Open Journal of Anesthesiology
Vol.3 No.3(2013), Article ID:31724,4 pages DOI:10.4236/ojanes.2013.33044
Early and Late Postoperative Pain and Side Effects after Mastectomy: A Comparison of Ketamine and Thiamylal Administered for Anesthetic Induction
![]()
1Department of Anesthesiology, Sasebo Kyosai Hospital, Nagasaki, Japan; 2Department of Anesthesiology, Nagasaki University School of Medicine, Nagasaki, Japan.
Email: tscat@fb3.so-net.ne.jp
Copyright © 2013 Tadasuke Use et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 11th, 2013; revised April 19th, 2013; accepted May 6th, 2013
Keywords: Preventive Analgesia; Ketamine; Mastectomy; Postoperative Pain; Anesthetic Induction
ABSTRACT
Objective: To compare acute and long-term postoperative pain and side effects in patients undergoing mastectomy for breast cancer under general anesthesia induced with ketamine or thiamylal. Methods: Twenty four ASA physical status I-III patients undergoing mastectomy were randomly assigned to one of two groups. Ketamine group received intravenous ketamine, 1 mg/kg, and thiamylal group received intravenous thiamylal, 4 mg/kg, at the induction of general anesthesia. Anesthesia was maintained with sevoflurane, N2O and fentanyl. The intensity of pain was assessed by using visual analog scale (VAS) 3 and 16 hr and 2, 3 and 4 weeks after surgery. Postoperative side effects, including nausea, vomiting and hallucination were also recorded. Results: At 16 hr after surgery, VAS in ketamine group was significantly lower than that in thiamylal group. However, there were no statistically significant differences between the two groups in the VAS at 3 hr and 2, 3 and 4 weeks after surgery. There were no differences in the incidence of side effects such as nausea, vomiting and hallucination between the two groups. Conclusion: Intravenous ketamine at the induction of anesthesia could reduce acute postoperative pain but not long-term pain after mastectomy.
1. Introduction
The recent studies suggested that the incidence of chronic pain following breast cancer surgery was close to 50%, and that the intensity of acute postoperative pain was predictive factor of the chronic pain after mastectomy [1].
Perioperative noxious input causes central sensitization, which is brought about mainly by activation of Nmethyl-D-aspartate (NMDA) receptors in dorsal horn of spinal cord [2]. NMDA antagonists have been shown to prevent the induction of central sensitization, and abolish the hypersensitivity once it is established [3]. Ketamine, a non-competitive NMDA antagonist, has analgesic properties at subanesthetic doses [4].
In the recent systematic review, McCartney et al. [5] demonstrated the strongest evidence that the perioperative administration of ketamine reduces the early postoperative pain beyond its pharmacological duration of action, indicating preventive analgesia. However, there are few studies that evaluated the effect of perioperative administration of ketamine on the late postoperative pain.
Ketamine is not only analgesic drug but also anesthetic agent by itself and the intravenous administration of 0.5 - 1.5 mg/kg is recommended for induction of general anesthesia [6]. Ngan Kee et al. [7] demonstrated that patients who received ketamine, 1 mg/kg, for anesthetic induction required less postoperative morphine than patients who received thiopental in cesarean section without increasing the psychotomimetic side effects.
The present study was carried out to compare the early and late postoperative pain and side effects in patients who underwent mastectomy for breast cancer under general anesthesia induced with ketamine or thiamylal.
2. Materials and Methods
After institutional approval of Sasebo Kyosai Hospital was obtained along with written informed consent, 24 ASA physical status I-III women undergoing elective mastectomy with axillar lymph node resection (Auchin closs method) for breast cancer were recruited into this prospective, randomized and controlled study. Patients were excluded if they had allergic history of study drugs or used analgesic. They were randomly allocated to one of two groups, i.e. groups K and T according to a computer-generated table of random numbers. Before anesthetic induction, an anesthesiologist not involved in the evaluation of the patients’ symptom prepared the syringes sealed with opaque adhesive tapes of either ketamine, 1 mg/kg, or thiamylal, 4 mg/kg, diluted to 20 ml with saline. General anesthesia was induced with ketamine, 1 mg/kg, (group K) or thiamylal, 4 mg/kg, (group T) combined with fentanyl, 2 μg/kg. Vecuronium was used to facilitate tracheal intubation and maintain muscle relaxation. Anesthesia was maintained with sevoflurane, 1% - 2.5% end-tidal, 60% N2O in oxygen and a fixed dose of fentanyl, 4 μg/kg. At skin closure, patients received rectal dicrofenac sodium, 1 mg/kg.
