Journal of Cancer Therapy
Vol.4 No.1A(2013), Article ID:27102,8 pages DOI:10.4236/jct.2013.41A015
OPRT Is a Potential Predictive Factor for the Response to S-1 in Gastric Cancer*
![]()
1Department of General Surgery, Chinese PLA General Hospital, Beijing, China; 2Department of Pathology, Chinese PLA General Hospital, Beijing, China.
Email: #chenlinbj@vip.sina.com
Received November 17th, 2012; revised December 18th, 2012; accepted December 27th, 2012
Keywords: S-1; Chemotherapy; Gastric Cancer; Orotate Phosphoribosyltransferase; Biomarker
ABSTRACT
Objective: To analyze the impact of mRNA expression of oral fluoropyrimidine (S-1) metabolism (thymidylate synthase, dihydropyrimidine dehydrogenase, thymidine phosphorylase, and orotate phosphoribosyltransferase [OPRT]), on treatment outcomes in locally advanced gastric cancer patients receiving preoperative S-1 combined with oxaliplatin chemotherapy. Methods: Preoperative stage III gastric cancer patients received S-1 (80 mg/m2/day; days 1-14) and oxaliplatin (130 mg/m2; day 1) every 3 weeks and subsequently received gastrectomy with D1/D2 lymphadenectomy. Paired tumor and normal fresh frozen tissues were collected to evaluate mRNA levels of thymidylate synthase, thymidine phosphorylase, dihydropyrimidine dehydrogenase, and orotate phosphoribosyltransferase using quantitative reverse-transcriptase polymerase chain reaction. Results: Between December 2009 and October 2010, thirty-five patients were enrolled in this study. 24 (68.5%) patients had clinical tumor response and 10 (28.6%) patients achieved histological response. Quantitative reverse-transcriptase polymerase chain reaction results showed that orotate phosphoribosyltransferase (OPRT) mRNA expression was significantly higher in histological responders than non-responders (3.75 vs. 1.81, P = 0.005). Diffuse-type gastric cancer patients demonstrated higher orotate phosphoribosyltransferase (OPRT) expression levels than intestinal-type ones (2.79 vs. 1.60, P = 0.014). Similar results were not found when comparing thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase expression levels. Conclusion: Orotate phosphoribosyltransferase (OPRT) expression level may be a potential predictive biomarker in advanced gastric cancer patients treated with oral fluoropyrimidine (S-1) based chemotherapy. Mini Abstract: Orotate phosphoribosyltransferase (OPRT) expression level may be a potential predictive biomarker in advanced gastric cancer patients treated with oral fluoropyrimidine (S-1) based chemotherapy.
1. Introduction
Gastric cancer is an aggressive disease with high incidence and poor prognosis in Eastern Asia, South America, Eastern Europe, and is ranked the second most common cause of cancer death [1,2]. Although surgical resection remains the only curative method in the treatment of gastric cancer, various clinical trials have shown significant benefits of multimodal treatments represented by perioperative chemotherapy [3], postoperative adjuvant chemotherapy [4] or chemoradiotherapy [5].
S-1 is a novel oral fluoropyrimidine, which is a combination of tegafur, gimeracil and oteracil potassium in a molar ratio of 1.0:0.4:1.0. It has been demonstrated to have a higher response rate than other oral anticancer agents against advanced gastric cancer [6]. Oxaliplatin, a third generation platinum compound, has shown activity in combination with fluoropyrimidines in patients with advanced gastric cancer in phase II studies [7,8]. It was found that the combination of S-1 with oxaliplatin (SOX regimen) was effective for unresectable or recurrent gastric cancer patients with a tumor response rate of 59% [9]. It was noted, however, that not all patients could benefit from S-1 based chemotherapy because of individual differences.
Now, many investigators are seeking new methods to precisely identify patients who may benefit from given anticancer agents to achieve individualized therapy. Identification of enzymes involved in fluoropyrimidine metabolism to predict 5-FU chemosensitivity has made it possible to achieve these goals [10]. The first step of S-1 metabolism is its conversion into 5-FU. 80% - 90% of administered 5-FU is converted to the inactive metabolite by dihydropyrimidine dehydrogenase (DPD) and its expression had been reported to correlate with drug resistance as well as toxicity [11]. Fluoropyrimidine is converted to active metabolites by phosphorylation through three different pathways. Thymidine phosphlorylase (TP) and orotate phosphoribosyltransferase (OPRT) are the key enzymes in these processes [12]. Thymidylate synthase (TS) is an essential DNA synthesis enzyme suppressed by 5-fluoro-deoxyrudine-monophosphae (FdUMP), an active metabolite of 5-FU. Expression of these enzymes, alone or in combination, have been shown to have the potential to be predictive parameters of 5-FU based chemotherapy sensitivity in gastric cancer patients [13].
So far, there were no reports detailing 5-FU metabolic enzymes predicting chemotherapy response in advanced gastric cancer patients treated with preoperative S-1 combined with oxaliplatin chemotherapy. In this study, we examined the expression of TS, TP, DPD and OPRT mRNAs in fresh frozen tissues, so as to evaluate whether they are associated with chemotherapeutical treatment outcomes.
