Journal of Modern Physics
Vol. 2 No. 10 (2011) , Article ID: 8059 , 7 pages DOI:10.4236/jmp.2011.210150
Room Temperature Ammonia Gas Sensing Using MnO2-Modified ZnO Thick Film Resistors
Nanomaterials Research Lab, Department of Physics, Pratap College, Amalner, India
E-mail: *plalchand_phy_aml@yahoo.co.in
Received January 30, 2011; revised May 28, 2011; accepted June 12, 2011
Keywords: MnO2 Modified ZnO, Thick Films, Room Temperature Sensing, NH3-Gas Sensor
ABSTRACT
Pure ZnO thick film, prepared by screen-printing technique, was almost insensitive to NH3. Pure ZnO thick films were surface modified with MnO2 by dipping them into 0.01 M aqueous solution of manganese chloride (MnCl2) for different intervals of time and fired at 500˚C for 12 h. The grains of MnO2 would disperse around the grains of ZnO base material. The MnO2 modified ZnO films dipped for 30 min were observed to be sensitive and highly selective to NH3 gas at room temperature. An exceptional sensitivity was found to low concentration (50 ppm) of NH3 gas at room temperature and no cross sensitivity was observed even to high concentrations of other hazardous and polluting gases. The effects of surface microstructure and MnO2 concentrations on the sensitivity, selectivity, response and recovery of the sensor in the presence of NH3 and other gases were studied and discussed. The better performance could be attributed to an optimum number of surface misfits in terms of MnO2 on the ZnO films.
1. Introduction
Ammonia is produced and utilized extensively in many chemical industries, fertilizer factories, refrigeration systems, food processing, medical diagnosis, fire power plants etc. A leak in the system can result the health hazards. Ammonia is harmful and toxic [1-5] in nature. Therefore all industries working on and for ammonia should have an alarm system detecting and warning for dangerous ammonia concentration levels. Detection of low concentration of ammonia is not only important from the points discussed above but also, it is very important from the view of chemical pollution in the production of silicon devices in clean rooms. It is therefore necessary to monitor ammonia gas and to develop the ammonia gas sensors. Efforts are made to develop the ZnO -based gas sensors, which should detect ammonia at room temperature.
Among various materials ZnO [6-8] is the most promising semiconductor to detect toxic and hazardous gases. In fact pure ZnO was reported to have poor gas sensitivity. Various ammonia sensors reported basically work at higher temperature (>300˚C) [9-11], but it is not convenient to work at such high temperature while sensing. The room temperature sensors are therefore necessary. Few room temperature ammonia sensors are already available [12,13], but having comparatively low response. The electrolytic techniques using diaphragm electrodes are generally used for the detection of ammonia. However, this method is expensive and does not have sufficient response and selectivity for ammonia [14,15]. Another technique utilizes a Pd-metal oxide semiconductor MOS device. This device is sensitive to ammonia but it suffers from poor selectivity. A few sensor models are also available for detecting ammonia gas. They are Figaro gas sensor model TGS 824 (detection range 300 ppm) and Sierra gas monitor model CM 99-447 (electrochemical type, detection range 200 ppm).
Despite of high sensitivity, selectivity and long-term stability, the main drawback of such sensors is that they are operated at high temperature (>300˚C). Also, the noble metal additives like Pt, Pd, Au, and Ag to a base material like ZnO, for the modification, increases the cost of the sensors. Therefore the applicability of these sensors remains limited. Hence the sensors operable at room temperature with low cost metal additives must be developed for large applicability. In the present work, the efforts are made to develop a room temperature sensor with a low cost additive (MnO2) using dipping technique —a simplest method of modification.
2. Experimental Procedure
2.1. Powder and Paste Preparation
AR grade (99.9% pure) zinc oxide powder was ball milled to ensure sufficiently fine particle size. The fine powder was calcined at 1100˚C for 24 h in air and re-ground. The thixotropic paste was formulated as explained elsewhere [16,17]. The ratio of inorganic to organic part was kept as 75:25 in formulating the paste. The paste was then used to prepare thick films.
