Journal of Environmental Protection
Vol. 4 No. 10 (2013) , Article ID: 38682 , 8 pages DOI:10.4236/jep.2013.410134
Coupling between the Changes in CO2 Concentration and Sediment Biogeochemistry in Mudflat of Salinas de San Pedro, California, USA
![]()
1Geosciences and Environment Department, California State University, Los Angeles, USA; 2University of California, San Diego, Scripps Institution of Oceanography, La Jolla, USA.
Email: *mrezaie@calstatela.edu
Copyright © 2013 Mohammad Hassan Rezaie-Boroon et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 21st, 2013; revised August 23rd, 2013; accepted September 26th, 2013
Keywords: Biogeochemical Factors; Elevated CO2; Degassing; Gas Discharge; Fault; Sulfate Reduction; Salinas de San Pedro; Climate Change
ABSTRACT
We investigated the effects of elevated carbon dioxide (CO2) on biogeochemistry of marsh sediment including speciation of selected heavy metals in Salinas de San Pedro mudflat in California. The Salinas de San Pedro mudflat has higher carbon (C) content than the vast majority of fully-vegetated salt marshes even with the higher tidal action in the mudflat. Sources for CO2 were identified as atmospheric CO2 as well as due to local fault degassing process. We measured carbon dioxide, methane, total organic carbon, dissolved oxygen, salinity, and heavy metal concentration in various salt marsh locations. Overall, our results showed that CO2 concentration ranging from 418.7 to 436.9 (ppm), which are slightly different in various chambers but are in good agreement with some heavy metal concentrations values in mudflat at or around the same location. The selected metal concentration values (ppm) ranging from 0.003 - 0.011 (As); 0.001 - 0.005 (Cd); 0.04 - 0.02 (Cr); 0.13 - 0.38 (Cu); 0.11 - 0.38 (Pb); 0.0009 - 0.020 (Se); and 0.188 - 0.321 (Zn). The low dissolved oxygen (ppm) in the pore water sediment indicated suboxic environment. Additionally, CO2 (ppm) and loss on ignition (LOI) (%) correlated inversely; the higher CO2 content, the lower was the LOI (%); that is to say the excess CO2 causes higher rates of decomposition and therefore it leads to lower LOI (%) on the mudflat surface. It appears that the elevated CO2 makes changes in salt marsh pore water chemistry for instance the free ionic metal (Cu2+, Pb2+, etc.) speciation is one of the most reactive form because simply assimilated by the non-decayed or alive organisms in sediment of salt marsh and/or in water. This means that CO2 not only is a sign of improvement in plant productivity, but also activates microbial decomposition through increases in dissolved organic carbon availability. CO2 also increases acidification processes such as anaerobic degradation of microorganism and oxidation of reduced components. The heavy metal concentrations in sediment samples were slightly higher in suboxic layer, yet it appears that salt marsh sediments in Salinas de San Pedro act like a sink for nutrient and carbon by maximizing carbon sequestration.
1. Introduction
Developing an effective way to sequestrate carbon dioxide (CO2) and other greenhouse gases has gained interest in the last two decades due to the worldwide concern about global climate changes. Therefore, wetland and salt marsh ecosystems have triggered interest due to the fact that they are a source for methane (CH4) and nitrous oxide (NO2) production carrying on a biologically active surface, and operating as greenhouse gas sink through photosynthetic absorbing and incorporating of CO2 and consequently storage in long-lasting biomass. The salt marsh biota trap sediment (mostly clay and silt) fine particles, organic material, and trace metals, allowing large amount of plant growth. The accumulation of soil organic carbon is primarily from plant yield and through microbial respiration [1] promoting microbiological reactions causing the elevated atmospheric concentrations of CO2. The latter can stimulate sulfate reduction [2,3] along with changes in pore water concentrations of CH4 and dissolved organic carbon in the grass dominated system.
According to Forster et al. [4], atmospheric CO2 concentration has increased worldwide over the last 2 centuries due to anthropogenic activities. Some consequences include sea level rise and some changes in carbon cycling [5]. Moreover to mitigate the latter impact, the capture and storage of CO2 in carbon pools (sequestration) is possible by the buildup of biomass and soil organic matter such as wetland and marshes [6]. In order to stay even with sea level rise [7], the salt marshes and wetlands should grow new soil and biologically active surface area; as a result the volume of soil will be increased and more organic carbon can be stored and sequestered. Carbon sequestration rate is estimated for tidal salt marsh 210 g CO2 m−2∙y−1, which this is per area volume much higher than northern peat land ecosystem [8].