2. Materials and Methods
2.1. Patient Eligibility
Eligibility criteria: histologically proven gastric adenocarcinoma; AJCC Stage III diseases by computer tomography (CT) and endoscopic ultrasonography (EUS); measurable or assessable tumor as Response Evaluation Criteria in Solid Tumors (RECIST, 1.1 Ed.) [14]; 20 - 70 years of age; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 - 2; no distant or peritoneal metastases and negative cytology by contrast enhanced CT and laparoscopy exploration, positron emission tomography (PET)/CT was optional; no previous chemotherapy or radiotherapy; no previous cancer history; no signs of organ failure, as assessed by white blood cell (WBC) count 4000/mm3 - 12,000/mm3, platelet count of 100,000/mm3 or above, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) less than three times the upper limit of normal, total bilirubin 1.5 mg/dl or less, creatinine 1.2 mg/dl or less and creatinine clearance 60 ml/min or above, and hemoglobin 10.0 g/dl or more; arterial partial pressure of oxygen (PaO2) of 70 mmHg or above; negative serology for viral hepatitis and no past history of hepatitis. All patients gave written informed consent and this study was approved by the institutional review board.
2.2. Preoperative Chemotherapy
S-1 80 mg/m2 was administered orally on day 1 to day 14 and an intravenous 2 h bolus of oxaliplatin 130 mg/m2 was given on day 1. This regimen was repeated every 21 days for a total of 2 - 4 cycles. If the patient had WBC of 4000/mm3 or less, platelet of 10,000/mm3 or less, abnormal peripheral nerve feeling, diarrhea, or nausea of Grade 2 or higher,, this regimen was modified by decreasing S-1 from 120 mg/d to 100 mg/d or 80 mg/d and oxaliplatin from 130 mg/m2 to 100 mg/m2or 85 mg/m2. If the adverse event was worse, the dose was postponed until recovery. After 2 cycles of chemotherapy, the patients’ tumor response was evaluated based on CT and EUS findings by RECIST 1.1 criteria. If radical resection was considered possible or if tumor response was considered progressive disease, then the patients received surgery immediately. If not, l or 2 additional cycles of chemotherapy were given before surgery. All chemotherapeutic adverse events were recorded by a medical nurse and were classified according to the National Cancer Institute Common Toxicity Criteria version 4.0 (NCICTC 4.0).
2.3. Surgery and Pathology
All patients underwent surgical resection within 4 weeks after the last chemotherapy cycle. Gastrectomy with D1/ D2 lymphadenectomy was performed according to Japanese Gastric Cancer Association (JGCA) criteria [15].
An experienced pathologist examined all surgical specimens. Postoperative pathology was reported as follows: 1) Lauren pathological classification; 2) Positive and negative lymph nodes in each group; 3) Resection margins were classified as R0 (no cancer at the resection margins), R1 (microscopically involved margin), and R2 (macroscopically involved margin); 4) Histological response was evaluated and graded according to the proportion of tumor affected by degeneration or necrosis [15]: Grade 0, no evidence of effect; Grade 1a, viable tumor cells occupy more than 2/3 of the tumorous area; Grade 1b, viable tumor cells remain in more than 1/3 but less than 2/3 of the tumorous area; Grade 2, viable tumor cells remain in less than 1/3 of the tumorous area; Grade 3, no viable tumor cells remain. Grade 2 or Grade 3 histological responses are defined as responders, while grade 1a and 1b are non-responders. Archived fresh frozen samples were obtained from the primary paired tumor and normal tissues at the time of surgery and stored at −80˚C until RNA extraction.
2.4. RNA Extraction, cDNA Synthesis, and Quantitative Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA from tumor and normal samples was isolated using TRIzol® Reagent kit (Invitrogen-GIBCO BRL, Life Technologies, USA) according to the manufacturer’s instructions. After RNA isolation, cDNA was derived from each sample and target cDNA sequences were amplified by quantitative real-time PCR using a fluorescence-based detection method (Bio-Rad MiniOpticonTM Real-Time PCR system, Hercules, CA). TS, TP, DPD and OPRT primers were used to perform PCR amplification by Bio-Rad iQTM Multiplex Powermix. The PCR conditions were 50˚C for 2 minutes and 95˚C for 5 minutes, followed by 41 cycles at 95˚C for 15 seconds and 60˚C for 1 minute.
Quantitative relative expressions of mRNA were obtained from the CT number at which the increase in the signal associated with the exponential growth of the PCR products were able to be detected (using PE Biosystems analysis software, according to the manufacturer’s manual) and calculated using the comparative CT method [16,17]. All data were controlled for the quantity of RNA input by measuring an endogenous reference gene, β- actin. Briefly, the analysis was performed as follows: for each sample, the difference in the CT values (ΔCT) was calculated for each mRNA by taking the mean CT of duplicate wells and subtracting the mean CT of duplicate wells for the reference RNA (β-actin), measured in an aliquot from the same RT reaction. Thus, ΔCT (target gene) = CT (target gene) − CT (β-actin), based on the methods of Latil et al. [16]. We termed the relative expression of the target gene as “N target,” as determined by the formula N target= 2−ΔCT(target gene). The relative expression compared tumor tissue with normal tissue [T/N ratio] and was calculated by the formula N target[T/N] =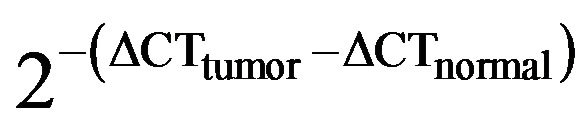 . If N target(T/N) is more than 1, this means that the mRNA expression in tumor tissue is higher than in normal tissue. This calculation assumes that all PCR reactions occurred at 100% efficiency. All PCR efficiencies were found to be >95%; therefore, any errors arising from this assumption were minimal.
