Advances in Bioscience and Biotechnology
Vol.3 No.1(2012), Article ID:17204,6 pages DOI:10.4236/abb.2012.31015
Moderate anxiety in pregnant women does not compromise gestational immune-endocrine status and outcome, but renders mothers to be susceptible for diseased states development: A preliminary report
![]()
1Grupo de Biología y Salud Integral, Instituto de Investigaciones Biológicas, Universidad Veracruzana, Veracruz, México
2Reproduction, Development and Sexual Function Department of Anatomy and Cell Biology, Queen’s University, Kingston, Canada
3Instituto de Neuroetología, Universidad Veracruzana, Veracruz, México
4Laboratorio de Neuroinmunología, Departamento de Medicina Experimental, Facultad de Medicina, Universidad Nacional Autónoma de México, Ciudad de México, México
5Laboratorio de Biología de Sistemas, Departamento de Biología Celular, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Ciudad de México, México
Email: *tromogonzalez@uv.mx
Received 16 October 2011; revised 17 November 2011; accepted 22 December 2011
Keywords: Gestation; Pregnancy; Anxiety, Progesterone; Estradiol; Pro-Inflammatory Cytokines; Anti-Inflammatory Cytokines; Immune Tolerance; Endocrine Deficiency
ABSTRACT
High anxiety levels during pregnancy commonly lead to clinical complications that affect the mother/child’s prenatal and perinatal health. Such complications are thought to result from combining deficiencies of the endocrine milieu and decreased immune tolerance that support pregnancy. It is yet unclear whether pregnant women subjected to moderate anxiety develop a similar state of endocrine deficits and compromised immune tolerance. Here, we evaluated this issue by monitoring endocrine and immune parameters during pregnancy in a sample of women suffering of moderate anxiety. The health of mothers and children, as well as the outcome of the pregnancies were also revised and recorded. Moderately anxious pregnant women present increments of state anxiety, serum cortisol and progesterone, but not of estradiol, as pregnancy progressed. No variations of leukocyte antiand pro-inflammatory cytokine mRNA expression were found along pregnancy. However, these pregnant women did show an increased frequency of gestational and perinatal complications, conditions that had no major consequences for their health and that of their children at birth. Hence, moderate anxiety renders the mother to be susceptible to develop diseased states during or by the end of pregnancy, while keeping the endocrine milieu and immune tolerance reasonably in place.
1. INTRODUCTION
During the past three decades, we have witnessed an increment in the number cases of maternal and fetal/infant preand perinatal complications worldwide [1]. Although nutritional factors, infections of various sorts, stressful circumstances-whether social, biological or bothand a wealth of physiological (including behavioral) disorders alone or combined may predispose or trigger pathophysiological states during gestation and delivery, many intricacies of the mechanisms underlying them are still poorly understood.
Anxiety disorders are now recognized to have harmful effects on the course of gestation and on the mother/child prenatal and postnatal health status [2-6]. Such deleterious effects seem to result from immune-endocrine unbalances [7,8]. Most of the studies conducted up to this date, however, have documented high anxiety levels to be related with negative gestational health outcomes [5,9, 10]. To our knowledge, it is yet unclear whether pregnant women subjected to moderate anxiety develop a state of endocrine deficiency and compromised immunological tolerance as that described for pregnant women living under high anxiety levels. Here, we evaluated this issue by monitoring endocrine and immune parameters during pregnancy and the impact of moderate anxiety on the pregnancy outcome in a sample of pregnant women.
2. METHODS
2.1. Participants
This pilot study was conducted in a total of ten women (17 - 33 years old) having moderate anxiety. Volunteers were primiparous, mestizo, literate middle class pregnant women (n = 10; Table 1) attending to prenatal care. The physical exploration and routine laboratory analyses revealed no abnormalities or underlying pathologies. Family status, pregnancy outcome and pre/perinatal health were followed and/or supervised. All the participants signed the corresponding informed consent and the protocols used for sample withdrawing and handling were approved by the Ethics Committee at the Hospital Escuela, Universidad Veracruzana and concurred with the Norma Oficial Mexicana [11], section NOM-007-SSA2- 1993.