2.2. Thick Film Preparation
The thixotropic paste was screen printed on a glass substrate in desired patterns. Fluidity of the paste depends up on extent of organic part, which goes in its formulation, i.e., on solid to liquid ratio. Paste must exhibit a certain degree of yield such that after flow occurs under squeegee pressure, it should stiffen and remain in position to have sharp line definitions of patterns to be printed. In other words, it should exhibit thixotropic properties. The films prepared were fired at 500˚C for 12 h. These films were surface modified by dipping them into a 0.01 M aqueous solution of manganese chloride (MnCl2) for different intervals of time such as 5 min, 15 min, 30 min and 45 min, and were dried at 90˚C, followed by firing at 500˚C for 12 h in ambient air. The particles of manganese chloride dispersed on the films would be transformed to manganese oxide (MnO2) during firing process, and sensor elements with different mass% of MnO2 were obtained. These surface modified films are termed as MnO2 modified films. Silver contacts were made by vacuum evaporation for electrical measurements.
2.3. Characterizations
The microstructure and chemical composition of the films were analyzed using a scanning electron microscope (JOEL JED 2300) coupled with an energy dispersive spectrometer (6360 LA). The polycrystalline structures of the films were analyzed with X-ray diffractogram (RIGAKU DMAX 2500) using CuKa radiation with a wavelength 1.5418 Å. Thickness measurements were carried out using a Taylor-Hobson (Talystep, UK) system. Electrical and gas sensing characteristics were measured using a static gas sensing system.
2.4. Details of the Gas Sensing System
There were electrical feeds through the base plate. The heater was fixed on the base plate to heat the sample under test, up to required operating temperatures. The current passing through the heating element was monitored using a relay with adjustable ON and OFF time intervals. A Cr-Al thermocouple was used to sense the operating temperature of the sensor. The output of the thermocouple was connected to a digital temperature indicator. A gas inlet valve was fitted at one of the ports of the base plate. The required gas concentration inside the static system was achieved by injecting a known volume of test gas using a gas-injecting syringe. A constant voltage was applied to the sensor, and current was measured by a digital Pico-ammeter. Air was allowed to pass into the glass dome after every NH3 gas exposure cycle.
3. Materials Characterizations
3.1. Structural Properties (X-Ray Diffraction Studies)
Figure 1 depicts the XRD of MnO2-modified ZnO film. The observed peaks are matching well with ASTM reported data of ZnO. The sharp peaks of the XRD pattern correspond to ZnO and are observed to be microcrystalline in nature. The average grain size was determined using Scherrer formula and was estimated to be 190 nm. The crystals show anisotropy because different directions within the repeating pattern interact differently with incident radiations. In case of dipped films, no peaks corresponding to MnO2 were observed, which may be due to its very small mass% dispersed on the surface of ZnO thick films.
3.2. Microstructure-SEM
Figure 2 depicts the SEM images of unmodified (pure) and two MnO2-modified ZnO films (dipped for 30 min, which was most sensitive and for 45 min, the longest dipping time). The pure ZnO film (Figure 2(a)) consists of randomly distributed grains with smaller size and shape distribution. Figure 2(b) depicts the microstructure of MnO2-modified ZnO film (30 min) consisting of
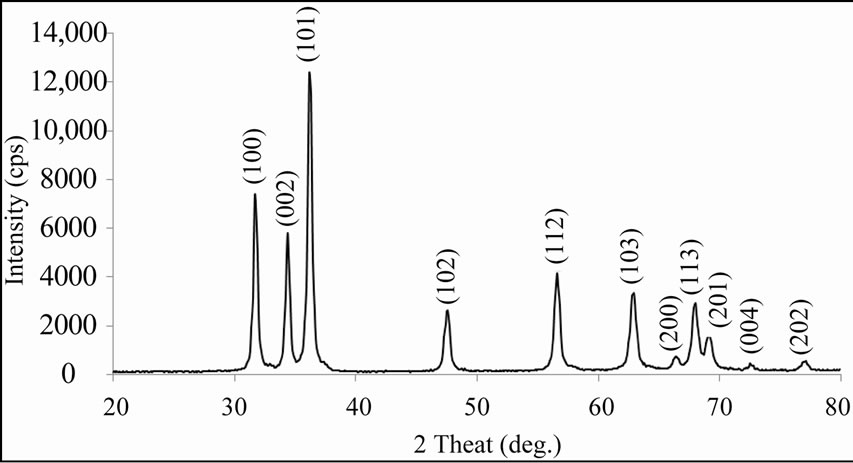
Figure 1. XRD of MnO2-modified ZnO (30 min).