. If N target(T/N) is more than 1, this means that the mRNA expression in tumor tissue is higher than in normal tissue. This calculation assumes that all PCR reactions occurred at 100% efficiency. All PCR efficiencies were found to be >95%; therefore, any errors arising from this assumption were minimal.
2.5. Statistical Analysis
Differences of mRNA T/N expression ratio between responders and nonresponders in terms of their relative gene expression to “N target” were analyzed using Wilcoxon test. Chi-square test was used to compare percentages in cross-tabulations. Two-sided P values less than 0.05 were assumed to be significant. Data were analyzed using SPSS 15.0 for Windows software (SPSS Inc., Chicago, IL).
3. Results
3.1. Patient Characteristics
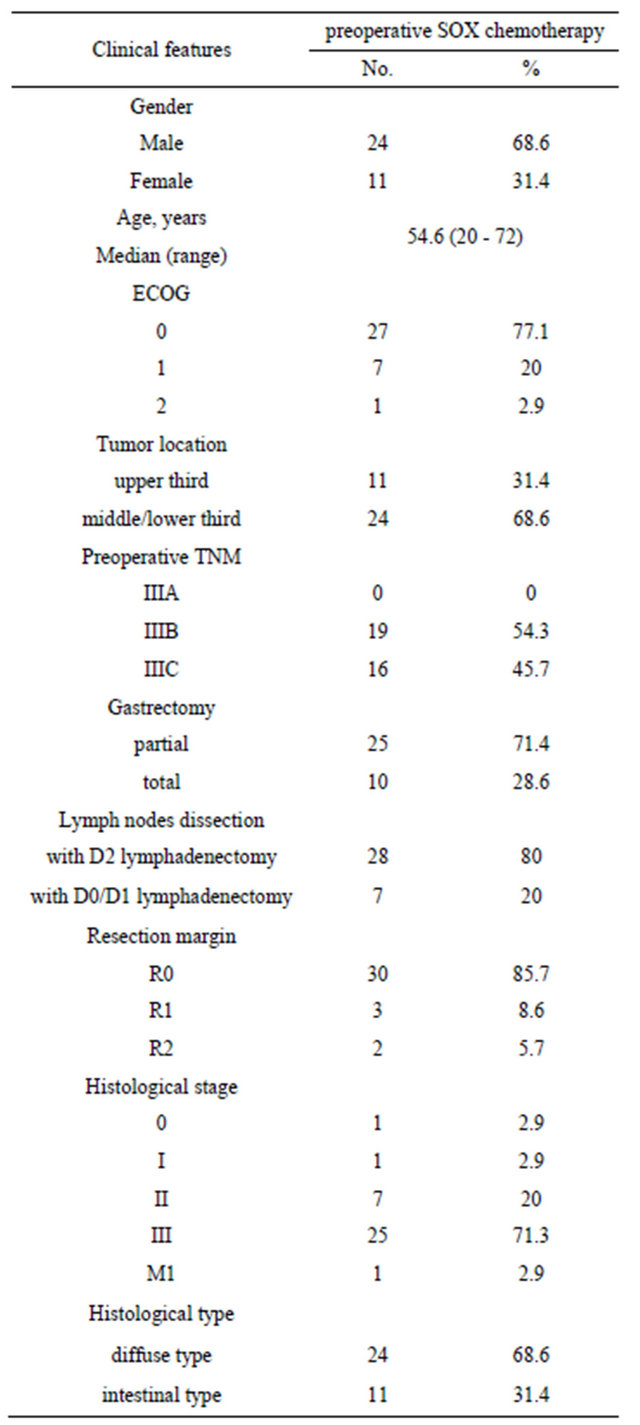
Between December 2009 and October 2010, 35 patients with stage III gastric cancer were enrolled in this study. The characteristics of patients were summarized in Table 1. Median age was 54.6 (range, 20 - 72). There were 24 male and 11 female patients, and 34 patients had ECOG PS of 0 - 1.
Table 1. Baseline demographic and patient characteristics.
3.2. Efficacy and Safety
All enrolled patients received at least two cycles of preoperative SOX chemotherapy. One stage IIIC patient achieved complete response (CR), and 23 patients received partial response (PR), resulting in a tumor response rate (RR) of 68.5%. 9 patients had stable disease (SD) and 2 patients suffered from progressive disease (PD). Toxicities were well tolerated and manageable in almost all cases. No Grade 4 adverse effects were found.