The intensity of postoperative pain at rest was assessed using visual analog scale (VAS) at 3 and 16 hrs and 2, 3 and 4 weeks after surgery. The VAS was graded on a scale from 0 to 100, in which 0 = no pain, 100 = worse pain imaginable. Only when patients request, pain and adverse effects were treated. Pain and nausea at operative and first postoperative day were treated with pentazocine, 15 mg i.m. and metoclopramide, 10 mg i.v., respectively. After the second postoperative day, oral loxoprofen sodium (180 mg/day) was administered. We recorded the time from the end of skin closure to extubation. Postoperative side effects, including nausea, vomiting and hallucination were also recorded. These assessments and treatments were carried out by an anesthesiologist and nurses who were not aware of the assignment of patients to the treatment groups.
Data were analyzed using Mann-Whitney U-test and Fisher’s exact test as applicable. Data were presented as mean ± SD. P < 0.05 was considered statistically significant.
3. Results
All patients completed the study. Duration of surgery was longer in group K than in group T, but all of demographic and postoperative data did not differ significantly between two groups (Table 1).
VAS at 16 hr after surgery was significantly less in group K (5.5 ± 6.6) than in group T (17.7 ± 16.0). In contrast, VAS at 3 hr and 2, 3 and 4 weeks after surgery were not significantly different between two groups (group T: 22.7 ± 19.5, 12.0 ± 12.7, 12.6 ± 14.1and 9.8 ± 10.5; group K: 17.0 ± 16.2, 10.1 ± 13.7, 5.6 ± 9.3 and 5.4 ± 8.0, respectively) (Figure 1).
There were no significant differences in the number of patients who had requested the postoperative analgesic or antiemetic between two groups. There were no significant differences in the incidence of nausea, vomiting and hallucination between the groups (Table 2).
4. Discussion
The present study investigated the postoperative pain after mastectomy, and the results show that the patients who received ketamine for anesthetic induction had lower
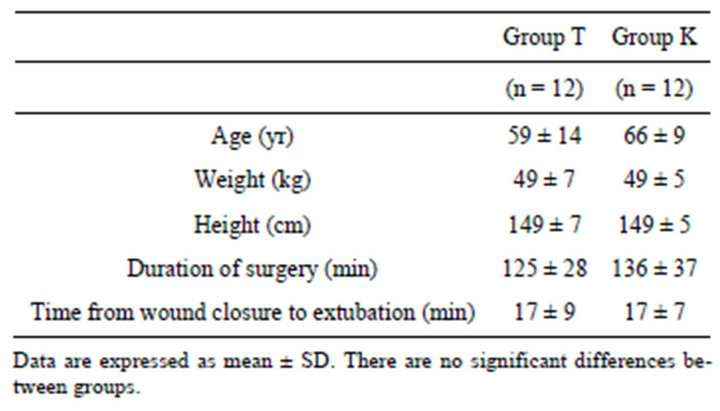
Table 1. Demographic and perioperative data.

Figure 1. Visual analog scale (VAS) pain scores (0 - 100 mm) in the two groups 3, 16 hrs and 2, 3 weeks after surgery. VAS at 16 hr after surgery was significantly less in group K than in group T. In contrast, VAS at 3 hr and 2, 3 and 4 weeks after surgery were not significant difference. *P < 0.05 versus group T.
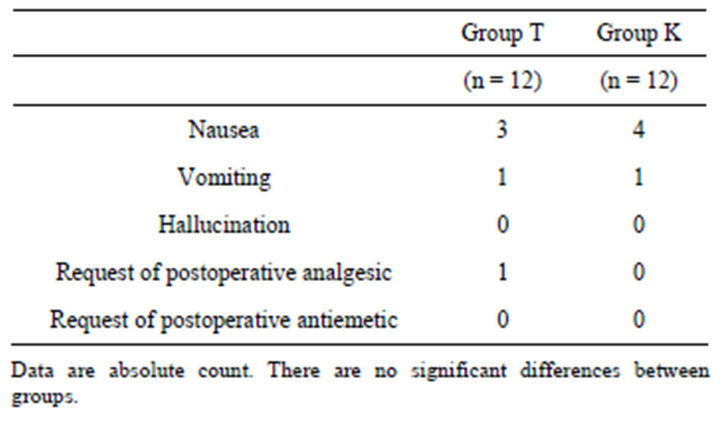
Table 2. Postoperative side effects and request of analgesics.