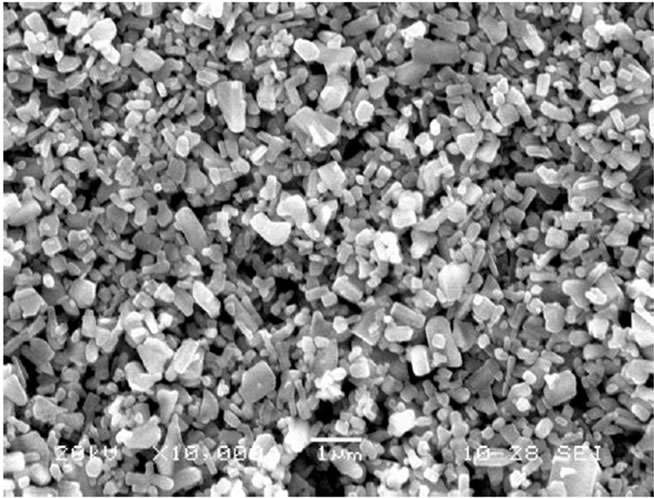 (a)
(a)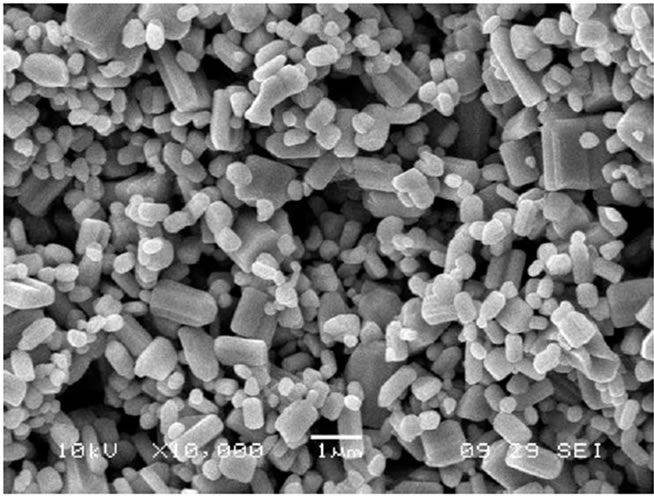 (b)
(b) (c)
(c)
Figure 2. (a) Micrographs of pure ZnO sample; (b) Micrographs of MnO2-modified (30 min) ZnO sample; (c) Micrographs of MnO2-modified (45 min) ZnO sample.
some larger particles distributed around smaller grains. Figure 2(c) depicts the microstructure of MnO2-modified ZnO film (45 min) consisting of some larger particles distributed around smaller grains, which may be more as compared with the grains in Figure 2(b).
3.3. Thickness Measurement
The thicknesses of the films were observed to be in the range from 30 to 35 mm. The reproducibility of the film thickness was achieved by maintaining the proper rheology and thixotropy of the paste.
3.4. Quantitative Elemental Analysis
The quantitative elemental compositions of MnO2- modified ZnO films were analysed using an energy dispersive spectrometer and mass% of Zn, O, MnO2 and ZnO are represented in Table 1. Pure ZnO is expected to be stoichiometric and showing insulating properties. The fine powder of pure ZnO calcined at higher temperature (1100˚C for 24 h) losses oxygen, resulting in deficiency of oxygen. Stoichiometrically expected mass% of Zn and O (in ZnO) are 80.34 and 19.66, respectively. The mass% of Zn and O in each samples were not as per the stoichiometric proportion, leading to semiconducting nature (Table 1). It is clear from Table 1 that, the mass% of MnO2 goes on increasing with the dipping time, reaches to maximum and decreases further. The film dipped for 30 min was found to be most oxygen deficient. This may be the reason of showing most sensing performance for this film.
4. Electrical Properties of the Sensor
4.1. I-V Characteristics
Figure 3 depicts the I-V characteristics of the pure and MnO2 modified ZnO films. It is clear from the symmetrical I-V characteristics that the silver contacts on the films were ohmic in nature.

Table 1. Elemental analysis of pure and MnO2 modified ZnO films.