3.3. Surgical and Pathological Results
Twenty-eight patients (28/35, 80%) underwent gastrictomy with D2 lymphadenectomy. Seven patients (7/35, 20%) did not receive D2 surgery because of tumor invasion of major vessels or intraperitoneal metastasis.
An experienced GI pathologist carefully inspected all surgical specimens. 30 (85.7%) patients achieved negative margins (R0 resection). 24 (68.6%) patients were classified as diffuse type and 11 (31.4%) patients were intestinal type (Table 1). 10 (28.6%) patients achieved considerable histological response as responders. The correlation of pathological Lauren classification with histological response is shown in Table 2.
3.4. RT-PCR Results
The relative mRNA expression (T/N ratio) levels of four candidate genes, TS, TP, OPRT and DPD were tested using real-time RT-PCR in 35 paired tumor and normal fresh tissues (Table 3). Expression of TS, TP and DPD did not correlate with histological response, however, the relative mRNA level of OPRT was significantly higher in responders than in non-responders (3.75 vs. 1.81, P = 0.005) (Figure 1). One patient with histological complete response after preoperative chemotherapy was found to have a very high OPRT relative expression ratio exceeding 9.0.
When the associations between TS, TP, OPRT and DPD expression with Lauren pathology classification were analyzed, a significantly higher OPRT level was found in diffuse-type patients than in intestinal-type ones (2.79 vs. 1.60, P = 0.014). TS, TP and DPD expression did not follow the same pattern (Figure 2).
4. Discussion
Preoperative chemotherapy seems to be an effective method to treat resectable gastric cancer through primary tumor downstaging and thus can produce a higher cure rate after subsequent surgery [18-21]. S-1 is a DPD-inhibitory fluoropyrimidine, which produced the highest response rate among oral anticancer agents against advanced gastric cancer, showing a 44.6% response rate [22]. Recent studies have shown that S-1 monotherapy or combined regimen as preoperative chemotherapy are effective for advanced gastric cancer patients and prove to be valuable for the improvement of surgical outcomes [23-26]. However, despite these promising clinical results, there are still many patients who do not benefit from preoperative chemotherapy because of individual differences. If particular molecular markers predict S-1 efficacy, S-1 based chemotherapy or surrogate regimen
Table 2. Histological response and lauren classification.
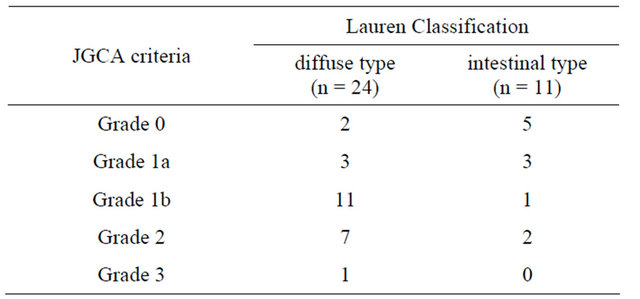
Note: Histological Response; responders: Grade 2 + Grade 3; non-responders: Grade 1a + Grade 1b.
Table 3. PCR primers and probes.

could be modified for each individual treatment plan.
Intratumoral gene expression or activities of some enzymes related to the metabolism of 5-FU have been shown to correlate with the sensitivity to this anticancer agent in the treatment of various cancers [27,28]. OPRT is one of the key enzymes that metabolize 5-FU to its active metabolite 5-fluoro-2’-deoxyuridine-5’-monophosphate (FdUMP), which suppresses thymidylate synthase (TS). Fujii et al. have revealed that OPRT enzyme activity is higher in 5-FU-sensitive tissues than in nonsensitive ones using in vitro chemosensitivity tests [29]. Ichikawa et al. reported that OPRT expression could be used to predict tumor shrinkage and survival in response to UFT and LV adjuvant chemotherapy [30]. Similar results were also found in urinary bladder cancer [31]. All these studies demonstrated that OPRT has the potential to be a valuable predictive biomarker associated with sensitivity of 5-FU. In the current study, it was demonstrated that OPRT relative mRNA levels were related to higher histological response after preoperative SOX chemotherapy (3.75 vs. 1.81, P = 0.005). However, mRNA levels of TS, TP, DPD were not found to be correlated with histological response, although they have definite roles in the metabolism of 5-FU. This result contradicts some studies that analyzed the TS, DPD and TP expression in gastric
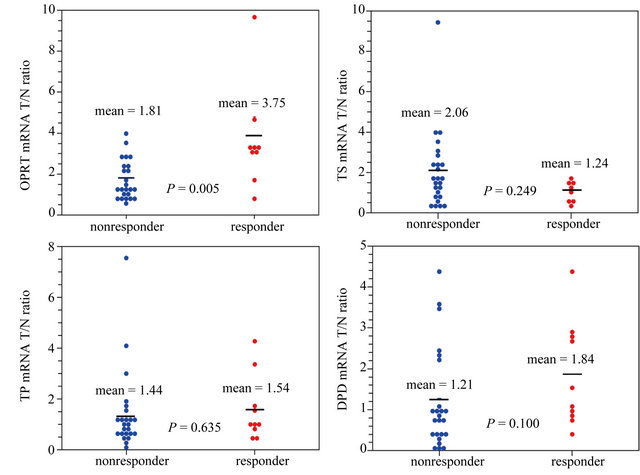
Figure 1. The relative mRNA level of OPRT was significantly higher in responders than in non-responders (3.75 vs. 1.81, P = 0.005).