There are few systematic studies on the chemistry and flux of CO2 from various sources in estuarine and coastal waters tidal salt marshes over a large salinity range. Natural CO2 fluxes through degassing process from local fault line are significant in salt marsh ecology in tectonically active area such as southern CA, yet these fluxes has not been quantified [12]. Nevertheless, we need to differentiate the linkages between the elevated CO2 on mudflat surface due to microbial processes/responses, with gas discharge from local fault line. All things considered, the Salinas de San Pedro is a seriously impacted urban marsh located in San Pedro, California near Long Beach Harbor having these both unique conditions for research. For determining the sediment condition in this urban habitat of southern CA, sediment chemistry, toxicity, and benthic macrofaunal community condition (benthos) need to be evaluated. Barnett et al. [9] report that the southern CA urban coastal habitat has the greatest severity of impacts including inner/outer Los Angeles Harbor and Long Beach Harbors. Overall, southern California is an area of bigger and denser industrial, commercial, and population concentration. Barnett et al. [9] and Schiff et al. [10] state that southern California sediment and water contaminants are more widespread and higher concentrated in compare to northern CA. Greater Los Angeles urban ecosystem provide habitat for diverse array of native and non native plants and animals. In this highly populated area the ecosystem responses to urbanization and pollution across climatic and societal gradients; yet knowledge of ecosystem responses to urbanization and of the urban socioecosystems themselves is based, at present, on individual and often peculiar case studies [11].
Thus, the Salinas tidal flat habitat provides an attracttive research project in metal and trace elements biogeochemistry in both low and high tide level conditions [12]. Here we measure CO2, CH4, LOI, DO, and heavy metal concentration in various salt marsh locations of the marsh.
Our objectives were to 1) understand the link between total CO2 volume on salt marsh surface (including CO2 emission from fault line and total organic carbon) and heavy metal concentration and speciation in salt marsh sediments and 2) analyze and interpret tidal marsh CO2 and CH4 emission in the context of carbon sequestration rate and metal speciation.
2. Materials and Methods
2.1. Site Description
The Salinas de San Pedro salt marsh is located in southern part of Long Beach Harbor, in Los Angeles County, California, USA. The salt marsh was created in 1985 to restore missing shallow-bottom fish habitat. Our study area has a kidney shape; 3.75 acre (~15,175 m2) surface area; 1 - 2 m deep; soft bottom, which is enclosed on one end and is connected to the open ocean from other side. It represents a vital habitat for many responsive groups of macro and micro-organism such as the benthic bivalves. Salinas de San Pedro is a polyhaline salt marsh (salinity > 18‰) entirely manmade, which is anthropogenically impacted by severe manufacturing activities in the local harbor during the last 30 years (Figure 1) [12].
2.2. Analysis and Sampling
To quantify soil organic carbon, 11 mud grab samples were collected from salt marsh in low tide (ebb) condition in 2009 (Figure 1). The sediment sampling procedure was conducted according to EPA sediment sampling guideline and methods. In order to reduce water and
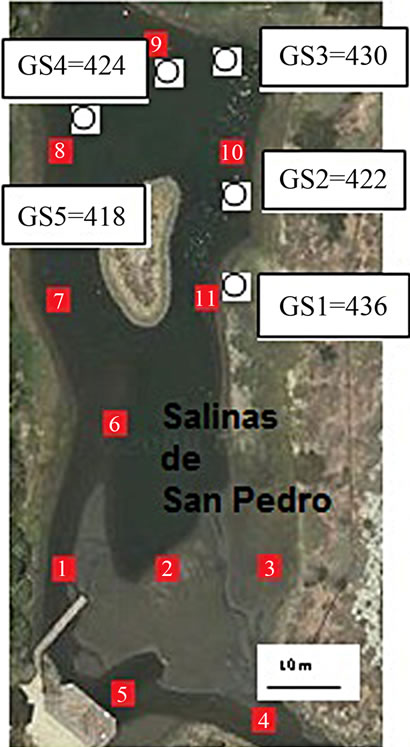
Figure 1. Bulk sediment sampling location (red box) and CO2 sampling location (white circles).
moisture loss by twisting and compaction we used scoop samples (using hand shovel cylinder) to minimize water loss during bulk sediment sampling. Upon sampling, samples were transported to the mud lab at California State University, Los Angeles for further analysis including LOI (%). We dried samples to a constant mass at 60˚C for 2 weeks and then weighed to determine bulk density (g∙dry∙mass/cm3). Subsamples dried sediments were homogenized using a silicon mortar and pestle and subsequently ground to a fine powder. The organic matter content of mud samples were determined as LOI (%) at 450˚C for 12 hours and converted to organic carbon content on a percent mass using Craft et al. method [13].
To measure the Net Ecosystem Respiration (NER) and to quantify degassed CO2 from fault line in Salinas de San Pedro salt marsh, we used EPA method 8021 [14] to measure NER as the net flux of CO2 using 10 ml disposable syringes equipped with stopcock to allow for gas sampling. Two head escape samples were collected in 15 - 30 min interval for each chamber from six locations. Samples were transported on the same day of collected gas samples to the School of Earth and Environmental Sciences Lab, Chapman University, Orange, CA for CO2 and CH4 analysis using gas chromatograph equipped with a flame ionization detector (SRI Instrument/1-mL sample loop, 100˚C column temperature/using GHG Standard = 1 ppm CH4; 351 ppm CO2). Laboratory blank was analyzed at the end of field gas sampling day along with five laboratory duplicates on June 13th, 2013. Laboratory duplicates were performed by injecting a second aliquot from a field syringe sample into the GC instrument. The CO2 concentration values (ppm) showed good agreement between replicates.