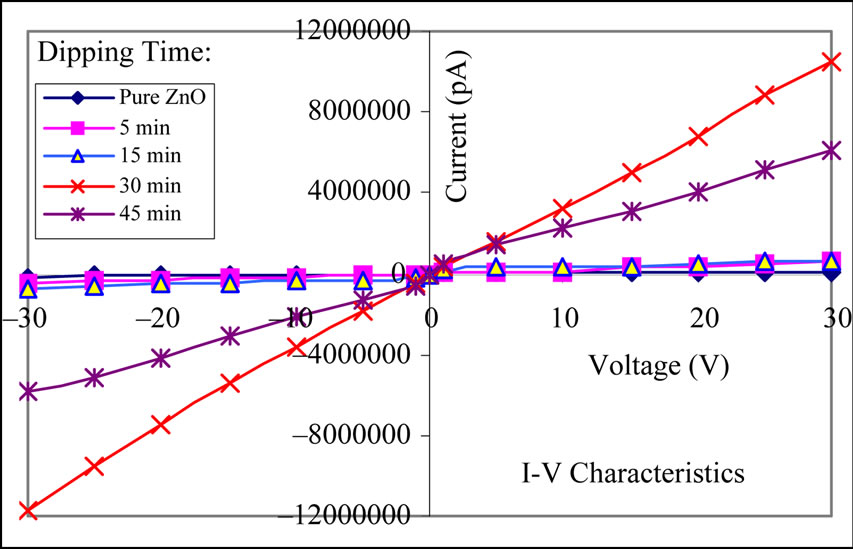
Figure 3. I-V characteristics of pure and MnO2 modified ZnO films.
4.2. Electrical Conductivity
Figure 4 shows the variation of log (conductivity) with reciprocal of temperature. The conductivity values of all samples increase with operating temperature. They are nearly linear to 1/T in the range from 100˚C to 250˚C. The increase in conductivity with increasing temperature could be attributed to the negative temperature coefficient of resistance and semiconducting nature of the MnO2 modified ZnO films. The conductivity increases during 250˚C to 350˚C and suddenly increases above 350˚C. It is observed from Figure 4 that the electrical conductivity of the pure ZnO film was larger than modified ZnO films in dry air. It may be due to the intergranular potential barrier. Pure ZnO has only one kind of grains, where in the case of activated films there would be two types of the grains MnO2 and ZnO. The modification causes the formation of heterogeneous intergrain boundaries of MnO2-ZnO. Thus increased barrier heights of the intergranular regions of modified ZnO may be responsible to decrease the conductivity.
5. Sensing Performance of the Sensor
5.1. Measurement of Gas Response, Selectivity, Response and Recovery Time
The relative response to a target gas was defined as the ratio of the change in conductance of a sample upon exposure to the gas to the original conductance in air. The gas response can be written as:
Gas response = 
where Ga = conductance in air and Gg = conductance in a target gas.
Specificity or selectivity can be defined as the ability of a sensor to respond to a certain gas in the presence of different gases. Response time (RST) was defined as the time required for a sensor to attain the 90% of the maximum increase in conductance after exposure of the sensor surface to a test gas, while recovery time (RCT) as the time taken to get back 90% of the maximum conductance [17] in air.
5.2. Sensing Performance of Pure ZnO Thick Films
5.2.1. Effect of Operating Temperature
Figure 5 shows the variation of response of pure ZnO thick film to 1000 ppm NH3 gas with operating tempera
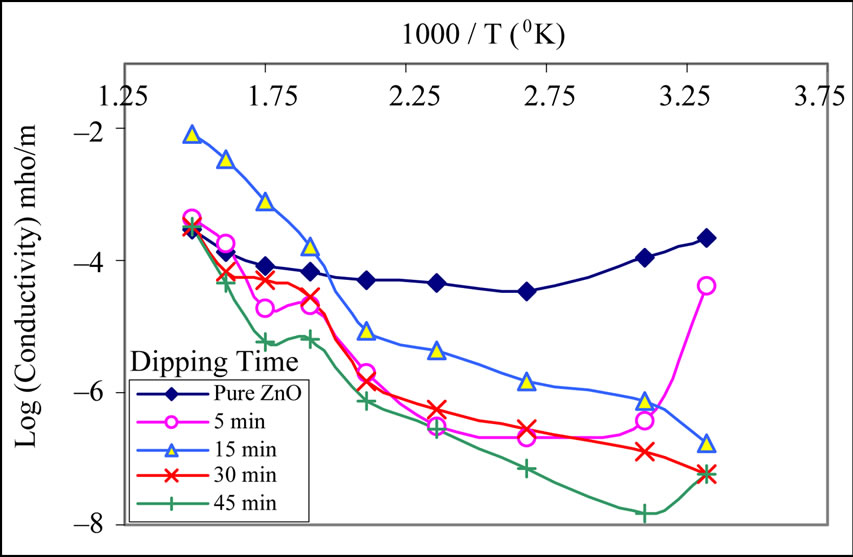
Figure 4. Conductivity-temperature profiles of MnO2 modified ZnO samples.