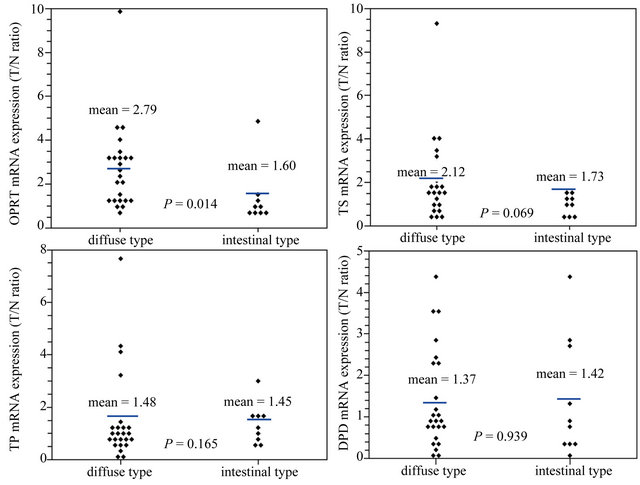
Figure 2. A significantly higher OPRT level was found in diffuse-type patients than in intestinal-type ones (2.79 vs. 1.60, P = 0.014). TS, TP and DPD expression did not follow the same pattern.
cancer in comparison to the response to 5-FU or S-1 therapy [6,17,32-34]. The lack of association of these enzymes with treatment outcomes has several explanations. First, differences in the results may be attributed to differences in methodology. Second, the predictability of these enzymes might be influenced by combined oxaliplatin. Ichikawa et al. reported that the predictive power of TS was overcome by irinotecan combination with S-1 [35]. In addition, S-1 has antitumor activity, even in tumors with a high expression of DPD [36]. This could be explained by the fact that DPD activity is inhibited by gimeracil, which is contained in S-1. These findings suggest that this chemosensitivity mechanism is multifactorial and that prediction is unlikely to be accomplished through a single enzyme evaluation. Gene expression profiles combined with drug activity would be extremely valuable for the identification of molecular mechanisms of cellular drug sensitivity and resistance [37].
Diffuse-type gastric carcinoma, according to the Lauren classification, is highly metastatic and characterized clinically by rapid disease progression and poor prognosis. Studies have shown that diffuse-type gastric cancer patients were more sensitive to S-1 chemotherapy than intestinal-type ones [38-41]. However, there are no reports demonstrating the correlation of Lauren classification with 5-FU metabolic enzymes. In our study, diffuse-type gastric cancer patients were found to have OPRT overexpression in comparison to intestinal-type ones (2.79 vs. 1.60, P = 0.014]. No significant differences were observed in the expression of the other three enzymes. The possible explanation is the loss of expression of the cell adhesion protein E-cadherin in diffusetype gastric cancer patients. E-cadherin affects the regulation of cell proliferation and differentiation, and can be related to decreased chemosensitivity. Chemosensitivity of cancer is affected by the state of cell adhesion and expression of intercellular adhesion molecules [42,43]. In addition, there might be a negative correlation between OPRT and E-cadherin.
There are some limitations in this study. First, there are many methods to detect enzymatic activities using fresh or formalin-fixed and paraffin-embedded (FFPE) samples. However, some questions remain as to whether the mRNA levels could predict actual enzymatic activities. Even for fresh samples, the results might be unstable and highly dependent on how promptly the samples were collected and stored. Taking into account this reason, extracting mRNA from fresh samples is still one of optimal targets for evaluation of enzymatic activities. Second, in this study, we just evaluated the association of four 5-FU metabolic enzymes with treatment outcomes. Whether oxaliplatin would impact these results is still unknown. In addition, small sample size was another limitation of this study, and thus significant differences of other biomarkers might not have been detected.
In conclusion, our study shows OPRT may play a leading role among the four representative 5-FU metabolic enzymes, indicating its potential to be a predictive marker in advanced gastric cancer patients treated with S-1 combined with oxaliplatin. Large-scale studies with the most appropriate testing method are still needed to confirm the results of the current study and to develop tailored treatment to achieve individual therapy in advanced gastric cancer patients.