2.2. Anxiety Evaluation
The magnitude of anxiety was scored by using the state-trait anxiety inventory (STAI) [12] http://www.mhs. com/product.aspx?gr=cli&prod=stai&id=overview#). This inventory has been used extensively in basic neuropsychological research, clinical settings and cross-cultural studies [10,13]. Recently it was adapted to the obstetric population to measure subjective feelings of tension, apprehension, nervousness and worries [14]. STAI measures both state and trait anxiety by assessing how subjects feel by the time of testing as well as how they felt at a particular time in the past. The inventory consists of 40 items presented as a Likert scale of almost—never to almost—always. In our case, women were asked to fill out the questionnaires with no time limit, working in undisturbed classroom within the hospital around 10 o’clock after breakfast. The questionnaire was applied when women first arrived to the hospital seeking for medical care then re-applied at 12 (first trimester) and 38 (third trimester) weeks of pregnancy. Anxiety scores were compared between the first and third trimesters taking the former as the reference value, since scores between the first (upon arrival to the hospital) and the second application (by the first trimester) did not differ. Statistical differences between first and third trimester anxiety scores were explored by using one way ANOVA tests (p < 0.05).
2.3. Hormonal Profiling
A progressive increase of cortisol, estradiol and progesterone serum availability is required for maintaining pregnancy [2-6,15,16]. During gestation, however, ab-

Table 1. Main description of 10 female participants.
normal rises of cortisol serum levels may down regulate estradiol and progesterone thus increasing the risk of abortion or premature delivery [17,18]. High anxiety is frequently associated with increased cortisol serum levels [18]. However, the relationship between progestational hormone levels and moderate anxiety in pregnant women has not been assessed before. We then determined cortisol, estrogen and progesterone serum levels (blood samples were withdrawn between 8:00 and 9:00 a.m.) in pregnant women living under moderate anxiety at 12, 27 - 29 and 38 - 40 weeks of pregnancy by clinical conventional means and standard protocols. The serum obtained was used to determine hormonal concentrations (ng/ml) by ELISA following the protocol provided by the supplier (Sensitivity 0.11 µg/dl and intra-assay variability coefficient <3 percent; VITROS Kit; OrthoClinical Diagnostics; Johnson & Johnson). Temporal differences of hormonal concentrations were assessed by using repeated measures one-way ANOVA tests (p < 0.05).
2.4. Cytokine Profiling
Experimental evidence suggests that sources different from the placenta, likely maternal leukocytes, release cytokines that mediate inflammatory states during high risk pregnancies [19], including those subjected to high anxiety levels [7]. Since no previous work has studied the immunological profile of pregnant women living under moderate anxiety, we evaluated levels of mRNA expression of pro-(INF-γ, TNF-α, IL-12) and anti-inflammatory (TGF-β, IL-4, IL10) cytokines in peripheral leukocytes. Lymphocyte mRNA was extracted by using the RNAeasy Mini Kit following the protocol recommended by the supplier (QIAGEN). The integrity, purity and concentration of mRNA was estimated following conventional protocols having as an internal control actin mRNA [20]. Then, cDNA was obtained [21] and used to amplify cytokine genes by multiple PCRs (37 cycles, aligning temperature 59˚C) following the protocol provided by the manufacturer (Maximbio). Densitometric, semi-quantitative analyses (LabWorks 4.5) of the amplified products relative to GADPH expression were then carried out using digitized photographs (Bio Doc It; UVP Co., USA) of ethidium bromide stained polyacrylamide gels. We then pulled together, summed up and averaged intensity values of all cytokines per cytokine group analyzed for each trimester. Although we recognized that this manipulation masks cytokine individual variations along pregnancy, it provides a global, easy to compare and interpret, picture on the behavior of the proand anti-inflammatory immunological profiles throughout pregnancy. The average values referred above were also used to estimate the anti-/pro-inflammatory cytokine ratio (i.e., Th2/Th1 response balance; for a similar approach see [17]). Statistical differences of both parameters over time were tested by using repeated measures one-way ANOVA tests (p < 0.05).