To measure the heavy metal concentration of mudflat sediments, another set of samples were taken in 2009, using a core sampling device to collect samples at depth of 0 - 10 and 10 - 20 cm. Each sample was analyzed on many parameters selectively after SW-846, 40 CFR Part 136, EPA method. The chemical analysis of metals including (As, Cd, Cr, Cu, Pb, Se, and Zn) using Inductively Coupled Plasma Optical Emission spectrometry (ICP-OES/Optima 3000 XL-Perkin Elmer) at the Scripps Institution of Oceanography, UC, San Diego. Detailed information on the distribution and concentrations of these and additional chemicals were reported in previous publication [12].
To measure physiochemical analysis including dissolved oxygen (DO), salinity we used YSI 550A device. We also measured pH, water temperature using pH-meter (Accumet-AP71) in ebb condition. All instruments were calibrated (2-point calibration) on daily basis, on site, before every run using calibration standard solution (Fisher Scientific). Quality assurance (QA) and quality control
(QC) were assessed using duplicates, method blanks and standard reference materials according to EPA standard.
3. Results
The CO2 measurement showed the CO2 concentration changes from 419 - 437 ppm with an average of 425 ppm or 0.01 mol/l (n = 10). Gas samples (GS) ranked by decreasing concentration value showed GS1 > GS4 > GS3 > GS2 > GS5. The gas sample chambers GS1 (437 ppm) and GS4 (431 ppm) showed higher concentration of CO2 than other locations. GS2, GS3 and GS5 showed lower concentration (ppm) with 421, 423, and 418 ppm respectively. The correlation of CO2 (ppm) and LOI (%) was inversely significant at the 0.01 level (2-tailed) with correlation coefficient of r2 = 0.91. Hence, the lower was the CO2 concentration the higher was recorded the LOI (%) values. The CO2 and Cr concentration were correlated significantly; the higher was the CO2 (ppm) the higher was Cr concentration (r2 = 0.92). The CO2 concentration was moderately to weakly correlated with Pb (r2 = 0.60) and Zn (r2 = 0.51) and As (r2 = 0.49) (Figure 2).
