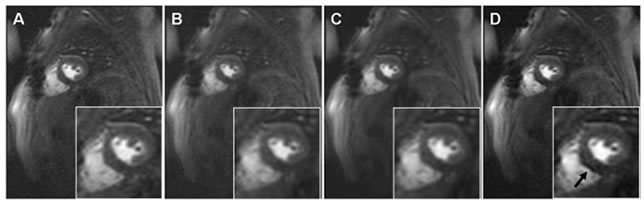
Figure 5. Examples of perfusion images at the same dynamic phase: (A) original, (B) registered, (C) registered and KLT filtered and (D) only KLT filtered image. Observe the filter induced artifact (arrow, D) that may be mistakenly classified as a perfusion defect, while the protective effect of registration against the filter induced artifact is clearly seen in C.
4. DISCUSSION
In this study, a non-rigid registration method was combined with a KLT filter method to test the improvement in SNR/CNR of the GRE-EPI first pass perfusion acquisition.
MR myocardial perfusion imaging offers a non-invasive, high resolution method to assess tissue perfusion and detect myocardial ischemia [16,17] without the use of ionizing radiation. Assessment of myocardial perfusion is generally based on visual evaluation and detection of perfusion abnormalities during the first-pass of a contrast bolus. While the introduction of parallel imaging and the EPI readout improved considerably the speed of acquisition allowing for more complete heart coverage and reduction of characteristic “dark rim artifacts”, both strategies are applied at the expense of image SNR. Our choice of GRE-EPI first pass perfusion images to test the combined effect of non-rigid registration and KLT filtering was inspired by the superior diagnostic confidence and fewer image artifacts resulting from this technique (despite its lower calculated SNR) as demonstrated in a direct side-by-side comparative study with GRE and SSFP MR first pass perfusion sequences [18].
In this study we demonstrated that the SNR and CNR of GRE-EPI first-pass perfusion images could be significantly increased through the combination of motion correction and KLT filtering. The data showed that prior non-rigid registration of the dynamic first-pass images prevented filter induced artifacts, opening the door for the use of the KLT denoising filter in myocardial perfusion imaging. Successful non-rigid registration improved the temporal correlation between images, and was shown to preserve more information in the eigenimages retained by the KLT filtering process. Based on subjective image evaluation, KLT filtering without prior motion correction caused discernible artifacts in 76% of the image series; with registration, this percentage decreased to only 1.8%. While no formal comparison between the original data sets and filtered and registered perfusion images was performed, the measurable improvements in SNR and CNR as the result of registration and filtering were visually obvious, and are expected to improve both diagnostic accuracy and confidence.
We note that both Principal Component Analysis (PCA) [19] and temporal-spatial wavelet denoising [4] have been applied to improve the SNR of first pass perfusion images with promising results in canine models of myocardial ischemia. These methods however were only shown to work well on animal data where respiratory miss-registration was not a factor [4].
The success of KLT filtering on perfusion images is based on the exploitation of the temporal correlation between dynamic images. Previous experience with this filter in dynamic cine imaging showed a large reduction of temporally random noise without degradation of image sharpness or introduction of artifacts [9]. In this scenario, no motion correction was needed and the temporal correlation was sufficient for the success of KLT filtering, probably due to the repetitive nature of the cardiac cycle and a consistent image contrast [20]. However, in firstpass perfusion MR imaging, the image contrast changes with the arrival of contrast agent, and complex motion patterns introduced by respiration and heart beat irregularities can disrupt the temporal correlation of the images and diminish the effectiveness of temporal filtering methods.
Motion correction is also a critical step in quantitative evaluation of signal enhancement and tissue kinetics. Various methods have been proposed to correct for motion of the left ventricle in first-pass imaging, starting with the manual shifting of images to a reference frame [21] and continuing with semi-automatic and automatic registration approaches [5-8,22] that either use anatomical landmarks for myocardium registration or attempt to take into consideration the changes in contrast and signal intensity of the myocardium in a perfusion MR scan. While any of these methods may work for a specific circumstance, there is currently no generally accepted approach of myocardium registration and/or image filtering in perfusion MRI.
Our study has a few limitations. First, we were unable to provide any SNR/CNR assessments between the original data sets, the KLT-image sets and KLT-motion corrected data sets. This was prevented by the different position of the myocardium between original and nonrigid registered data sets. Second, we only used retrospectively evaluated patients and from those only the slices with obvious perfusion defects for the SNR/CNR evaluation. Another limitation could be the use of handdrawn ROI rather than standard myocardial segmentation method to evaluate the quantitative SNR/CNR improvements.
Future work will focus on determining the clinical utility of this technique that needs to be evaluated in a blinded clinical assessment of different approaches used to detect perfusion defects. It would be interesting also to find out how the current results would generalize to a series that covered the entire myocardium, especially with regard to the key-frame selection and processing.
In conclusion, our approach for denoising GRE-EPI perfusion images using non-rigid registration and KLT filtering significantly increased SNR and CNR and is anticipated to further add to the superior diagnostic advantage of the GRE-EPI sequence. The improvement in image quality may favor both qualitative interpretation and quantitative evaluation of first-pass perfusion images.