2.5. Additional Statistical Analyses
To investigate possible temporal correlations among all parameters measured, we used Pearson’s correlative analyses (p < 0.05).
3. RESULTS
As shown in Figure 1(a), pregnant women with moderate anxiety increased their state anxiety as pregnancy progressed. In spite of these change, cortisol and progesterone serum concentrations increased in the course of pregnancy (Figure 1(b)). In contrast, estradiol serum levels remained relatively constant during gestation (Figure 1(b)). Moderate anxiety did not modify antiand pro-inflammatory cytokine mRNA profile expression during pregnancy (Figure 1(c)). With the exception of gestational antiand pro-inflammatory cytokine profile patterns (Pearson’s correlation coefficient 0.79 at p < 0.05), no interaction among anxiety, endocrine and immune variables were found.
Table 2 shows pregnancy complications and outcomes developed by the mothers. Seven out of ten of these women developed complications throughout pregnancy or labor. Four out of these seven women acquired urinary tract infections; two had hypertensive states, two presented premature labor and one an upper respiratory tract
 (a)
(a)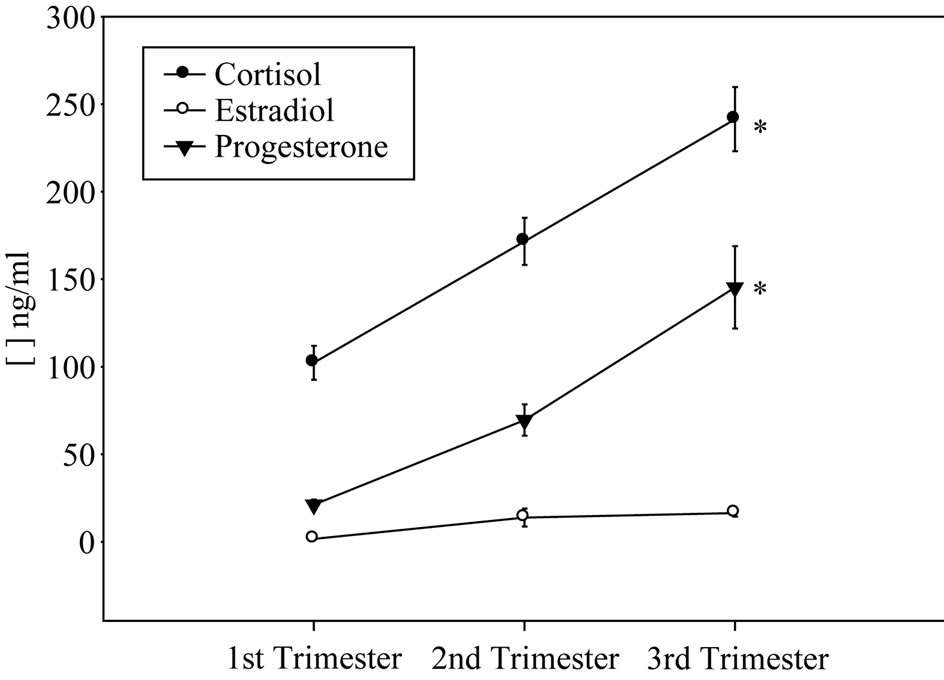 (b)
(b)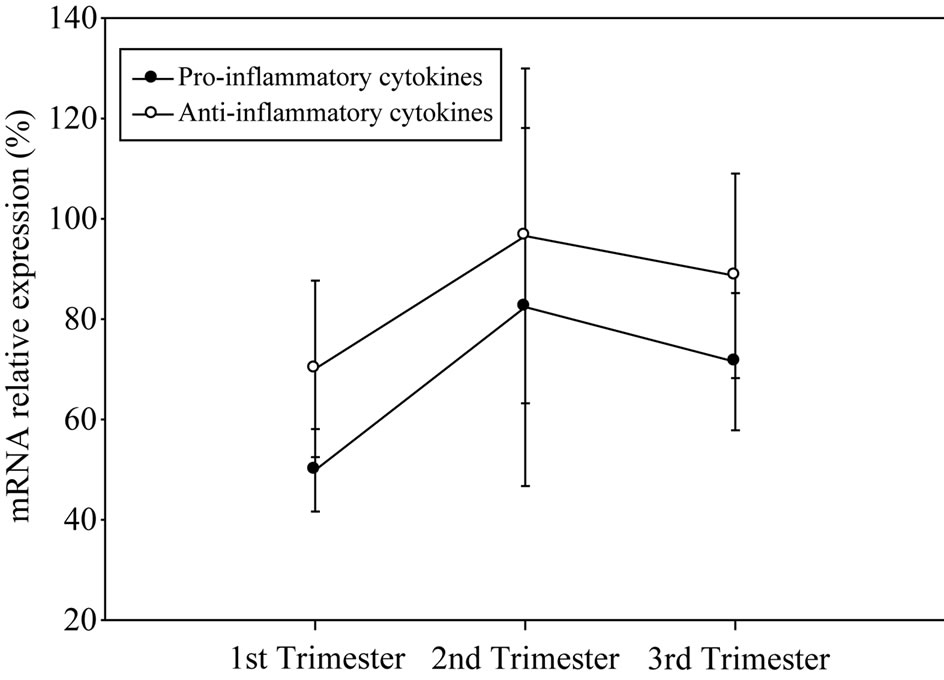 (c)
(c)