The mean percent of LOI (%) was very high at the surface in recently stored salt marsh. However, differences between sites locations varied by the sampling site and sediment grain size. In all sites, the highest LOI (%) was seen in very fine silt and mud fraction (closer to the surface; 0 - 10 cm) with 72% (SD = 4.5). Considering the entire lagoon and all sampling locations minimum and maximum values for organic content was recorded as 64% to 79% respectively. The L4 and L5 samples showed the highest value (%) (LOI = 79% and LOI = 78%) and L4 sample showed lowest value [%] percent with LOI = 75%. The sampling locations of salt marsh, where the gas samples were taken include L8, L9, L10, and L11. These sample locations ranked by decreasing percentage value L11 > L8 > L9 > L10 (Table 1).
Net CH4 flux was negligible in all locations except near fault zone (5.5 ppm), which was recorded higher than the other locations further away from approximate gas discharge locations (Table 1). In this study we have collected CH4 but have not focused on the results, thus we will need to sample CH4 using more adequate device to catch larger amount of CH4 for the future study.
As for heavy metals and metalloid concentration the results showed the highest concentration for Cu (0.325 - 0135 ppm), Cr (0.214 - 0.041 ppm), Pb (0.388 - 0.115 ppm), and Zn (0.32 - 0.18 ppm). Mean concentration rankings of sediments samples were in Cu > Zn > Pb > Cr > As > Cd > Se. We also compared the data from this study with the reference data from Carpenteria Marsh, CA, USA. Our study results showed higher concentration (ppm) for Cd, Cu, Cr, and Pb (Table 1). The results of Carpinteria marsh showed much higher concentration of
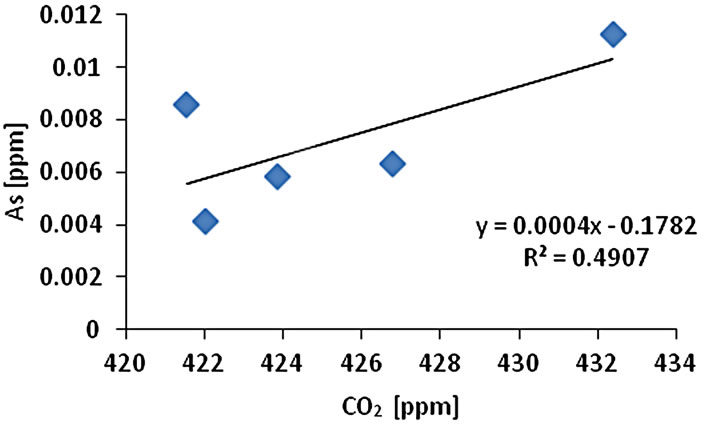
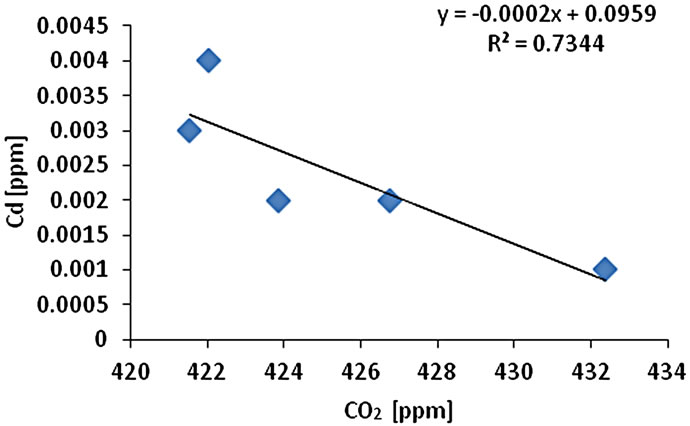
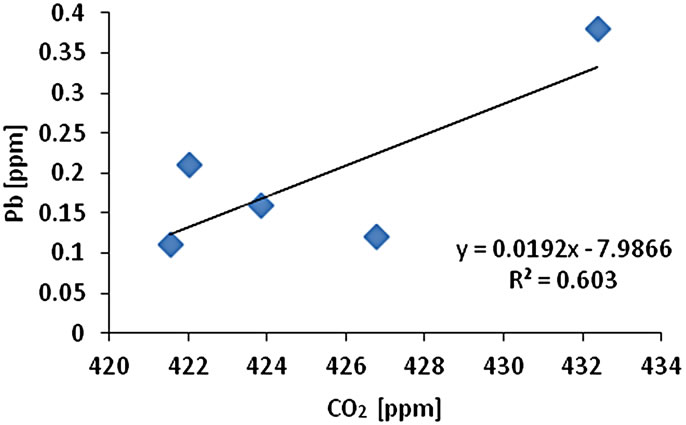


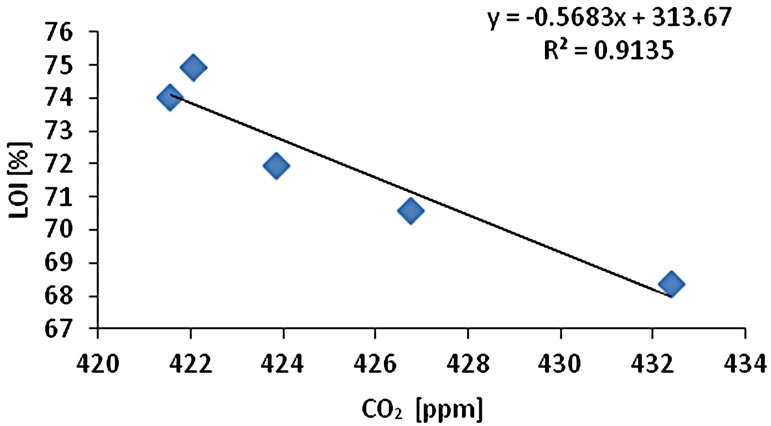
Figure 2. Correlation of various metals and LOI with CO2.
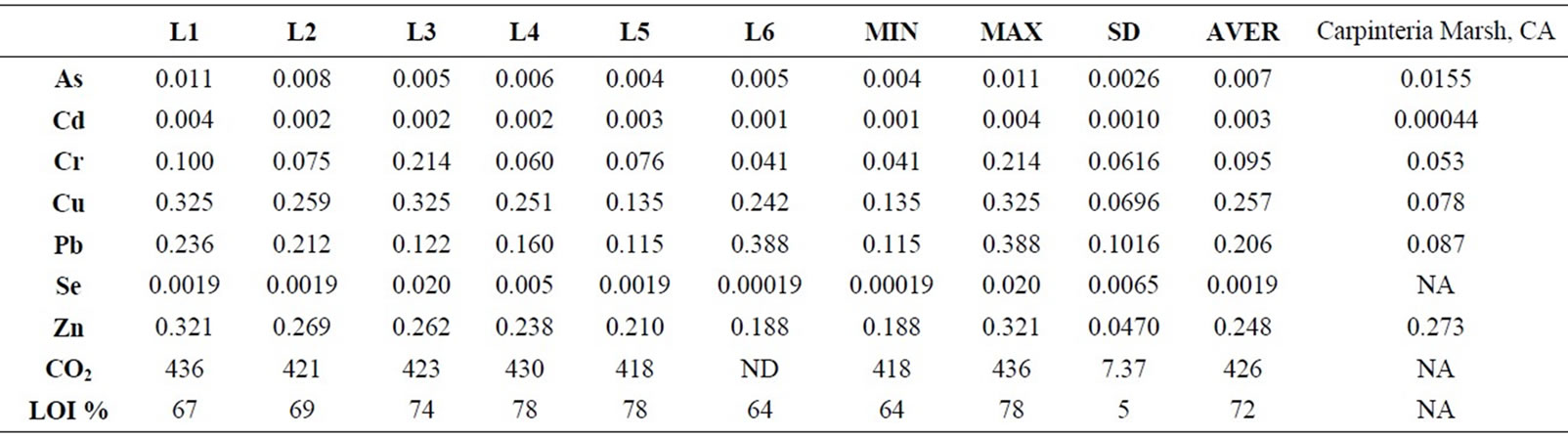
Table 1. Metal concentrations [ppm] of highly urbanized Carpinteria marsh in CA after [22] is compared with this data from Salinas de San Pedro study.
