Food and Nutrition Sciences
Vol. 3 No. 10 (2012) , Article ID: 23398 , 5 pages DOI:10.4236/fns.2012.310191
IR Spectral Analysis of Diterpene Glycosides Isolated from Stevia rebaudiana
![]()
1Organic Chemistry Department, The Coca-Cola Company, Global Research and Development, One Coca-Cola Plaza, Atlanta, USA; 2Analytical Chemistry Department, The Coca-Cola Company, Global Research and Development, One Coca-Cola Plaza, Atlanta, USA.
Email: *vchaturvedula@coca-cola.com
Received September 6th, 2012; revised October 6th, 2012; accepted October 13th, 2012
Keywords: Stevia rebaudiana; Diterpene Glycosides; IR Spectral Data
ABSTRACT
The objective is to identify the Infra-Red (IR) spectral analysis of the diterpene glycosides present in the commercial extracts of Stevia rebaudiana was achieved by PerkinElmer Spectrum 400 Fourier Transform (FT) spectrometer employing a PerkinElmer Universal Attenuated Total Reflection (ATR) accessory. Using this technique the IR spectral pattern of 15 steviol glycosides which belongs to three different classes of ent-kaurane diterpene glycosides namely ent-13-hydroxykaur-16-en-19-oic acid, ent-13-hydroxykaur-15-en-19-oic acid, and 13-methyl-16-oxo-17-nor-ent-kauran-19-oic acid were identified. From the wave numbers found for all 15 steviol glycosides, it was observed that that though there are differences in the number of sugar units, nature of sugar units, and their attachments; there are not any notable differences in the IR values.
1. Introduction
Stevia rebaudiana (Bertoni) is a perennial shrub belonging to the family of Asteraceae (Compositae) native to Brazil and Paraguay, but now grown commercially in a number of countries, particularly in Japan, Taiwan, Korea, Thailand and Indonesia [1,2]. Extracts of the leaves of S. rebaudiana have been used for decades to sweeten food and beverages in Japan, South America and China. The major constituents in the leaves of S. rebaudiana are the potently sweet diterpenoid glycosides namely stevioside, and rebaudioside A; which are glycosides of the diterpene steviol, ent-13-hydroxykaur-16-en-19-oic acid [3]. These compounds are also known as Stevia sweeteners; rebaudioside F is about 200 times sweeter compared to sucrose and is non-caloric.
In our continuing research to discover natural sweeteners, we have collected commercial extracts of S. rebaudiana from various suppliers all over the World and isolated several novel diterpene glycosides [4-10]. Apart from isolating novel compounds from S. rebaudiana and utilizing them as possible natural sweeteners or sweetness enhancers, we are also engaged in understanding the stability of the steviol glycosides in various systems of interest and identification of degradation products using various spectroscopic techniques [11-13] as well as synthesis using naturally occurring starting materials [14]. In this article we are providing the IR spectral analysis achieved by PerkinElmer Spectrum 400 Fourier Transform (FT) spectrometer employing a PerkinElmer Universal Attenuated Total Reflection (ATR) accessory of 15 naturally occurring steviol glycosides identified from S. rebaudiana namely rebaudioside A (1), stevioside (2), dulcoside A (3), steviolbioside (4), rebaudioside F (5), rebaudioside D (6), 13-[(2-O-(3-O-α-D-glucopyranosyl)- β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy] ent-kauran-19-oic acid β-D-glucopyranosyl ester (7), 13-[(2-O-β-D-glucopyranosyl-3-O- (4-O-α-D-glucopyranosyl)-β-D-glucopyranosyl-β-D-gluco-pyranosyl)oxy] ent-kauran-19-oic acid β-D-glucopyranosyl ester (8), 13-[(3-O-β-D-glucopyranosyl-β- D-glucopyranosyl)oxy] ent-kauran-19-oic acid β-Dglucopyranosyl ester (9), 13-hydroxy-ent-kauran-19-oic acid β-D-glucopyranosyl ester (10), rubusoside (11), 13-[(2-O-β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]-15-ene-ent-kauran-19-oic acid β-D-glucopyra-nosyl ester (12), 13-[(2-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy] ent-kaur-15-en-19-oic acid β-D-glucopyranosyl ester (13), 13-[(2-O-β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]-17-hydroxy-ent-kaur-15-en-19-oic acid β-D-glucopyranosyl ester (14), and 13-methyl-16-oxo- 17-nor-ent-kauran-19-oic acid-β-D-glucopyranosyl ester (15). Further, the steviol glycosides studied belongs to three different classes of ent-kaurane diterpene glycosides: ent-13-hydroxykaur-16-en-19-oic acid (1 - 11, Figure 1), ent-13-hydroxykaur-15-en-19-oic acid (12 - 14, Figure 2), and 13-methyl-16-oxo-17-nor-ent-kauran19-oic acid (15, Figure 3).