Figure 1. Anxiety scores (a), cortisol, progesterone and estradiol (b) serum levels and anti-/pro-inflammatory mRNA cytokine expression (c) in pregnant women showing moderate anxiety during pregnancy.
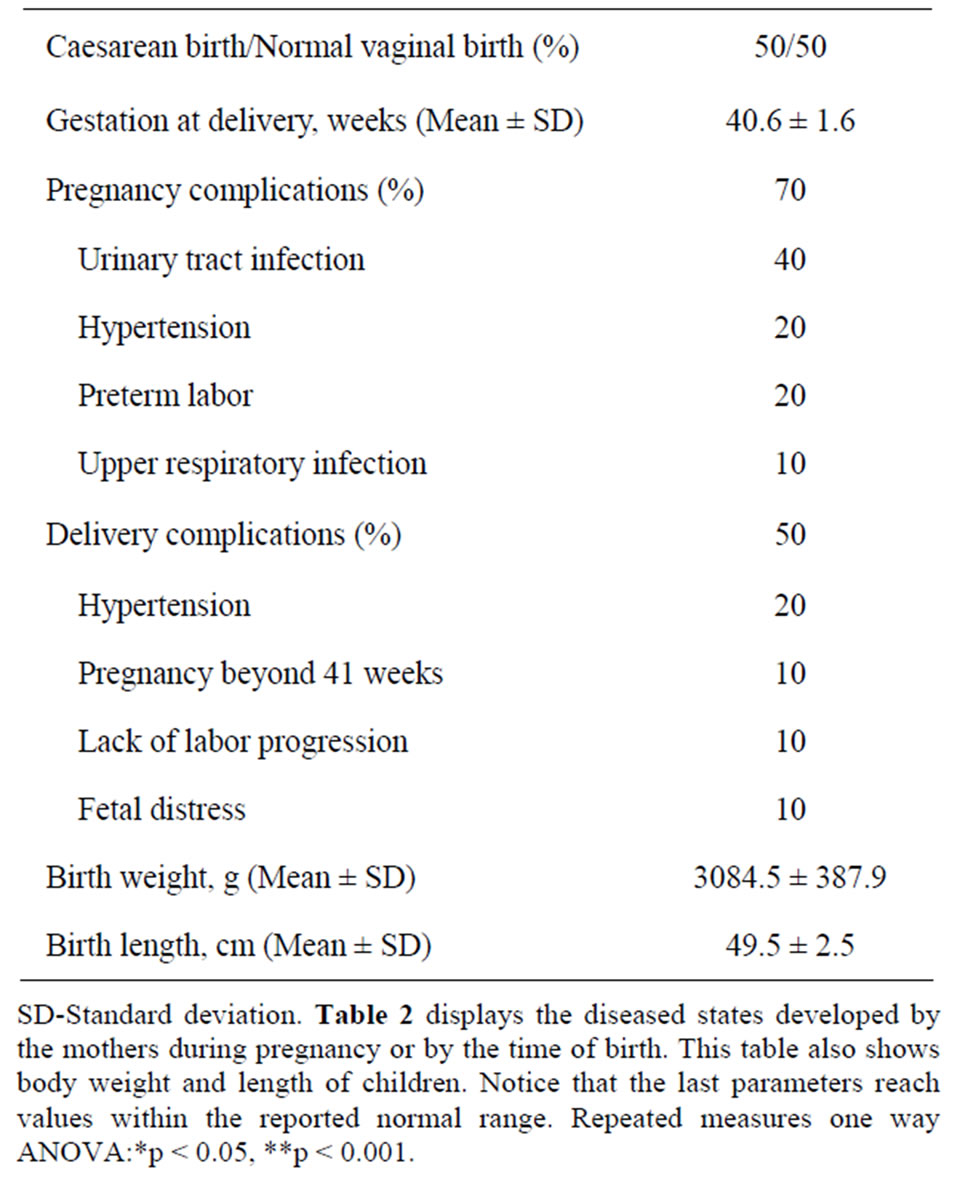
Table 2. Pregnancy and birth outcome details of ten the pregnant women.
infection. Interestingly, the women with urinary tract infection also presented hypertensive conditions and premature labor. Also, half of the participants-all of them developed pregnancy complications-had the highest anxiety scores (not shown) and were all subjected to cesarean sections. In spite of these pathophysiological findings, body weight and length of the children delivered by this group of women were normal (Table 2).
4. DISCUSSION
High anxiety during the course of gestation leads to a variety of clinical complications by the time of birth. Such deleterious effects have been attributed to endocrine deficiencies and reduced immune tolerance. However, another psychological condition that could affect pregnant women is moderate anxiety. To our knowledge, it is yet unclear whether pregnant women subjected to moderate anxiety develop a state of endocrine deficiency and compromised immune tolerance comparable to those observed in pregnant women with high anxiety levels. In this work we approached this issue by carrying out a preliminary study in a cohort of pregnant women having moderate anxiety. Our results show that even when moderately anxious women increased their anxiety scores as pregnancy progressed, overall endocrine and immune parameters behave as reported for women ongoing a normal pregnancy [22-26]. In our study moderate anxiety did not associated with a compromised gestational immune-endocrine status in contrast to that reported in pregnant women with high anxiety levels. It is interesting, however, that pregnant women in our study developed gestational and perinatal complications by the end of pregnancy, suggesting that moderate anxiety renders women to be susceptible to developed diseased states. Although the mechanism is currently uncertain, it may be that the lack of the gestational rising of estradiol serum levels observed. Indeed, the women studied increased their anxiety levels and developed hypertensive conditions, infectious diseases, preterm or delayed labor or lack of labor progression by the end of gestation. Estradiol is known to reduce anxiety [27,28], is a potent progestational hormone [29], an immune modulator [30] and a regulator of uterine and blood vessel contraction [7]. Hence, future studies must address this relationship.