Zn in compare to Salinas de San Pedro. Table 1 also shows that sample L1 exhibits much higher Cu and Zn in compare to other locations (Cu > Zn > Pb > Cr > As > Cd > Se). Table 2 shows the correlation between different metals. Arsenic (As) showed a significant correlation at 0.01 level (2-tailed) with Cd (r2 = 0.99), Cr (r2 = 0.88), Cu (r2 = 0.99), and Pb (r2 = 0.95). Cd showed also significant correlation at 0.01 (2-tailed) with Cu (r2 = 0.99),
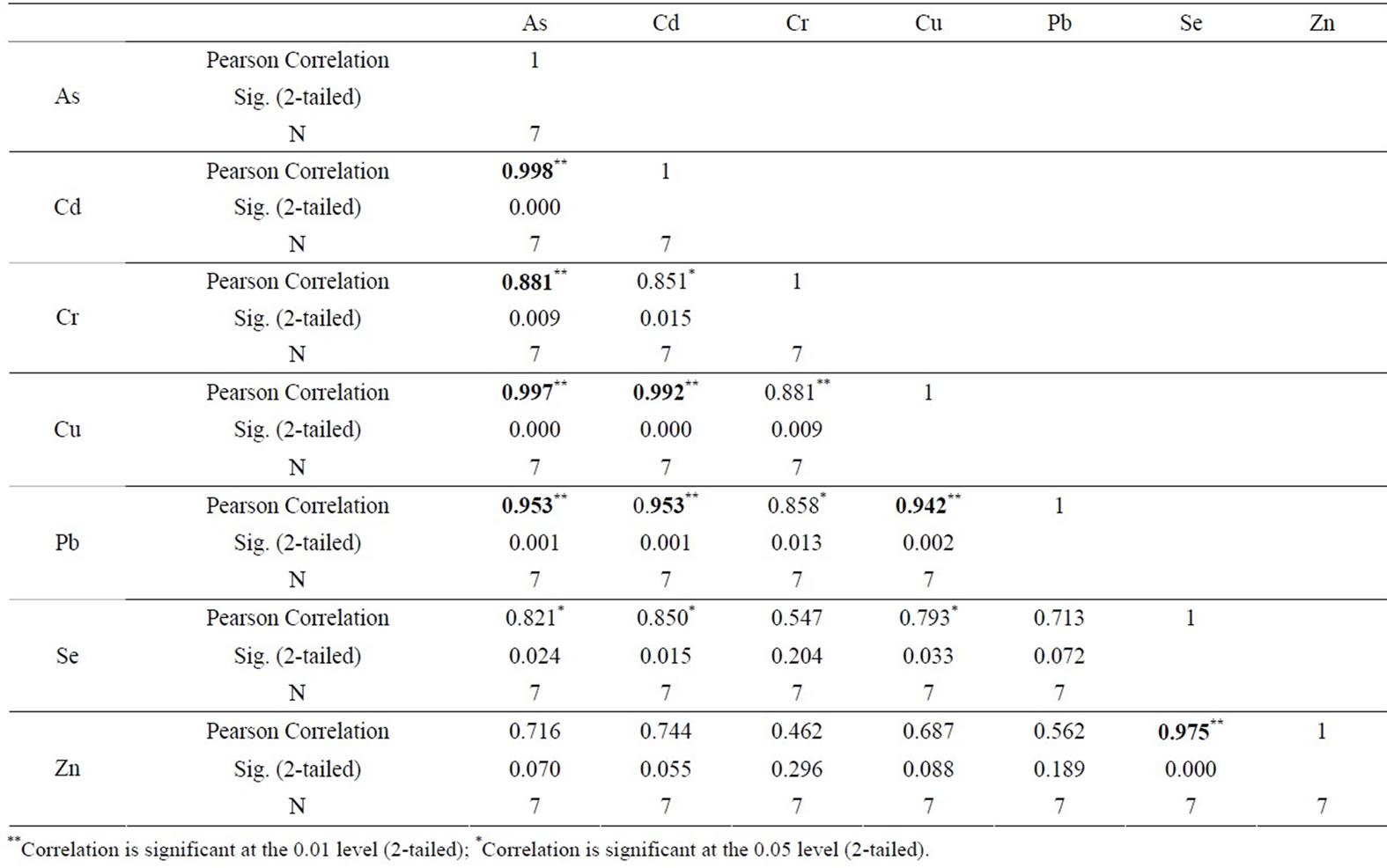
Table 2. Correlation of various metals with each other. Correlation is significant at the 0.01 level (2-tailed)** and 0.05 level (2-tailed)*.
and Pb (r2 = 0.95). Cr-Cu (r2 = 0.88) also showed significant correlation. Cr-Pb and Se-Zn showed a significant correlation at the 0.01 level (2 tailed) with r2 = 0.94 and r2 = 0.97 respectively. There was no clear association among As, Pb, and Zn concentrations with CO2 in the Salinas de San Pedro mudflat (Figure 2) (Table 2).