pain score 16 hr after surgery than the patients who received thiamylal. However, there were no significant differences in the pain score at 3 hr or 2, 3 or 4 weeks after surgery. These findings suggest that preoperative administration of ketamine can improve partially early postoperative pain, but not late postoperative pain after mastectomy.
Many studies demonstrated that intravenous subanesthetic ketamine, when added as adjunct to general anesthesia, reduced early postoperative pain and/or opioids requirement in a variety of surgery, such as cesarean section [7], laparoscopic gynecological surgery [8], life kidney donation [9], radical prostatectomy [10], knee arthroscopy [11], gastrectomy [12], tonsillectomy [13], rectal surgery [14] and thoracotomy [15]. In our study, patients who received ketamine, 1 mg/kg, at induction of general anesthesia had lower pain score 16 hr after surgery compared with patients who received thiamylal, 4 mg/kg. Our result is consistent with these studies demonstrating that perioperative administration of ketamine reduced early postoperative pain. In contrast, there was no significant difference in pain score 3 hr after surgery between two groups in our study. It might be related to the diclofenac sodium administered at the end of the surgery in all of our patients.
“Preventive analgesia” is confirmed if postoperative pain or analgesic consumption were significantly reduced five half-lives beyond the pharmacological duration of action of the target preventive drug compared with an untreated or placebo drug [5]. In our study, pain score 16 hr after surgery was significantly lower in patients receiving ketamine than those in patients receiving thiamylal. The half-life of ketamine is 3 hr [16]. Therefore, we assumed that preventive analgesia would be involved as a mechanism of the effect of ketamine.
Adam et al. [17] compared postoperative analgesic effect of preoperative administration of ketamine, 0.15 mg/ kg i.v., with postoperative administration of ketamine in patients undergoing mastectomy. They concluded that there was no preemptive analgesic action of ketamine. However, their study did not have a control group that did not receive ketamine preoperatively. The present study used thiamylal as a control group to evaluate the effect of preventive analgesia by ketamine on the postoperative pain. Kwok et al. [8] compared the postoperative analgesic effect of preoperative administration of ketamine, 0.15 mg/kg i.v., with placebo in the patients undergoing gynecologic laparoscopic surgery. They demonstrated that the patients receiving preoperative ketamine had lower postoperative pain scores compared with the patients receiving placebo.
Although administration of opioids typically would usually result in analgesia, a recent review suggests that opioids may cause hyperalgesia rather than analgesia (opioid-induced hyperalgesia) under a certain condition [18]. Chia et al. [19] reported that perioperative intravenous administration of fentanyl resulted in increased postoperative pain. In experimental study in rat model, single pretreatment of ketamine before fentanyl prevented fentanyl-induced hyperalgesia [20]. We used a fixed dose of fentanyl, 6 μg/kg, during anesthesia in the present study. Thus, the preoperative administration of ketamine might have prevented fentanyl-induced hyperalgesia in the present study.
Some clinical studies reported a beneficial effect of perioperative administration of ketamine on late postoperative pain. De Kock et al. [14] demonstrated that significantly less patients who received intravenous ketamine (bolus dose of 0.5 mg/kg followed by 0.25 mg/ kg/hr in intraoperative period) suffered from residual pain until 6 month after rectal surgery compared with the patients who received no ketamine. Suzuki et al. [15] demonstrated that continuous intravenous administration of ketamine at a dose of 0.05 mg/kg/hr for 3 days reduced postoperative pain 3 month after thoracotomy. In contrast, our study showed that ketamine could not reduce late postoperative pain. This difference may be explained by the following reason. We administered ketamine only at the induction of general anesthesia, and ketamine, 1 mg/kg, at induction of general anesthesia may be insufficient to reduce late postoperative pain.