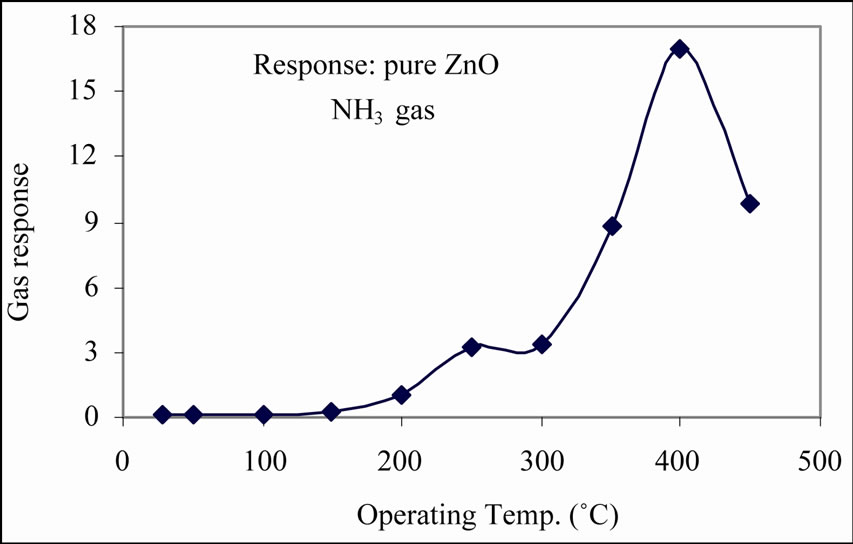
Figure 5. Variation of response of pure ZnO film with temperature.
ture. The gas response increases with operating temperature, reaches maximum at 400˚C and then decreases with a further increase in temperature. The response of pure ZnO to NH3 gas is 17 at 400˚C. The response of pure ZnO to NH3 gas is related, generally, to the oxygen adsorption on the surface of the film. If the film surface chemistry is favorable for adsorption, the response and selectivity would be enhanced. The oxygen adsorption is poor for pure ZnO and shows poor response to NH3. In addition to this, ZnO requires relatively larger operating temperature to adsorb the oxygen ions, and therefore it would have responded at higher operating temperature. To improve the sensing performance of ZnO, it is essential to modify pure ZnO.
5.2.2. Selectivity
It is observed from the Figure 6 that the pure ZnO shows maximum response to H2 at higher temperature (~400˚C). Also, it shows comparatively same response to NH3, ethanol and Cl2 gases. Thus, it is less selective to a particular gas among the different gases. This is the main drawback of pure ZnO thick film sensor.

Figure 6. Selectivity of pure ZnO thick film sensor.
5.3. Sensing Performance of MnO2 Modified ZnO Thick Films
5.3.1. Effect of Operating Temperature
Figure 7 depicts the variation of response to NH3 gas (50 ppm) with operating temperature of MnO2 modified ZnO thick films. The largest response of MnO2 modified ZnO was observed to be 100 at room temperature. The ammonia response at room temperature is expected to monitor by adsorption of moisture on the modified ZnO film. The cumulative effect would decrease the film resistance, giving a response to ammonia gas at room temperature. At room temperature, there would be no oxygen adsorption. Therefore the oxygen adsorptiondesorption mechanism is not employed to sense the NH3 gas. When raising the temperature above room temperature, the moisture from the film surface evaporates and hence the response would decrease further.
5.3.2. Active Region of the Sensor
The variation of gas response of MnO2 modified (30 min) ZnO sample with NH3 gas concentration at room tem-
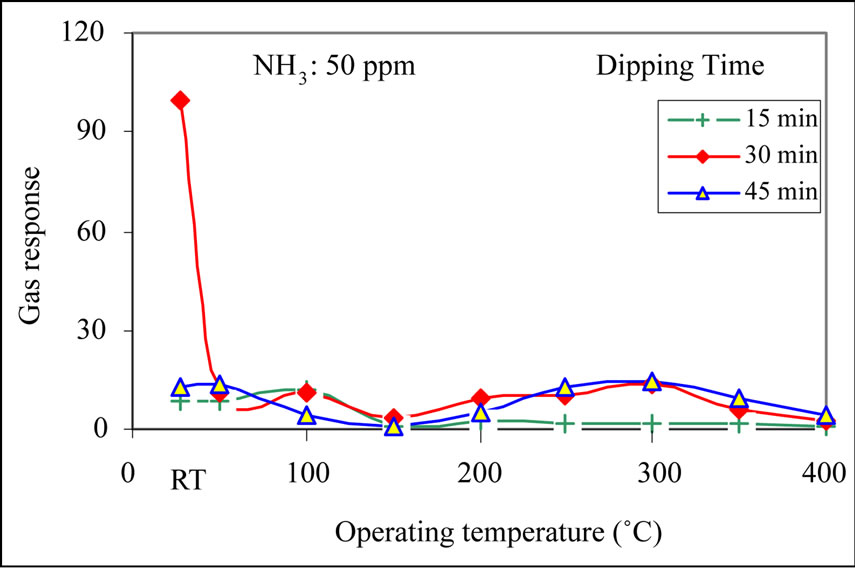
Figure 7. Variation of gas response with operating temperature.