REFERENCES
- A. Jemal, R. Siegel, E. Ward, T. Murray, J. Xu, C. Smigal, et al., “Cancer Statistics, 2006,” Cancer Journal for Clinicians, Vol. 56, No. 2, 2006, pp. 106-130. doi:10.3322/canjclin.56.2.106
- D. M. Parkin, F. Bray, J. Ferlay and P. Pisani, “Global Cancer Statistics, 2002,” Cancer Journal for Clinicians, Vol. 55, No. 2, 2005, pp. 74-108. doi:10.3322/canjclin.55.2.74
- D. Cunningham, W. H. Allum, S. P. Stenning, J. N. Thompson, C. J. Van de Velde, M. Nicolson, et al., “Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer,” The New England Journal of Medicine, Vol. 355, No. 1, 2006, pp. 11-20. doi:10.1056/NEJMoa055531
- S. Sakuramoto, M. Sasako, T. Yamaguchi, T. Kinoshita, M. Fujii, A. Nashimoto, et al., “Adjuvant Chemotherapy for Gastric Cancer with S-1, an Oral Fluoropyrimidine,” The New England Journal of Medicine, Vol. 357, No. 18, 2007, pp. 1810-1820. doi:10.1056/NEJMoa072252
- J. S. Macdonald, “Role of Post-Operative Chemoradiation in Resected Gastric Cancer,” Journal of Surgical Oncology, Vol. 90, No. 3, 2005, pp. 166-170. doi:10.1002/jso.20223
- W. Ichikawa and Y. Sasaki, “Challenges in Predicting the Clinical Outcome in S-1-Based Chemotherapy for Gastric Cancer Patients,” International Journal of Clinical Oncology, Vol. 13, No. 3, 2008, pp. 206-211. doi:10.1007/s10147-008-0786-y
- Y. H. Park, B. S. Kim, B. Y. Ryoo and S. H. Yang, “A Phase II Study of Capecitabine plus 3-Weekly Oxaliplatin as First-Line Therapy for Patients with Advanced Gastric Cancer,” British Journal of Cancer, Vol. 94, No. 7, 2006, pp. 959-963. doi:10.1038/sj.bjc.6603046
- S. E. Al-Batran, A. Atmaca, S. Hegewisch-Becker, D. Jaeger, S. Hahnfeld, M. J. Rummel, et al., “Phase II Trial of Biweekly Infusional Fluorouracil, Folinic Acid, and Oxaliplatin in Patients with Advanced Gastric Cancer,” Journal of Clinical Oncology, Vol. 22, No. 4, 2004, pp. 658- 663. doi:10.1200/JCO.2004.07.042
- W. Koizumi, H. Takiuchi, Y. Yamada, N. Boku, N. Fuse, K. Muro, et al., “Phase II Study of Oxaliplatin plus S-1 as First-Line Treatment for Advanced Gastric Cancer (GSOX Study),” Annals of Oncology, Vol. 21, No. 5, 2010, pp. 1001-1005. doi:10.1093/annonc/mdp464
- M. Iwahashi, M. Nakamori, M. Nakamura, K. Noguchi, K. Ueda, Y. Nakatani, et al., “Individualized Adjuvant Chemotherapy Guided by Chemosensitivity Test Sequential to Extended Surgery for Advanced Gastric Cancer,” Anticancer Research, Vol. 25, No. 5, 2005, pp. 3453- 3459.
- G. D. Heggie, J. P. Sommadossi, D. S. Cross, W. J. Huster and R. B. Diasio, “Clinical Pharmacokinetics of 5-Fluorouracil and Its Metabolites in Plasma, Urine, and Bile,” Cancer Research, Vol. 47, No. 8, 1987, pp. 2203-2206.
- G. J. Peters, E. Laurensse, A. Leyva, J. Lankelma and H. M. Pinedo, “Sensitivity of Human, Murine, and Rat Cells to 5-Fluorouracil and 5’-Deoxy-5-Fluorouridine in Relation to Drug-Metabolizing Enzymes,” Cancer Research, Vol. 46, No. 1, 1986, pp. 20-28.
- M. Kinoshita, Y. Kodera, K. Hibi, G. Nakayama, T. Inoue, N. Ohashi, et al., “Gene Expression Profile of 5-Fluorouracil Metabolic Enzymes in Primary Colorectal Cancer: Potential as Predictive Parameters for Response to Fluorouracil-Based Chemotherapy,” Anticancer Research, Vol. 27, No. 2, 2007, pp. 851-856.
- E. A. Eisenhauer, P. Therasse, J. Bogaerts, L. H. Schwartz, D. Sargent, R. Ford, et al., “New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1),” European Journal of Cancer, Vol. 45, No. 2, 2009, pp. 228-247. doi:10.1016/j.ejca.2008.10.026
- Japanese Gastric Cancer Association, “Japanese Gastric Cancer Treatment Guidelines 2010 (Ver.3),” Gastric Cancer, Vol. 14, No. 2, 2011, pp. 113-123. doi:10.1007/s10120-011-0042-4
- A. Latil, I. Bieche, L. Chene, I. Laurendeau, P. Berthon, O. Cussenot, et al., “Gene Expression Profiling in Clinically Localized Prostate Cancer: A Four-Gene Expression Model Predicts Clinical Behavior,” Clinical Cancer Research, Vol. 9, No. 15, 2003, pp. 5477-5485.