The high dose (>2 mg/kg i.v.) of ketamine sometimes causes psychomimetic symptoms such as hallucination, which limits the clinical usefulness of ketamine [21]. In contrast, a small dose of ketamine was reported to be not associated with psychomimetic side effects [8,9,11]. A recent review demonstrated that the risk of ketamineinduced hallucination was minimal in anesthetized patients [22]. In the present study, we used ketamine, 1 mg/kg, at induction of general anesthesia, and no patient reported the hallucination after surgery.
As the limitation in the present study, the sample population was very small. Small samples sometimes affect the statistical accuracy. If the sample had been larger, the differences between the two groups in the VAS at late postoperative time points might have become statistically significant. Therefore, future studies are needed to address this limitation.
In conclusion, intravenous administration of ketamine, 1 mg/kg, at induction of general anesthesia reduced early postoperative pain compared with thiamylal, 4 mg/kg, in the patient undergoing mastectomy. However, late postoperative pain did not differ between these two groups. Induction with ketamine, 1 mg/kg, did not cause the side effects such as hallucination, and did not delay the emergence from anesthesia.
REFERENCES
- F. M. Perkins and H. Kehlet, “Chronic Pain as an Outcome of Surgery. A Review of Predictive Factors,” Anesthesiology, Vol. 93, No. 4, 2000, pp. 1123-1133. doi:10.1097/00000542-200010000-00038
- A. B. Petrenko, T. Yamakura, H. Baba and K. Shimoji, “The Role of N-Methl-D Aspartate (NMDA) Receptors in Pain: A Review,” Anesthesia & Analgesia, Vol. 97, No. 4, 2003, pp. 1108-1116. doi:10.1213/01.ANE.0000081061.12235.55
- C. J. Woolf and S. W. Thompson, “The Induction and Maintenance of Central Sensitization Is Dependent on N-Methyl-D-Aspartic Receptor Activation; Implications for the Treatment of Post-Injury Pain Hypersensitivity States,” Pain, Vol. 44, No. 3, 1991, pp. 293-299. doi:10.1016/0304-3959(91)90100-C
- E. Kochs, E. Scharein, O. Mollenberg, B. Bromm and J. Schulte am Esch, “Analgesic Efficacy of Low-Dose Ketamine. Somatosensory-Evoked Responses in Relation to Subjective Pain Ratings,” Anesthesiology, Vol. 85, No. 2, 1996, pp. 304-314. doi:10.1097/00000542-199608000-00012
- C. J. McCartney, A. Sinha and J. Katz, “A Qualitative Systematic Review of the Role of N-Methyl-D-Aspartate Receptor Antagonists in Preventive Analgesia,” Anesthesia & Analgesia, Vol. 98, No. 5, 2004, pp. 1385-1400. doi:10.1213/01.ANE.0000108501.57073.38
- P. F. White, W. L. Way and A. J. Trevor, “Ketamine—Its Pharmacology and Therapeutic Uses,” Anesthesiology, Vol. 56, No. 2, 1982, pp. 119-136. doi:10.1097/00000542-198202000-00007
- W. D. Ngan Kee, K. S. Khaw, M. L. Ma, P. A. Mainland and T. Gin, “Postoperative Analgesic Requirement after Cesarean Section: A Comparison of Anesthetic Induction with Ketamine or Thiopental,” Anesthesia & Analgesia, Vol. 85, No. 6, 1997, pp. 1294-1298.
