World Journal of Cardiovascular Diseases
Vol. 2 No. 4 (2012) , Article ID: 23741 , 3 pages DOI:10.4236/wjcd.2012.24053
Very late pacemaker extrusion: A rare complication of permanent pacemaker implantation
![]()
Department of Cardiology, Advanced Cardiac Centre, Post Graduate Institute of Medical Education & Research, Chandigarh, India
Email: *rajeshvijay999@hotmail.com
Received 21 August 2012; revised 24 September 2012; accepted 3 October 2012
Keywords: Pacemaker Extrusion; Infection; Lead Fracture
ABSTRACT
Pacemaker extrusion is an uncommon complication of device implantation. Mostly, it happens either beuse of infection or secondary to various mechanical factors. It usually occurs days to months after the implantation, however very late pacemaker extrusion has not been described in the literature. We present a case of 70 years old male, who had pacemaker extrusion 11 years after its implantation. The various management issues related with such patient is discussed in the article.
1. INTRODUCTION
Pacemaker extrusion is a known complication of device implantation. Most of the extrusion occurs within days to months after the implantation. The management of these cases is technically demanding. We hereby report an unusual case of a very late pacemaker extrusion and discuss various management issues involved in such situation.
2. CASE REPORT
A 70-year-old non-diabetic male presented in November 2009 with pacemaker generator extrusion of 20 days duration. There was no history of fever, any systemic infection, pacemaker site trauma or local site boil formation. He had permanent pacemaker (Telectronics Quadra 9221 model, DDD mode) implantation in November 1989 for symptomatic sinus bradycadia and frequent sinus pauses of more than 2.5 seconds. In March 1994, he had asymptomatic ventricular lead fracture (lead resistance >3000 ohms), for which the pacemaker mode was changed from DDD to AAI mode. He remained asymptomatic in following years till November 1998, when he had elective replacement of pacemaker generator following battery depletion (Medtronic Prevail 8084 model, AAI mode). The fractured ventricular lead was not removed and normal functioning atrial lead was connected with the new generator. Thereafter, he remained asymptomatic for 11 years, and now presented with pacemaker extrusion (Figure 1A).
There was no discharge from the extrusion site. The pocket did not show any sign of active inflammation/ infection. The culture swab taken from the local site did not show any bacterial infection. The blood culture was also sterile twice; one sample was taken 24 hours before and another one 24 hours after the procedure. The ECG showed atrial pacing with PR interval of 280 msecs (Figure 2A). Transthoracic echocardiography showed left ventricular ejection fraction of 0.60, no regional wall motion abnormality, no tricuspid regurgitation, and no vegetation on any of the two leads. An ultrasound Doppler revealed patent superior vena cava and right subclavian vein with no evidence of thrombosis or stenosis. After a written informed consent, he was subjected for pacemaker removal. The right side pocket was thoroughly cleaned and debrided after generator removal, and wound was partially sutured for secondary healing. The 21-year-old implanted atrial and ventricular leads could not be removed by simple traction, hence left insitu. Both the leads were cut near the right subclavian vein entry point and were well secured to prevent its dislodgement in superficial veins. In the same sitting, a new pacemaker unit (Medtronic Relia REVDD01 model, VDD mode) was implanted via left subclavian approach (Figures 2B and 3) on left infra-clavicular region. A 7-day-course of intravenous antibiotics-teicoplanin and amikacin was given following the procedure. He had uneventful recovery and discharged after 8 days of implantation. He remained asymptomatic on follow-up with no discharge from right side. At one and half years of follow-up in April 2011, both the sites were absolutely healthy (Figure 1B). During the last out-patient visit in August 2012, he was asymptomatic with atrial sense and ventricular paced rhythm.