2. Materials and Methods
2.1. IR Spectral Analysis
Infrared measurements were performed on a PerkinElmer
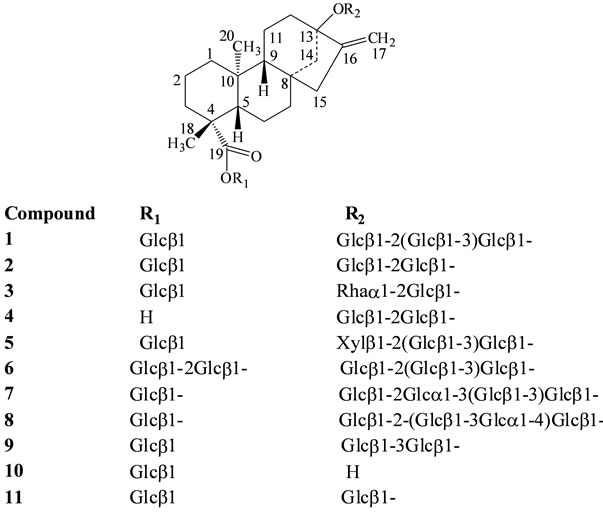
Figure 1. Structures of ent-13-hydroxykaur-16-en-19-oic acid glyco-sides.

Figure 2. Structures of ent-13-hydroxykaur-15-en-19-oic acid glyco-sides.
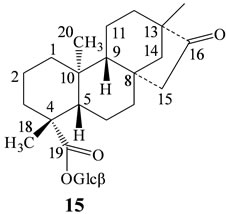
Figure 3: Structure of 13-methyl-16-oxo-17-nor-ent-kauran- 19-oic acid glycoside. Glcβ: β-D-glucopyranosyl; Glca: α-Dglucopyranosyl; Rhaa: α-L-rhamnopyranosyl; Xylβ: β-DXylopyranosyl; 6-Deoxyglcβ: 6-Deoxy-β-D-gluco-Pyranosyl.
Spectrum 400 FT-IR spectrometer employing a PerkinElmer Universal Attenuated Total Reflection (ATR) accessory. The spectrometer is fitted with potassium bromide (KBr) optics and a medium band MCT detector. The ATR accessory contains a single reflection Diamond/ZnSe crystal top-plate. All spectra were acquired over a scan range of 4000 - 650 cm−1 at a resolution of 4 cm−1 and a scan speed of 1 cm/s. Before acquisition, the sample was placed on the ATR diamond top-plate and using the interactive pressure control, approximately 150 N of force was applied to ensure optimum contact is made between the sample and the diamond. Atmospheric vapour compensation function was enabled in the PerkinElmer Spectrum software to reduce the effects of unwanted atmospheric absorptions in sample spectra. Thirtytwo scans were collected and averaged in each acquisition.
2.2. Steviol Glycosides 1-15
The steviol glycosides, rebaudioside A (1), stevioside (2), dulcoside A (3), steviolbioside (4), rebaudioside F (5), rebaudioside D (6), and rubusoside (11), were isolated by AMRI (Bothell, WA) or obtained from Chromadex (Irvine, CA). The other steviol glycosides 7 - 10, and 12 - 15 were isolated by The Coca-Cola Company as reported in the literature [4-14].
3. Results and Discussion
The primary objective of this study is to utilize Infrared (IR) spectroscopy to find variations in wavenumbers of the steviol glycosides 1-15 of S. rebaudiana.
The IR spectral data for all the 15 steviol glycosides which belongs to ent-kaurane diterpene glycosides: ent- 13-hydroxykaur-16-en-19-oic acid (1-11), ent-13-hydroxykaur-15-en-19-oic acid (12-14), and 13-methyl- 16-oxo-17-nor-ent-kauran-19-oic acid (15) was performed under the same condition and the wavenumbers for the stretching of hydroxyl (-OH), alkane (sp3-CH), carbonyl (-C=O), alkene (-C=C-), ether (-C-O-C-) groups; and alkene (-C=C-) bending vibrations were identified by employing the method described above which were given in Table 1.