perature is represented in Figure 8. This film was exposed to varying concentrations of NH3. For the MnO2 modified ZnO samples, the response values were observed to increase continuously with increasing the gas concentration up to 50 ppm at room temperature. The rate of increase in response was relatively larger up to 50 ppm, but smaller during 50 and 100 ppm. Thus, the active region of the sensor would be up to 50 ppm. At lower gas concentrations, the unimolecular layer of gas would be formed on the surface of the sensor, which could interact more actively giving larger response. The multilayers of gas molecules on the sensor surface, at the higher gas concentrations, would result into saturation in response beyond 50 ppm gas.
5.3.3. Effect of Dipping Time
The response of MnO2 modified ZnO films to 50 ppm NH3, as a function of the dipping time is shown in Figure 9. The sample dipped for 30 min in 0.01 M aqueous solution of manganese chloride was observed to be the most sensitive film at room temperature. It showed the response of 100 to 50 ppm NH3 gas at room temperature. Elemental analysis (Table 1) shows that the mass% of MnO2 goes on increasing with dipping time, reaches

Figure 8. Variation in response with NH3 gas concentration.
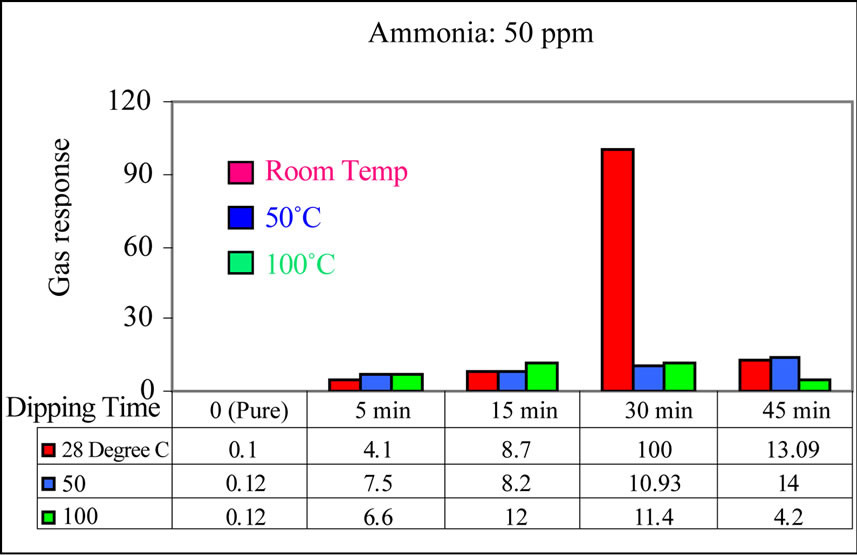
Figure 9. Variation of gas response with dipping time.
maximum (0.76) at 30 min dipping time and decreases with further increase in dipping time. The higher response of this sample may be attributed to the maximum number of MnO2 grains dispersed on the surface of thick film as islands. This would create maximum number of MnO2-ZnO heterojunctions. If the amount of MnO2 dispersed on the film surface was smaller, then the number of MnO2 islands on the surface would be less and less number of MnO2-ZnO heterojunctions would be formed. This would facilitate the weak interaction of target gas, giving smaller response. It doesn’t mean that if MnO2 covers the whole surface of ZnO thick film, it will give more response to NH3 at room temperature. If this may be the case, MnO2 grains would mask the whole surface of ZnO thick film, which resists the gas to reach to interstitial sites of thick film, giving less response.