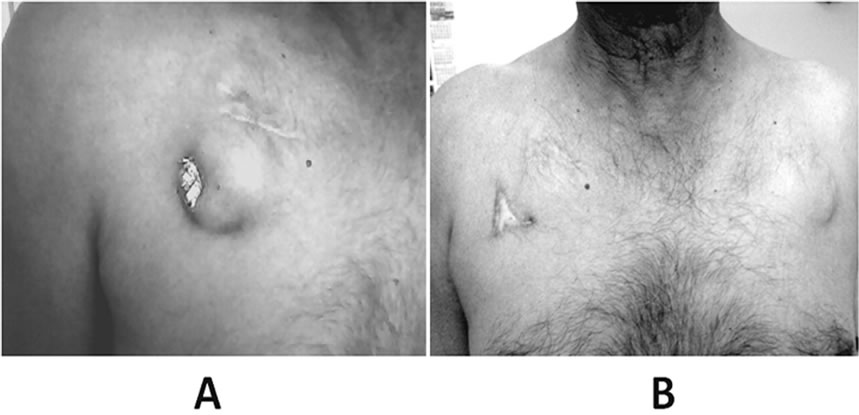
Figure 1. A: Pacemaker extrusion from the pocket, which was implanted 11 years back. B: Perfectly healthy right side of chest with no discharge and normal left side pacemaker implanted site, at one and half years of follow-up.
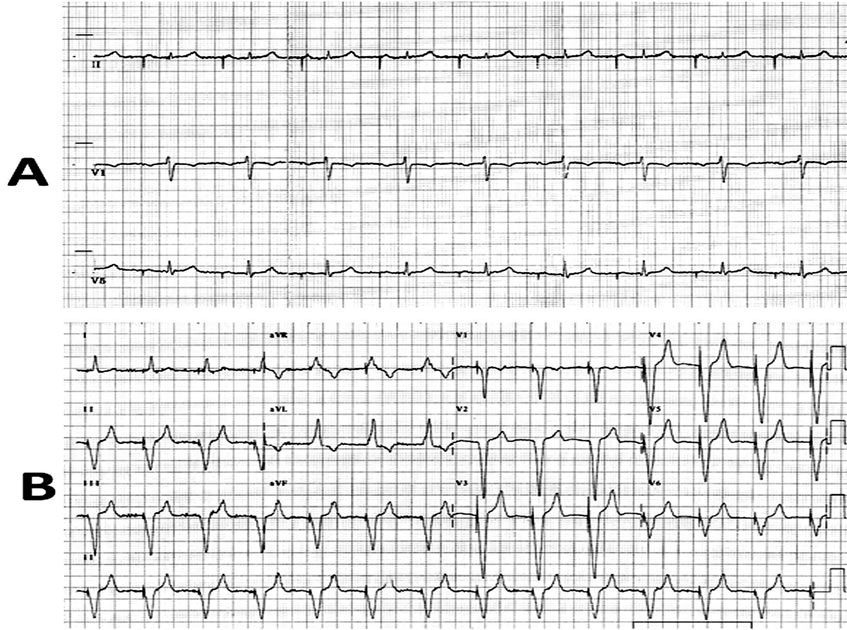
Figure 2. A: ECG shows AAI pacing with PR interval of 280 msecs. B: ECG showing atrial sense, ventricular pace rhythm following VDD pacing.
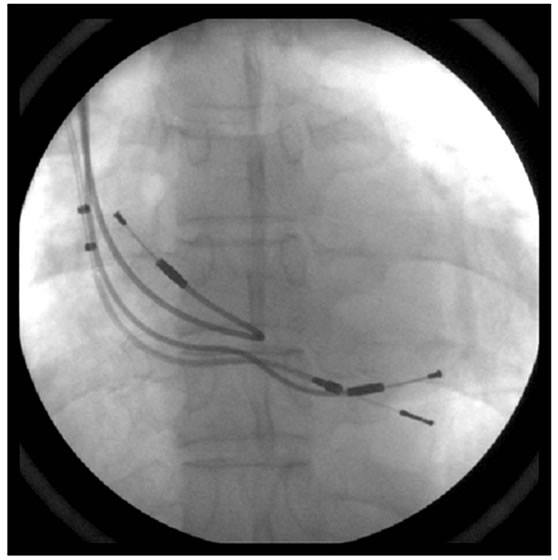
Figure 3. Chest X-ray shows one VDD lead and two superfluous atrial & ventricular leads.
3. DISCUSSION
Pacemaker extrusion is a known complication of device implantation. It’s true incidence is variable ranging from 0.9% - 4%, when early pocket infection is also included [1-3]. The main cause of extrusion is either because of pocket infection or secondary to pressure/ischemic necrosis of overlying skin [2]. Mostly, pacemaker extrusion occurs in months time following implantation [1-3], however the index case is unusual because of extrusion occurred 11 years after the generator replacement. The possible reasons for a delayed extrusion may be local tissue vulnerability in elderly patients secondary to thin subcutaneous layer of fat, erosive action of device, poor local hygiene, depressed immunity, associated diabetes, etc. [4,5].