Temperature, pH, dissolved oxygen (DO), salinity monitored during the sampling events displayed only small differences between different locations, which were statistically significant. Daily change of DO can be explained by photosynthesis and respiration. The pore water DO was recorded from range of 3 - 7 mg/l for all locations (n = 6), which was slightly higher in some location closer to ocean water entrance in the lagoon. The pore water temperature was measured with a digital thermometer (18.5˚C - 19.25˚C). Air temperature also was recorded with a range of 19˚C - 22˚C on sampling days and locations. Temperature reflects length and strength of exposing to the sunlight at daily time-scale. Temperature values were generally comparable at locations and time; some shown detectable thermal gradients because of the daily patterns with lower temperature early morning and higher temperatures during noon and after noon hours. The pH was very consistent among various locations with values generally (average) between 7.21 and 7.51 throughout salt marsh. The pH remained around 7, suggesting the presence of bicarbonates [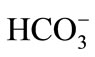 ] as a pH-buffer.
] as a pH-buffer.
Average salinity values were recorded in pore water 33.7‰ recorded during the lowest low tide level or ebb condition. In this condition the evaporation rate was slightly higher where sediments were exposed to the intense sunlight especially in summer. The results showed that there was a negative correlation between the pore water salinity and methane flux. Considering Salinas de San Pedro a polyhaline salt marsh (salinity > 18‰) the methane values were significantly lower and were consistent with prior works.
4. Discussion
Microbial Activity and Carbon Dynamics
The field data and results suggest that the excess CO2 in Salinas de San Pedro may a) due to the fault degassing process [12] and b) due to anaerobic respiration by bacteria in which organic matter oxidize to form CO2 attributing to anaerobic degradation of organic matter by microorganisms. In order to understand their relationship we need first to know what the significant sources of CO2 input to a typical intertidal marsh are. A few possible sources include pelagic respiration, benthic respiretion, and import of CO2 from the marshes, photodegradation, and simple mixing of seawater with polluted local runoff (slightly acidic water) (Figure 3).
As for the import of CO2 from the marshes, by examining overall gas exchanges and evaluating them in the context of a mass-balance model, Cai et al. [15] provided a valuable comparison of neritic and pelagic O2 and CO2 fluxes. Results indicate that respiratory activity in the marsh sediments and overlying water (at mud-water interference) during flood condition leaves a signal that is funneled back to the estuary during ebb tide. This can account for the estuarine gas concentrations and fluxes. In the other words, large CO2 emissions from salt-marsh sediments reported in low tide condition. In addition bottom resuspension may enhance the respiration rate as well [16] (Figure 3).
High evaporation rate, water temperature, and intense biomass productivity, degradation, and the breakdown of organic matter caused O2 deficiency in pore waterlogged sediment and cause dinitrification converting nitrate to nitrogen gas. Suboxic environment indicates elevated CO2 due to the low dissolved O2 in the pore water sediment. This will make changes in salt marsh water chemistry. That is to say, there is an enhancement in plankton yield, which more likely activates microbial decomposition by increasing dissolved organic carbon availability. Intense acidification processes including degradation of organic matter in an anaerobic condition and reduced components oxidation may be factors affecting the CO2 flux [17]. The acidification of sediments in mudflat may be caused by the oxidation of reduced sulfur compounds present in mudflat sediment [12]. This suggests an important change at the mud-water interface in supply and partitioning of metals, i.e. discharge of them into the dissolved phase as a result of biogeochemical processes (Figure 3). CO2 saturation near surface sediment in mudflat was recorded closer to the mouth of salt marsh (where the seawater enters and exits the marsh). Thus, we suggest that the fine grained mud is sufficiently rich in decaying organic matter and microbial decomposition are responsible for entrapping or retaining the metal by chemical complexes formation. In other words, organic debris, known to concentrate metals, may be the source for these metals. As it was stated before, CO2 (ppm) and LOI (%) correlate inversely (Figure 2). In brief, the higher is CO2 content, the lower is the LOI (%); in other words, the excess CO2 may causes higher rates of decomposition and therefore it leads to lower soil organic matter on the mudflat surface. However, we would suggest there is anaerobic degradation of organic matter in subsurface that make the retention of heavy metals (such as Cu, Pb, and Zn) strongly; particularly, when the fraction of decaying organic matter underneath is high. The major part of Cu, Pb, and Zn seems bond to the decayed organic matter. In the same way may complexation by organic matter would direct to a stable fixation state, leading to low mobility of metals. When plants are present, the metal bioavailability can change [18]. Overall, finer particles show evidence of greater binding site density, and thus lower bioavailability [19], which may retain Cu, Pb, and Zn not only by adsorbing on decayed organic matter but also on metallic hydroxides, either by cation exchange [20] or by fixation on carbonates. In natural ecosystems, mainly in soil, chemical speciation of metals is depending on numerous physico-chemical and/or biological parameters, which can be subjected to wide variations. The chemical speciation of some elements or metals is defined as all the chemical forms/sorts of these elements or metals in a natural environment. Some ligands (inorganic or organic) are able to condition the speciation of these metals by the formation of more or less stable complexes. The toxicity of element is dependent on its speciation [21]. Equally important, the free ionic metal (Cu2+, Pb2+, etc.) speciation is one of the most reactive forms with the neutral species, because more easily assimilated by the non-decayed or alive organisms. The metal (Cu, Cd, Pb, Zn,) speciation is also dependent on a large number of physical and chemical parameters such as temperature, pressure, pH, ionic strength, concentrations in major elements, complexing ligands.