- R. F. Kwok, J. Lim, M. T. Chan, T. Gin and W. K. Chiu, “Preopetative Ketamine Improves Postoperative Analgesia after Gynecologic Laparoscopic Surgery,” Anesthesia & Analgesia, Vol. 98, No. 4, 2004, pp. 1044-1049. doi:10.1213/01.ANE.0000105911.66089.59
- A. Stubhaug, H. Breivik, P. K. Eide, M. Kreunen and A. Foss, “Mapping of Punctuate Hyperalgesia around a Surgical Incision Demonstrates That Ketamine Is a Powerful Suppressor of Central Sensitization to Pain Following Surgery,” Acta Anaesthesiologica Scandinavica, Vol. 41, No. 9, 1997, pp. 1124-1132. doi:10.1111/j.1399-6576.1997.tb04854.x
- D. G. Snijdelaar, H. B. Cornelisse, R. L. Schmid and J. Katz, “A Randomized Controlled Study of Peri-Operative Low Dose s(+)-Ketamine in Combination with Postoperative Patient-Controlled s(+)-Ketamine and Morphine after Radical Prostatectomy,” Anaesthesia, Vol. 59, No. 3, 2004, pp. 222-228. doi:10.1111/j.1365-2044.2003.03620.x
- C. Menigaux, B. Guignard, D. Fletcher, D. I. Sessler, X. Dupont and M. Chauvin, “Intraoperative Small-Dose Ketamine Enhances Analgesia after Outpatient Knee Arthroscopy,” Anesthesia & Analgesia, Vol. 93, No. 3, 2001, pp. 606-612. doi:10.1097/00000539-200109000-00016
- S. Aida, H. Baba, T. Yamakura, K. Taga, S. Fukuda and K. Shimoji, “The Effectiveness of Preemptive Analgesia Varies According to the Type of Surgery: A Randomized, Double-Blind Study,” Anesthesia & Analgesia, Vol. 89, No. 3, 1999, pp. 711-716.
- O. N. Aydin, B. Ugur, S. Ozgun, H. Eyigor and O. Copcu, “Pain Prevention with Intraoperative Ketamine in Outpatient Children Undergoing Tonsillectomy or Tonsillectomy and Adenotomy,” Journal of Clinical Anesthesia, Vol. 19, No. 2, 2007, pp. 115-119. doi:10.1016/j.jclinane.2006.06.003
- M. De Kock, P. Lavand’homme and H. Waterloos, “‘Balanced Analgesia’ in the Postoperative Period: Is There a Place for Ketamine?” Pain, Vol. 92, No. 3, 2001, pp. 373- 380. doi:10.1016/S0304-3959(01)00278-0
- M. Suzuki, S. Haraguti, K. Sugimoto, T. Kikutani, Y. Shimada and A. Sakamoto, “Low-Dose Intravenous Ketamine Potentiates Epidural Analgesia after Thoracotomy,” Anesthesiology, Vol. 105, No. 1, 2006, pp. 111-119. doi:10.1097/00000542-200607000-00020
- J. A. Clements, W. S. Nimmo and I. S. Grant, “Bioavailability, Pharmacokinetics, and Analgesic Activity of Ketamine in Humans,” Journal of Pharmaceutical Sciences, Vol. 71, No. 5, 1982, pp. 539-542. doi:10.1002/jps.2600710516
- F. Adam, M. Libier, T. Oszustowicz, D. Lefebvre, J. Beal and J. Meynadier, “Preoperative Small-Dose Ketamine Has No Preemptive Analgesic Effect in Patients Undergoing Total Mastectomy,” Anesthesia & Analgesia, Vol. 89, No. 2, 1999, pp. 444-447.
- M. S. Angst and J. D. Clark, “Opioid-Induced Hyperalgesia: A Qualitative Systematic Review,” Anesthesiology, Vol. 104, No. 3, 2006, pp. 570-587. doi:10.1097/00000542-200603000-00025
- Y. Y. Chia, K. Liu, J. J. Wang, M. C. Kuo and S. T. Ho, “Intraoperative High Dose Fentanyl Induces Postoperative Fentanyl Tolerance,” Canadian Journal of Anesthesia, Vol. 46, No. 9, 1999, pp. 872-877. doi:10.1007/BF03012978
- J. P. Laulin, P. Maurette, J. B. Corcuff, C. Rivat, M. Chauvin and G. Simonnet, “The Role of Ketamine in Preventing Fentanyl-Induced Hyperalgesia and Subsequent Acute Morphine Tolerance,” Anesthesia & Analgesia, Vol. 94, No. 5, 2002, pp. 1263-1269. doi:10.1097/00000539-200205000-00040
- R. L. Schmid, A. N. Sandler and J. Katz, “Use and Efficacy of Low-Dose Ketamine in the Management of Acute Postoperative Pain: A Review of Current Techniques and Outcomes,” Pain, Vol. 82, No. 2, 1999, pp. 111-125. doi:10.1016/S0304-3959(99)00044-5
- N. Elia and M. R. Tramer, “Ketamine and Postoperative Pain—A Quantitative Systematic Review of Randomized Trials,” Pain, Vol. 113, No. 1-2, 2005, pp. 61-70. doi:10.1016/j.pain.2004.09.036