Any eroded pocket with exposed device has the risk of device infection and its related complications like infective endocarditis and septicaemia. In the index case, though there was no evidence of infection, we decided to put a newer device on opposite side, instead of re-implantation at same site after adequate debridement and irrigation of the pocket as reported by other authors [2,6]. The newer device was implanted in same sitting along with debridement of exposed pocket, instead of making it a staged procedure as recommended for infected pocket [7]. The right side two leads-fractured ventricular lead and normally functioning atrial lead were not extracted by described percutaneous methods [7], considering 21 years duration of implantation. The old leads like in index case, are likely to have firm encapsulation, fibrosis and calcification, which in turn can result into major complication on extraction [8]. The newer device implanted from left side had the VDD mode, which have the advantage of atrio-ventricular synchronous pacing even with single transvenous lead. An AAI pacemaker was not implanted as the PR interval in the ECG was 280 msecs, which have the future risk of complete heart block [9]. A dual chamber pacemaker from left side resulting into total four leads (2 superfluous and 2 new leads) theoretically have more complication than the 3 leads in index case. Increasing number of leads have the higher risk of complications like clot formation, vascular occlusion and obliteration, inter-lead adherence and electrical interference [8]. Another option was to make a conduit to tunnel the normally functioning right side atrial lead to left side pacemaker pocket and put a dual chamber pacemaker with new ventricular lead from left side,[8] which would have resulted into total 3 leads (1 superfluous fractured ventricular lead, 1 old atrial lead and 1 new ventricular lead). However, tunnelling was avoided in the index case, considering the risk of pocket infection of left side and also the requirement of general anaesthesia and surgical support in this elderly patient. The above discussed line of management is individualized and is likely to differ depending upon risk-benefit ratio, associated co-morbid illness, various technical issues and operator’s experience.
REFERENCES
- Kiviniemi, M.S., Pirnes, M.A., Eränen, H.J., Kettunen, R.V. and Hartikainen, J.E. (1999) Complications related to permanent pacemaker therapy. Pacing and Clinical Electrophysiology, 22, 711-720. doi:10.1111/j.1540-8159.1999.tb00534.x
- Griffith, M.J., Mounsey, J.P., Bexton, R.S. and Holden, M.P. (1994) Mechanical, but not infective, pacemaker erosion may be successfully managed by re-implantation of pacemakers. British Heart Journal, 71, 202-205. doi:10.1136/hrt.71.2.202
- Fleck, T., Khazen, C., Wolner, E. and Grabenwoger, M. (2006) The incidence of reoperations in pacemaker recipients. Heart Surgery Forum, 9, 779-782. doi:10.1532/HSF98.20061057
- Harcombe, A.A., Newell, S.A., Ludman, P.F., Wistow, T.E., Sharples, L.D., Schofield, P.M., et al. (1998) Late complications following permanent pacemaker implantation or elective unit replacement. Heart, 80, 240-244.
- Yuksel, S., Demir, S. and Sahin, M. (2012) Total extrusion of a normally functioning pacemaker. Texas Heart Institute Journal, 39, 156-157. doi:10.1097/01.sap.0000261846.73531.2e
- Kolker, A.R., Redstone, J.S. and Tutela, J.P. (2007) Salvage of exposed implantable cardiac electrical devices and lead systems with pocket change and local flap coverage. Annals of Plastic Surgery, 59, 26-29.
- Wilkoff, B.L., Love, C.J., Byrd, C.L., Bongiorni, M.G., Carrillo, R.G., Crossley, G.H. 3rd, Epstein, L.M., Friedman, R.A., Kennergren, C.E., Mitkowski, P., Schaerf, R.H. and Wazni, O.M. (2009) Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management. Heart Rhythm, 6, 1085-1104. doi:10.1016/j.hrthm.2009.05.020
- Byrd, C.L. (2007) Managing device related complications and transvenous lead extraction. In: Ellenbogen, K.A., Kay, G.N., Lau, C.P., Wilkoff, B.L., Eds., Clinical cardiac pacing, defibrillation and resynchronization therapy. 3rd Edition, Saunders Elsevier Publication, 855-930.
- Vijayvergiya, R. and Grover, A. (2002) Acquired atrioventricular block in a patient with sinus node disease on single-chamber atrial pacing. Indian Heart Journal, 54, 435-436.
NOTES
*Corresponding author.