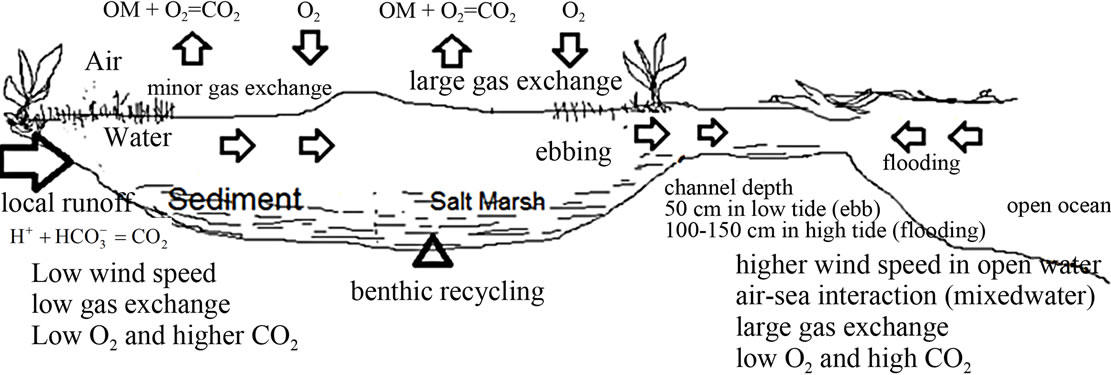
Figure 3. This figure shows a conceptual model of gas exchange in the salt marsh. This vertical profile shows the change of respiration rate from a large area of intertidal marsh (near open ocean), where environmental conditions support higher gas exchange rates into a much smaller surface area of the salt marsh where conditions support low rate of gas exchange rates.
To conclude, Salinas de San Pedro marsh plays a dual role in local marine environment since 1) it acts as a biological pump for the ambient CO2, part of which may have been discharged from adjacent fault zone beneath the salt marsh and 2) it is a natural bed for microbial retention, uptake and fixation, and import of nitrogen-rich material in the salt marsh environment. Both could lead to speciation of metals and trace elements in salt marsh environment by biogeochemical processes. To understand and clarify these processes we recommend extending these preliminary results by analyzing C/N ratio in salt marsh sediment.
5. Acknowledgements
The authors thank Dr. Jason Keller at School of Earth and Environmental Sciences at Chapman University, Orange, CA for measuring CO2 and CH4 in his environmental lab. We thank the Drs Latz-Deheyn Laboratory at Scripps Institution of Oceanography, UC San Diego for ICP analysis. We would like to thank Dr. Ramirez at CSU, Los Angeles for using mud lab for grain size analysis. We thank also Cabrillo Marine Aquarium administration and staff for allowing us to sample the Salinas de San Pedro in the last four years.
REFERENCES
- J. K. Keller, A. A. Wolf, P. B. Weisenhorn, B. G. Drake and J. P. Megonigal, “Elevated CO2 Affects Porewater Chemistry in a Brackish Marsh,” Biogeochemistry, Vol. 96, No. 1-3, 2009, pp. 101-117.
- J. K. Keller, K. K. Takagi, M. E. Brown, Kellie N. Stump, C. G. Takahashi, W. Joo, K. L. Au, C. C. Calhoun, R. K. Chundu, K. M. Hokutan, M. Jessica and K. Roy, “Soil Organic Carbon Storage in Restored Salt Marshes in Huntington Beach, California,” Bulletin of Southern California Academy of Sciences, Vol. 111, No. 2, 2012, pp. 153-161.
- J. K. Keller, A. E. Sutton-Grier, A, L. Bullock and J. P. Megonigal, “Anaerobic Metabolism in Tidal Freshwater Wetlands: I. Plant Removal Effects on Iron Reduction and Methanogenesis,” Estuaries and Coasts, Vol. 36, No. 3, 2013, pp. 457-470.
- P. V. Forster, P. Ramaswamy, T. Artaxo, B. R. Betts, D. W. Fahey, J. Haywood, J. Lean, D. C. Lowe, G. Myhre, J. Nganga, R. Prinn, G. Raga, M. Schulz and R. Van Dorland, “Changes in Atmospheric Constituents and in Radiative Forcing,” In: S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor and H. L. Miller, Eds., Climate Change: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge and New York, 2007, pp. 131-234.
- K. L. Denman, G. Brasseur, A. Chidthaisong, P. Ciais, P. M. Cox, R. E. Dickinson, D. Hauglustaine, C. Heinze, E. Holland, D. Jacob, U. Lohmann, S. Ramachandran, P. L. da Silva Dias, S. C. Wofsy and X. Zhang, “Couplings between Changes in the Climate System and Biogeochemistry,” Climate Change 2007: The Physical Science Basis, Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate, Cambridge University Press, Cambridge and New York, 2007, pp. 499-587.