From the wave numbers observed in Table 1, it was evident that though there are differences in the number of sugar units, nature of sugar units, and their attachments; there are not any notable differences in the IR values of 1-11, and 12-14. The hydroxyl stretching for compounds 1-11, and 12-14 was observed between the wavenumbers 3319 - 3379 cm−1; alkane stretching was observed between 2918 - 2947 cm−1; carbonyl groups between 1719 - 1728 cm−1; alkene stretching between 1641 - 1662 cm−1; ether groups between 1054 - 1070 cm−1; and alkene bending between 870 - 895 cm−1. Compound 15 did not

Table 1. IR spectral data for the steviol glycsodies 1-15.
 cm−1
cm−1
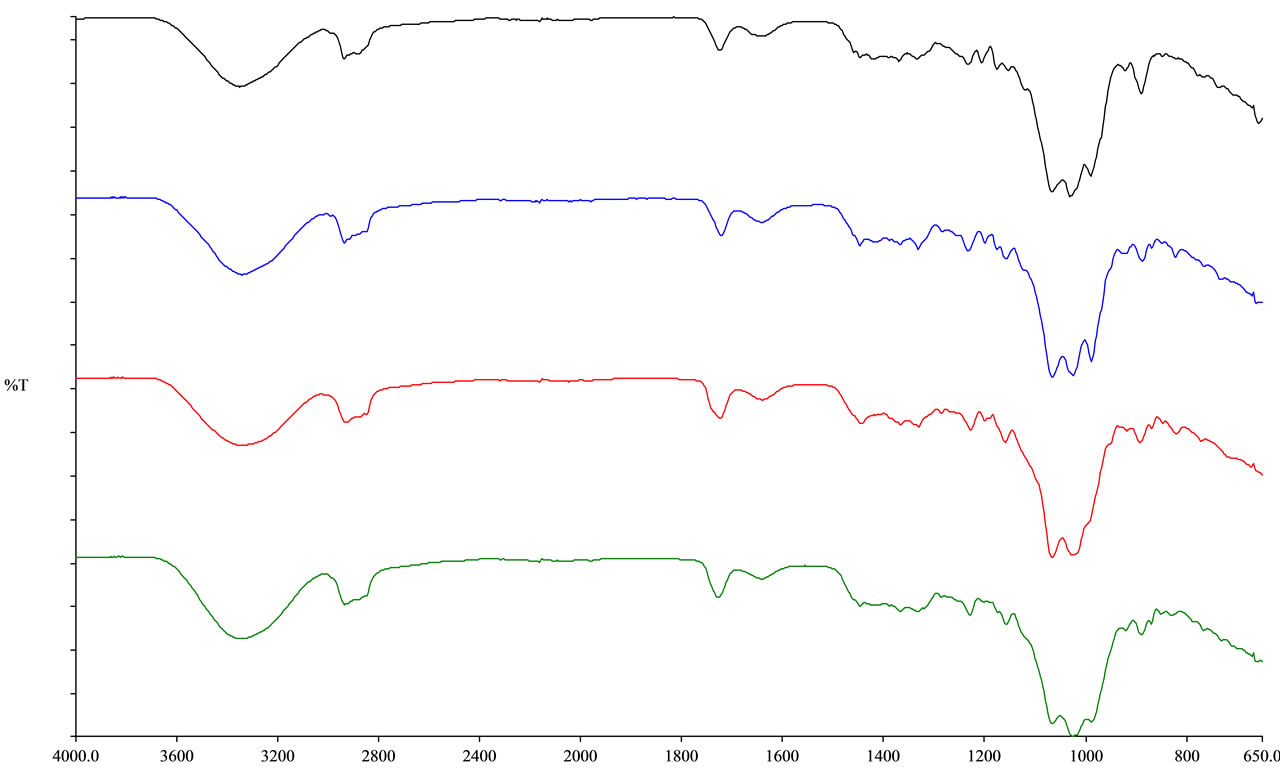
Figure 4. IR spectral data for compounds 1 (black), 4 (blue), 10 (red), and 11 (green).
 cm−1
cm−1
Figure 5. IR spectral data for compounds 1 (black), 12 (blue), 13 (red), and 14 (green).
 cm−1
cm−1
Figure 6. IR spectral data for compounds 1 (black), and 15 (blue).
show any vibrations corresponding to alkene stretching and bending, supported the IR pattern of steviol glycosides without having any alkene functionality. Also, it was observed that compound 15 showed an additional carbonyl group at 1741 cm−1 corresponding to the saturated carbonyl group at C-16 position of steviol glycoside. The IR spectral data for the steviol glycosides of the four classes of ent-kaurane diterpene glycosides: ent-13- hydroxykaur-16-en-19-oic acid (4, 10-11), ent-13-hydroxykaur-15-en-19-oic acid (12-14), and 13-methyl- 16-oxo-17-nor-ent-kauran-19-oic acid (15) overlapped with rebaudioside A (1) were given in Figures 4-6.