5. ACKNOWLEDGEMENTS
The project described was supported by Award Number R01HL102450 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
REFERENCES
- Gebker, R., Schwitter, J., Fleck, E. and Nagel, E. (2007) How we perform myocardial perfusion with cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance, 9, 539-547. doi:10.1080/10976640600897286
- Kellman, P. and Arai, A.E. (2007) Imaging sequences for first pass perfusion: A review. Journal of Cardiovascular Magnetic Resonance, 9, 525-537. doi:10.1080/10976640601187604
- Di Bella, E.V., Wu, Y.J., Alexander, A.L., Parker, D.L., Green, D. and McGann, C.J. (2003) Comparison of temporal filtering methods for dynamic contrast MRI myocardial perfusion studies. Magnetic Resonance in Medicine, 49, 895-902. doi:10.1002/mrm.10439
- Goldstein, T.A., Zhang, H., Misselwitz, B., Gropler, R.G. and Zheng, J. (2006) Improvement of quantification of myoardial first-pass perfusion mapping: A temporal and spatial wavelet denoising method. Magnetic Resonance in Medicine, 56, 439-445. doi:10.1002/mrm.20950
- Bidaut, L.M. and Vallee, J.P. (2001) Automated registration of dynamic MR images for the quantification of myocardial perfusion. Journal of Magnetic Resonance Imaging, 13, 648-655. doi:10.1002/jmri.1092
- Dornier, C., Ivancevic, M.K., Thevenaz, P. and Vallee, J.P. (2003) Improvement in the quantification of myocardial perfusion using an automatic spline-based registration algorithm. Journal of Magnetic Resonance Imaging, 18, 160-168. doi:10.1002/jmri.10351
- Gallippi, C.M., Kramer, C.M., Hu, Y.L., Vido, D.A., Reichek, N. and Rogers, W.J. (2002) Fully automated Registration and warping of contrast-enhanced first-pass perfusion images. Journal of Cardiovascular Magnetic Resonance, 4, 459-469. doi:10.1081/JCMR-120016384
- Gupta, S.N., Solaiyappan, M., Beache, G.M., Arai, A.E. and Foo, T.K. (2003) Fast method for correcting image misregistration due to organ motion in time-series MRI data. Magnetic Resonance in Medicine, 49, 506-514. doi:10.1002/mrm.10394
- Ding, Y., Chung, Y.C., Raman, S.V. and Simonetti, O.P. (2009) Application of the Karhunen-Loeve transform temporal image filter to reduce noise in real-time cardiac cine MRI. Physics in Medicine and Biology, 54, 3909-3922. doi:10.1088/0031-9155/54/12/020
- Ding, Y., Chung, Y.-C., Raman, S. and Simonetti, O. (2008) Characteristics and performance of the KarhunenLoeve transform filter in dynamic magnetic resonance imaging. Proceedings of the 16th International Society of Magnetic Resonance in Medicine Annual Scientific Meeting & Exhibition, Toronto, 3-9 May 2008, 2858.
- Xue, H., Guehring, J., Srinivasan, L., et al. (2008) Evaluation of rigid and non-rigid motion compensation of cardiac perfusion MRI. Proceedings of the 11th International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 2008), New York, 6-10 September 2008, 35-43.
- Chefdhotel, C., Hermosillo, G. and Faugeras, O. (2002) Flows of diffeomorphisms for multimodal image registration. Proceedings of the 2002 IEEE International Symposium on Biomedical Imaging, Washington DC, 7-10 July 2002, 753-756. doi:10.1109/ISBI.2002.1029367
- Hermosillo, G., Chefdhotel, C. and Faugeras, O. (2002) Variational methods for multimodal image matching. International Journal of Computer Vision, 50, 329-343. doi:10.1023/A:1020830525823
- Golub, G. and Van Loan, C. (1996) Matrix computation. John Hopkins University Press, Baltimore, 1996.
- Jolliffe, I. (2002) Principal component analysis. Speringer, New York.
- Kim, R.J., Wu, E., Rafael, A., et al. (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. The New England Journal of Medicine, 343, 1445-1453. doi:10.1056/NEJM200011163432003
- Schwitter, J., Nanz, D., Kneifel, S., et al. (2001) Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation, 103, 2230-2235. doi:10.1161/01.CIR.103.18.2230
- Lyne, J.C., Gatehouse, P.D., Assomull, R.G., et al. (2007) Direct comparison of myocardial perfusion cardiovascular magnetic resonance sequences with parallel acquisition. Journal of Magnetic Resonance Imaging, 26, 1444-1451. doi:10.1002/jmri.21167
- Riabkov, D., Di Bella, E. and Sinusas, A. (2003) Denoising of the dymanic sequence of images for cardiac perfusion studies. International Society for Magnetic Resonance in Medicine, 11, 953.
- Ding, Y., Chung, Y.-C., Raman, S.V. and Simonetti, O.P. (2009) Application of the Karhunen-Loeve transform temporal image filter to reduce noise in real-time cardiac cine MRI. Physics in Medicine and Biology, 54, 3909-3922. doi:10.1088/0031-9155/54/12/020
- Vallee, J.P., Sostman, H.D., MacFall, J.R., et al. (1997) MRI quantitative myocardial perfusion with compartmental analysis: A rest and stress study. Magnetic Resonance in Medicine, 38, 981-989. doi:10.1002/mrm.1910380618
- Bansal, R. and Funka-Lea, G. (2002) Integrated image registration for cardiac MR perfusion data. Springer, Berlin, 659-666.