- J. P. Megonigal, M. E. Hines and P. T. Visscher, “An aerobic Metabolism: Linkages to Trace Gases and Aerobic Processes,” Treatise on Geochemistry, Vol. 8, 2003, pp. 317-424.
- S. M. Mudd, S. M. Howell and J. T. Morris, “Impact of Dynamic Feedbacks between Sedimentation, Sea Level Rise, and Biomass Production on Near Surface Marsh Stratigraphy and Carbon Accumulation,” Estuarine, Coastal and Shelf Science, Vol. 82, No. 3, 2009, pp. 377-389.
- G. L. Chmura, C. A. Shimon, D. R. Cahoon and J. C. Lynch, “Global Carbon Sequestration in Tidal Saline Wetland Soil,” Global Biogeochemical Cycles, Vol. 17, No. 4, 2003, pp. 1111-1120.
- A. M. Barnett, S. M. Bay, K. J. Ritter, S. L. Moore and S. B. Weisberg, “Sediment Quality in California Bays and Estuaries, Technical Report 522,” Southern California Coastal Water Research Project, Costa Mesa, 2008.
- K. Schiff, B. Luka, D. Gregorio and S. Gruber, “Assessing Water Quality in Marine Protected Areas from Southern California, USA,” Marine Pollution Bulletin, Vol. 62, No. 12, 2011, pp. 2780-2786. http://dx.doi.org/10.1016/j.marpolbul.2011.09.009
- N. B. Grimm, D. Foster, P. Groffman, J. M. Grove, C. S. Hopkinson, K. J. Nadelhoffer, D. E. Pataki and P. C. Peters, “The Changing Landscape: Ecosystem Responses to Urbanization and Pollution Across Climatic and Societal Gradients,” Frontiers in Ecology and the Environment, Vol. 6, No. 5, 2008, pp. 264-272.
- M. H. Rezaie-Boroon, V. Toress, S. Diaz, T. Lazzaretto, M. Tsang and D. D. Deheyn, “The Geochemistry of Heavy Metals in the Mudflat of Salinas de San Pedro Lagoon, California, USA,” Journal of Environmental Protection, Vol. 4, No. 1, 2013, pp. 12-25.
- C. B. Craft, E. D. Seneca and S. W. Broome, “Loss of Ignition and Kjedahl Digestion for Estimating Organic Carbon and Total Nitrogen in Estuarine Marsh Soils: Calibration with Dry Combustion,” Estuaries, Vol. 14, No. 2, 1991, pp. 175-179.
- EPA, “Final Project Report for the Development of an Active Soil Gas Sampling Method,” 2007. http://www.epa.gov/esd/cmb/pdf/203cmb07-Schmacher.pdf
- W.-J. Cai, L. R. Pomeroy, M. A. Moran and Y. Wang, “Oxygen and Carbon Dioxide Mass Balance for the Estuarine-Intertidal Marsh Complex of Five Rivers in the Southeastern US,” Limnology and Oceanography, Vol. 44, No. 3, 1999, pp. 639-649.
- S. C. Wainright, “Stimulation of Heterotrophic Microplankton Production by Resuspened Marine Sediments,” Science, Vol. 238, No. 4834, 1987, pp. 1710-1712.
- J. J. Middelburg, J. Nieuwenhuize, F. J. Slim and B. Ohowa, “Sediment Biogeochemistry in an East African Mangrove Forest (Gazi Bay, Kenya),” Biogeochemistry, Vol. 34, No. 3, 1996, pp. 133-155.
- C. M. R. Almeida, A. P. Mucha and M. T. S. D. Vasconcelos, “The Role of a Salt Marsh Plant on Trace Metal Bioavailability in Sediments Estimation by Different Chemical Approaches,” Environmental Science and Pollution Research, Vol. 12, No. 5, 2005, pp. 271-277.
- D. D. Deheyn and M. I. Latz, “Bioavailability of Metals along a Contamination Gradient in San Diego Bay (California, USA),” Chemosphere, Vol. 63, No. 5, 2006, pp. 818-834. http://dx.doi.org/10.1016/j.chemosphere.2005.07.066
- Y. J. M. Kone, G. Abril, B. Delille and A. V. Borges, “Seasonal Variability of Methane in the Rivers and Lagoons of Ivory Coast (West Africa),” Biogeochemistry, Vol. 100, No. 1, 2010, pp. 21-37.
- Y. L. Lai, M. Thirumavalavan and J. F. Lee, “Effective Adsorption of Heavy Metal Ions (Cu2+, Pb2+, Zn2+) from Aqueous Solution by Immobilization of Adsorbents on Ca-Alginate Beads,” Toxicological & Environmental Chemistry, Vol. 92, No. 4, 2010, pp. 697-705.
- H.-M. Hwang, P. G. Green, R. M. Higashi and T. M. Young, “Tidal Salt Marsh Sediment in California, USA. Part 2: Occurrence and Anthropogenic Input of Trace Metals,” Chemosphere, Vol. 64, No. 11, 2006, pp. 1899- 1909. http://dx.doi.org/10.1016/j.chemosphere.2006.01.053
NOTES
*Corresponding author.