- R. Matsuyama, S. Togo, D. Shimizu, N. Momiyama, T. Ishikawa, Y. Ichikawa, et al., “Predicting 5-Fluorouracil Chemosensitivity of Liver Metastases from Colorectal Cancer Using Primary Tumour Specimens: Three-Gene Expression Model Predicts Clinical Response,” International Journal of Cancer, Vol. 119, No. 2, 2006, pp. 406- 413. doi:10.1002/ijc.21843
- J. L. Dikken, J. W. van Sandick, H. Maurits Swellengrebel, P. A. Lind, H. Putter, E. P. Jansen, et al., “Neo-Adjuvant Chemotherapy Followed by Surgery and Chemotherapy or by Surgery and Chemoradiotherapy for Patients with Resectable Gastric Cancer (CRITICS),” BMC Cancer, Vol. 11, 2011, p. 329. doi:10.1186/1471-2407-11-329
- V. Valenti, J. L. Hernandez-Lizoain, M. C. Beorlegui, J. A. Diaz-Gozalez, F. M. Regueira, J. J. Rodriguez, et al., “Morbidity, Mortality, and Pathological Response in Patients with Gastric Cancer Preoperatively Treated with Chemotherapy or Chemoradiotherapy,” Journal of Surgical Oncology, Vol. 104, No. 2, 2011, pp. 124-129. doi:10.1002/jso.21947
- D. Cunningham, W. H. Allum, S. P. Stenning, J. N. Thompson, C. J. Van de Velde, M. Nicolson, et al., “Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer,” The New England Journal of Medicine, Vol. 355, No. 1, 2006, pp. 11-20. doi:10.1056/NEJMoa055531
- M. Ychou, V. Boige, J. P. Pignon, T. Conroy, O. Bouche, G. Lebreton, et al., “Perioperative Chemotherapy Compared with Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial,” Journal of Clinical Oncology, Vol. 29, No. 13, 2011, pp. 1715-1721. doi:10.1200/JCO.2010.33.0597
- Y. Sakata, A. Ohtsu, N. Horikoshi, K. Sugimachi, Y. Mitachi, T. Taguchi, “Late Phase II Study of Novel Oral Fluoropyrimidine Anticancer Drug S-1 (1 M Tegafur-0.4 M Gimestat-1 M Otastat Potassium) in Advanced Gastric Cancer Patients,” European Journal of Cancer, Vol. 34, No. 11, 1998, pp. 1715-1720. doi:10.1016/S0959-8049(98)00211-1
- T. Yoshikawa, K. Omura, O. Kobayashi, A. Nashimoto, A. Takabayashi, T. Yamada, et al., “A Phase II Study of Preoperative Chemotherapy with S-1 plus Cisplatin Followed by D2/D3 Gastrectomy for Clinically Serosa-Positive Gastric Cancer (JACCRO GC-01 Study),” European Journal of Surgical Oncology, Vol. 36, No. 6, 2010, pp. 546-551. doi:10.1016/j.ejso.2010.04.011
- T. Sato, H. Ozawa, K. Hatate, W. Onosato, M. Naito, T. Nakamura, et al., “A Phase II Trial of Neoadjuvant Preoperative Chemoradiotherapy with S-1 plus Irinotecan and Radiation in Patients with Locally Advanced Rectal Cancer: Clinical Feasibility and Response Rate,” International Journal of Radiation Oncology Biology Physics, Vol. 79, No. 3, 2011, pp. 677-683. doi:10.1016/j.ijrobp.2009.11.007
- A. Nashimoto, H. Yabusaki, S. Nakagawa, Y. Takii, Y. Tsuchiya and T. Otsuo, “Preoperative Chemotherapy with S-1 and Cisplatin for Highly Advanced Gastric Cancer,” Anticancer Research, Vol. 29, No. 11, 2009, pp. 4689- 4696.
- T. Kinoshita, M. Sasako, T. Sano, H. Katai, H. Furukawa, A. Tsuburaya, et al., “Phase II Trial of S-1 for Neoadjuvant Chemotherapy against Scirrhous Gastric Cancer (JCOG 0002),” Gastric Cancer, Vol. 12, No. 1, 2009, pp. 37-42. doi:10.1007/s10120-008-0496-1
- W. Ichikawa, “Prediction of Clinical Outcome of Fluoropyrimidine-Based Chemotherapy for Gastric Cancer Patients, in Terms of the 5-Fluorouracil Metabolic Pathway,” Gastric Cancer, Vol. 9, No. 3, 2006, pp. 145-155. doi:10.1007/s10120-006-0373-8
- J. L. Yen and H. L. McLeod, “Should DPD Analysis Be Required Prior to Prescribing Fluoropyrimidines?” European Journal of Cancer, Vol. 43, No. 6, 2007, pp. 1011- 1016. doi:10.1016/j.ejca.2007.01.030
- R. Fujii, A. Seshimo and S. Kameoka, “Relationships between the Expression of Thymidylate Synthase, Dihydropyrimidine Dehydrogenase, and Orotate Phosphoribosyltransferase and Cell Proliferative Activity and 5-Fluorouracil Sensitivity in Colorectal Carcinoma,” International Journal of Clinical Oncology, Vol. 8, No. 2, 2003, pp. 72-78. doi:10.1007/s101470300013