5.3.4. Selectivity for NH3 against Various Gases
Figure 10 depicts the selectivity of the MnO2 modified ZnO sensor for NH3 (50 ppm) gas at room temperature. The sensor showed crucial selectivity to NH3 gas against LPG, CO2, C2H5OH, H2 and Cl2 gases at room temperature.
6. Response and Recovery of Sensors
Figure 11 shows dynamic response of a sample, which is highly sensitive to NH3. The sensor showed rapid response (~10 s) and fast recovery (~50 s) to NH3 gas. Fast response and speedy recovery are the important qualities of this sensor.
7. Discussion
Gas sensing mechanism is generally explained in terms of conductance change either by adsorption of atmospheric oxygen on the surface at higher temperature and / or by direct reaction of lattice oxygen or interstitial oxygen with test gases. In the former case, the atmospheric oxygen adsorbs on the surface by extracting electrons from the conduction band to form superoxides or peroxides, which are mainly responsible for the detection of the test gases.
The surface reaction processes can explain the selective ammonia response of the sensor at room temperature. It is well known that the thick film of MnO2 modified ZnO consists of an largest number of grains of MnO2 distributed on the surface. Upon exposure to ammonia, a remarkable decrease in the resistance of the sensor was observed [12], which may be due to the surface reaction of ammonia with physisorbed H2O or by proton conductivity via NH4+ cations. MnO2 may generate the solid acidity on solid bases such as ZrO2 and ZnO. The solid
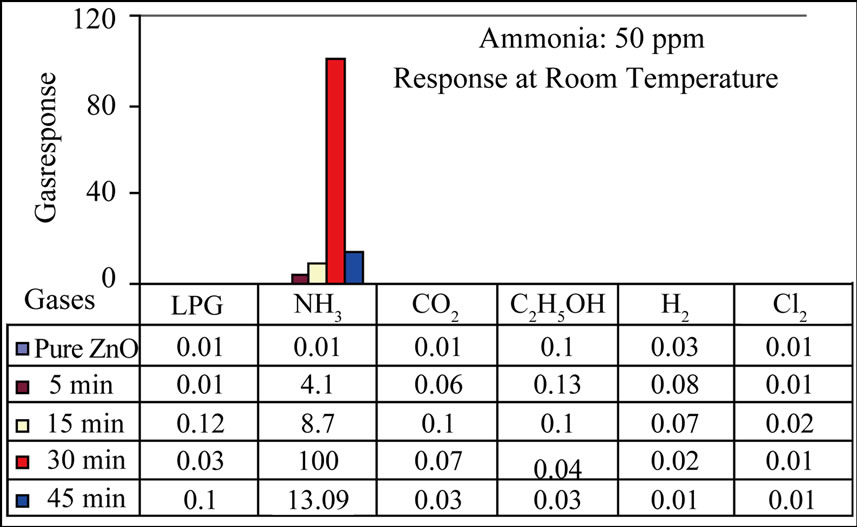
Figure 10. Selectivity to NH3 gas from the mixture of gases.

Figure 11. Response and recovery of Sensor on exposure of NH3.
acidity on the sensor surface would form NH4+ cations, which constitutes the proton conductivity leading to a crucial decrease of the resistance. This would decrease the barrier height among the MnO2-ZnO grains.
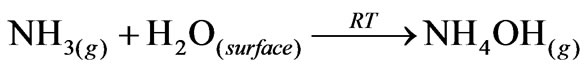
Ammonium hydroxide NH4OH produced during the surface reaction is volatile in nature. The high volatility of NH4OH explains the quick response and fast recovery of the sensor.
8. Summary
From the results obtained, the following statements can be made for the sensing performance of MnO2-activated ZnO sensors.
1) Pure ZnO thick films were observed to be less sensitive to NH3 gas even at higher temperature.
2) Surface properties of the pure ZnO thick film surfaces were conveniently customized (without affecting bulk properties) by modifying it with dipping technique.
3) MnO2 modified ZnO sensors showed crucial response to NH3 gas at room temperature.
4) The sensor was highly selective to NH3 gas (50 ppm) against other toxic gases of higher concentrations (1000 ppm).
The sensor showed very rapid response (~10 s) and recovery (~50 s) to NH3 gas.
9. Acknowledgements
Authors are grateful to the Principal, Pratap College, Amalner for providing laboratory facilities, and thankful to the Head of P. G. Department of Physics, Pratap College, Amalner for his keen interest in this research project.