4. Conclusion
Based on the IR analysis experiments utilized in this study, the spectral pattern of aglycone moieties from steviol glycosides were identified which belongs to three different classes of ent-kaurane diterpene glycosides namely ent-13-hydroxykaur-16-en-19-oic acid, ent-13-hydroxykaur-15-en-19-oic acid, and 13-methyl-16-oxo-17- nor-ent-kauran-19-oic acid.
REFERENCES
- E. Mosettig and W. R. Nes, “Stevioside. II. The Structure of the Aglucon,” Journal of Organic Chemistry, Vol. 20, No. 7, 1955, pp. 884-899. doi:10.1021/jo01125a013
- E. Mosettig, U. Beglinger, F. Dolder, H. Lichiti, P. Quitt and J. A. Waters, “The Absolute Configuration of Steviol and Isosteviol,” Journal of American Chemical Society, Vol. 85, No. 11, 1963, p. 2305. doi:10.1021/ja00898a025
- J. E. Brandle, A. N. Starrratt and M. Gijen, “Stevia rebaudiana: Its Agricultural, Biological and Chemical Properties,” Canadian Journal of Plant Sciences, Vol. 78, No. 4, 1998, pp. 527-536. doi:10.4141/P97-114
- V. S. P. Chaturvedula, J. Rhea, D. Milanowski, U. Mocek and I. Prakash, “Two Minor Diterpene Glycosides from the Leaves of Stevia rebaudiana,” Natural Product Communications, Vol. 6, No. 2, 2011, pp. 175-178.
- V. S. P. Chaturvedula, U. Mani and I. Prakash, “Diterpene Glycosides from Stevia rebaudiana,” Molecules, Vol. 16, No. 5, 2011, pp. 3552-3562. doi:10.3390/molecules16053552
- V. S. P. Chaturvedula and I. Prakash, “A New Diterpenoid Glycoside from Stevia rebaudiana,” Molecules, Vol. 16, No. 4, 2011, pp. 2937-2943. doi:10.3390/molecules16042937
- V. S. P. Chaturvedula and I. Prakash, “Structures of the Novel Diterpene Glycosides from Stevia rebaudiana,” Carbohydrate Research, Vol. 346, No. 8, 2011, pp. 1057- 1060. doi:10.1016/j.carres.2011.03.025
- V. S. P. Chaturvedula and I. Prakash, “Additional Minor Diterpene Glycosides from Stevia rebaudiana,” Natural Product Communications, Vol. 6, No. 6, 2011, pp. 1059- 1062.
- V. S. P. Chaturvedula, J. F. Clos, J. Rhea, D. Milanowski, U. Mocek, G. E. DuBois and I. Prakash, “Minor Diterpenoid Glycosides from the Leaves of Stevia rebaudiana,” Phytochemistry Letters, Vol. 4, No. 3, 2011, pp. 209-212. doi:10.1016/j.phytol.2011.01.002
- V. S. P. Chaturvedula, U. Mani and I. Prakash, “Structures of the Novel α-Glucosyl Linked Diterpene Glycosides from Stevia rebaudiana,” Carbohydrate Research, Vol. 346, No. 13, 2011, pp. 2034-2038. doi:10.1016/j.carres.2011.06.023
- V. S. P. Chaturvedula, J. F. Clos and I. Prakash, “Stability Study of Steviol Glycosides in Mock Beverages Using Fluorescent Light Exposure under ICH Guidelines,” International Journal of Pharmacy and Pharmaceutical Sciences, Vol. 3, No. 3, 2011, pp. 316-323.
- V. S. P. Chaturvedula, J. F. Clos and I. Prakash, “Stability of Steviol Glycosides in Mock Beverages under Acidic Conditions,” International Journal of Pharmacy and Pharmaceutical Sciences, Vol. 3, No. 5, 2011, pp. 421-425.
- V. S. P. Chaturvedula, J. F. Clos and I. Prakash, “Stability of Rebaudioside: A under Acidic Conditions and Its Degradation Products,” Food Research International, Vol. 48, No. 1, 2012, pp. 65-75. doi:10.1016/j.foodres.2012.02.015
- V. S. P. Chaturvedula, J. Klucik, U. Mani and I. Prakash, “Synthesis of ent-Kaurane Diterpene Glycosides,” Molecules, Vol. 16, No. 10, 2011, pp. 8402-8409. doi:10.1016/j.foodres.2012.02.015
NOTES
*Corresponding author.