- W. Ichikawa, H. Uetake, Y. Shirota, H. Yamada, T. Takahashi, Z. Nihei, et al., “Both Gene Expression for Orotate Phosphoribosyltransferase and Its Ratio to Dihydropyrimidine Dehydrogenase Influence Outcome Following Fluoropyrimidine-Based Chemotherapy for Metastatic Colorectal Cancer,” British Journal of Cancer, Vol. 89, No. 8, 2003, pp. 1486-1492. doi:10.1038/sj.bjc.6601335
- Y. Mizutani, H. Wada, M. Fukushima, O. Yoshida, H. Nakanishi, Y. N. Li, et al., “Prognostic Significance of Orotate Phosphoribosyltransferase Activity in Bladder Carcinoma,” Cancer, Vol. 100, No. 4, 2004, pp. 723-731. doi:10.1002/cncr.11955
- W. Ichikawa, T. Takahashi, K. Suto, Y. Shirota, Z. Nihei, M. Shimizu, et al., “Simple Combinations of 5-FU Pathway Genes Predict the Outcome of Metastatic Gastric Cancer Patients Treated by S-1,” International Journal of Cancer, Vol. 119, No. 8, 2006, pp. 1927-1933. doi:10.1002/ijc.22080
- K. Miyazaki, T. Shibahara, D. Sato, K. Uchida, H. Suzuki, H. Matsui, et al., “Influence of Chemotherapeutic Agents and Cytokines on the Expression of 5-Fluorouracil-Associated Enzymes in Human Colon Cancer Cell Lines,” Journal of Gastroenterology, Vol. 41, No. 2, 2006, pp. 140-150. doi:10.1007/s00535-005-1733-6
- K. Sato, Y. Kitajima, A. Miyoshi, Y. Koga and K. Miyazaki, “Deficient Expression of the DPD Gene Is Caused by Epigenetic Modification in Biliary Tract Cancer Cells, and Induces High Sensitivity to 5-FU Treatment,” International Journal of Oncology, Vol. 29, No. 2, 2006, pp. 429-435.
- W. Ichikawa, T. Takahashi, K. Suto, T. Yamashita, Z. Nihei, Y. Shirota, et al., “Thymidylate Synthase Predictive Power Is Overcome by Irinotecan Combination Therapy with S-1 for Gastric Cancer,” British Journal of Cancer, Vol. 91, No. 7, 2004, pp. 1245-1250. doi:10.1038/sj.bjc.6602139
- T. Shimizu, Y. Yamada, H. Yasui, K. Shirao and M. Fukuoka, “Clinical Application of Immunoreactivity of Dihydropyrimidine Dehydrogenase (DPD) in Gastric Scirrhous Carcinoma Treated with S-1, a New DPD Inhibitory Fluoropyrimidine,” Anticancer Research, Vol. 25, No. 4, 2005, pp. 2997-3001.
- L. Rickardson, M. Fryknas, S. Dhar, H. Lovborg, J. Gullbo, M. Rydaker, et al., “Identification of Molecular Mechanisms for Cellular Drug Resistance by Combining Drug Activity and Gene Expression Profiles,” British Journal of Cancer, Vol. 93, No. 4, 2005, pp. 483-492. doi:10.1038/sj.bjc.6602699
- N. Nakayama, W. Koizumi, S. Tanabe, T. Sasaki and K. Saigenji, “A Phase II Study of Combined Chemotherapy with Methotrexate, 5-Fluorouracil, and Low-Dose Cisplatin (MFP) for Histologically Diffuse-Type Advanced and Recurrent Gastric Cancer (KDOG9501),” Gastric Cancer, Vol. 9, No. 3, 2006, pp. 185-191. doi:10.1007/s10120-006-0371-x
- H. Kawai, A. Ohtsu, N. Boku, Y. Hamamoto, F. Nagashima, M. Muto, et al., “Efficacy and Safety Profile of S-1 in Patients with Metastatic Gastric Cancer in Clinical Practice: Results from a Post-Marketing Survey,” Gastric Cancer, Vol. 6, Suppl. 1, 2003, pp. 19-23. doi:10.1007/s10120-003-0216-9
- K. Shitara and K. Muro, “Histological Subtype of Gastric Cancer in Japan,” The Lancet Oncology, Vol. 11, No. 2, 2010, pp. 115-116. doi:10.1016/S1470-2045(09)70367-5
- Y. Jiang, “Advanced Gastric Cancer: A Disease of Diverse Clinical Biology,” Gastrointestinal Cancer Research, Vol. 3, No. 3, 2009, pp. 125-126.
- F. Graziano, A. Mandolesi, A. Ruzzo, I. Bearzi, E. Testa, F. Arduini, et al., “Predictive and Prognostic Role of E-Cadherin Protein Expression in Patients with Advanced Gastric Carcinomas Treated with Palliative Chemotherapy,” Tumor Biology, Vol. 25, No. 3, 2004, pp. 106-110. doi:10.1159/000079141
- T. Nakamura, Y. Kato, H. Fuji, T. Horiuchi, Y. Chiba and K. Tanaka, “E-Cadherin-Dependent Intercellular Adhesion Enhances Chemoresistance,” International Journal of Molecular Medicine, Vol. 12, No. 5, 2003, pp. 693- 700.
NOTES
*Conflict of interest: None.
#Corresponding author.