REFERENCES
- L. R. Narasimhan, W. Goodman, C. Kumar and N. Patel, “Correlation of Breath Ammonia with Blood Urea Nitrogen and Creatine during Hemodialysis,” Proceedings of the National Academy of Sciences, Vol. 98, No. 8, 2001, pp. 4617-4621. doi:10.1073/pnas.071057598
- R. E. de la Hoz, D. P. Schueter and W. N. Rom, “Chronic Lung Disease Secondary to Ammonia Inhalation Injury: A Report on Three Cases,” American Journal of Industrial Medicine, Vol. 29, 1996, pp. 209-214. doi:10.1002/(SICI)1097-0274(199602)29:2<209::AID-AJIM12>3.0.CO;2-7
- C. M. Leung and C. L. Foo, “Mass Ammonia Inhalation Burns-Experience in the Management of 12 Patients,” Annals Academy of Medicine Singapore, Vol. 21, 1992, pp. 624-629. doi:10.1289/ehp.99107617
- R. A. Michaels, “Emergency Planning and Acute Toxic Potency of Inhaled Ammonia,” Environmental Health Perspectives, Vol. 107, No. 8, 1999, pp. 617-627.
- L. G. Close, F. I. Catlin and A. M. Cohn, “Acute and Chronic Effects of Ammonia Burns on the Respiratory Track,” Archives of Otolaryngology, Vol. 106, No. 3, 1980, pp. 151-158.
- P. T. Moseley, “Materials Selection for Semiconductor Gas Sensors,” Sensors and Actuators B, Vol. 6, 1992, pp. 149-156. doi:10.1016/0925-4005(92)80047-2
- T. Seiyama and F. Era, “Gas Detecting Materials,” ZairyoKagaku Japanese, Vol. 8, 1971, pp. 232-239.
- S. Pizzini, N. Butta, D. Narducci and M. Palladino, “Thick film ZnO Resistive Gas Sensors,” Journal of the Electrochemical Society, Vol. 136, 1989, pp. 1945-1948. doi:10.1149/1.2097092
- D. R. Patil and L. A. Patil, “Preparation and Study of NH3 Gas Sensing Behavior of Fe2O3 Doped ZnO Thick Film Resistors,” Sensors and Transducers, Vol. 70, No. 8, 2006, pp. 661-670.
- D. R. Patil and L. A. Patil, “Ammonia Sensing Resistors Based on Fe2O3-Modified ZnO Thick Films,” Sensors IEEE, Vol. 7, 2007, pp. 434-439. doi:10.1109/JSEN.2006.886977
- M. S. Wagh, G. H. Jain, D. R. Patil, S. A. Patil and L. A. Patil, “Modified Zinc Oxide Thick Film Resistors as NH3 Gas Sensor,” Sensors and Actuators B, Vol. 115, 2006, pp. 128-133. doi:10.1016/j.snb.2005.08.030
- D. R. Patil, L. A. Patil and P. P. Patil, “Cr2O3-Activated ZnO Thick Film Resistors for Ammonia Gas Sensing Operable at Room Temperature,” Sensors and Actuators B, Vol. 126, 2007, pp. 368-374. doi:10.1016/j.snb.2007.03.028
- G. S. Trivikrama Rao and D. Tarakarama Rao, “Gas Sensitivity of ZnO Based Thick Film Sensor to NH3 at Room Temperature,” Sensors and Actuators B, Vol. 55, 1999, pp. 166-169. doi:10.1016/S0925-4005(99)00049-0
- H. Nanto, T. Minami and S. Takata, “Zinc Oxide Thin Film Ammonia Gas Sensors with High Sensitivity and Excellent Selectivity,” Journal of Applied Physics, Vol. 60, No. 2, 1986, pp. 482-484. doi:10.1063/1.337435
- P. T. Moseley and D. E. Williams, “A Selective Ammonia Sensor,” Sensors and Actuators B, Vol. 1, No. 1-6, 1990, pp. 113-115. doi:10.1016/0925-4005(90)80183-Z
- L. A. Patil and D. R. Patil, “Heterocontact Type CuOActivated SnO2 Sensor for the Detection of a ppm Level H2S Gas at Room Temperature,” Sensors and Actuators B, Vol. 120, 2006, pp. 316-323. doi:10.1016/j.snb.2006.02.022
- D. R. Patil and L. A. Patil, “Room Temperature Chlorine Gas Sensing Using Surface Modified ZnO Thick Film Resistors,” Sensors and Actuators B, Vol. 123, 2007, pp. 546-553. doi:10.1016/j.snb.2006.09.060