Another interesting observation was that moderate anxiety did not associate with child’s low body weight or short length at birth. What makes the embryos/fetus somehow resistant to the effects of moderate anxiety? Once again, the answer is yet unclear. Nevertheless, it may be that the increments of cortisol and progesterone serum levels and the maintenance of an adequate balance of pro/anti-inflammatory cytokines during pregnancy may suffice to procure a pro-gestational state in moderately anxious women. In addition, since estradiol can also stimulate pro-inflammatory processes under certain circumstances [7,17], a reduction of estradiol serum levels in pregnant women suffering of moderate anxiety could also be protective. Future experiments must address this important issue.
A surprising result was that maternal leukocyte antiand pro-inflammatory cytokine mRNA expression did not show variations along pregnancy in spite of the fact that anxiety levels and progesterone and cortisol serum concentrations increased significantly. Commonly, during implantation and labor the placenta releases pro-inflammatory cytokines that are counteracted by increments in anti-inflammatory cytokines secreted by maternal leukocytes and the placenta itself as pregnancy proceeds. This last response appears to depend upon increments of cortisol, progesterone and estradiol [7,8,17]. As the date of birth approaches cortisol increases further, pro-inflammatory cytokines also increase whereas progesterone and estradiol drop, a combination that precipitates labor [7,14,17,18]. So, it is puzzling that women included in our study did not modify their leukocyte cytokine expression during the course of their pregnancy. One possibility to explain this finding is that the code needed to trigger shifts of anti-/pro-inflammatory cytokine expression in maternal leukocytes heavily depends upon estrogen increments, a condition that was not present in pregnant women suffering of moderate anxiety levels. A second possibility is that the participation of cytokines released by maternal leukocytes is minor during the response of the immune system to moderate anxiety levels. Thirdly, moderate anxiety levels might decouple immune-endocrine relationships thus protecting the embryo/fetus, but certainly not the mother. Decoupling, however, might also be a sign that foresees worse things to come if anxiety levels are kept moderate or high for a longer time. Upcoming studies must sort out the merits of each of these possibilities.
5. CONCLUSIONS
Moderate anxiety in pregnant women associated with increments of anxiety levels, serum cortisol and progesterone, but no increase of estradiol, as pregnancy progressed. In addition, no variations of leukocyte antiand pro-inflammatory cytokine mRNA expression were found along pregnancy. However, these pregnant women presented an increased frequency of gestational and perinatal complications, conditions that had no major consequences for their health and that of their children at birth.
Consequently, pregnant women with moderate anxiety are more susceptible to develop complications during or by the end of pregnancy, while keeping the endocrine milieu and immune tolerance reasonably in place.
6. ACKNOWLEDGEMENTS
Authors thank the medical personnel of the Hospital Escuela, Universidad Veracruzana (UV), who assisted during the clinical procedures aimed at withdrawing and processing biological samples. Also we are grateful to Rocío Banderas by providing help during test application and to Warren Haid who assisted in the preparation, editing and proofreading of the manuscript. GGO has been awarded with visiting professor mini-grants provided for four years by the Coordinación de la Investigación Científica, Universidad Nacional Autónoma de México and the Departamento de Intercambio y Colaboración Académica de la Dirección de Desarrollo Académico de la Universidad Veracruzana. Funding was provided by PROMEP/103.5/07/2753 (PTC-262), Secretaría de Educación Pública, by the Instituto de Investigaciones Biológicas (UV) and by Hospital Escuela (UV).
REFERENCES
- Morbidity and Mortality Weekly Reports (1999) Preterm singleton births: United States 1989-1996. Morbidity and Mortality Weekly Reports, 48, 185-189.
- Senties, M. and Ortiz, G. (1993) Evaluación de los niveles de ansiedad y el estado emocional en mujeres embarazadas de bajo nivel socioeconómico. Psicología y salud Nueva Época, 2, 47-54.
- Rodríguez, J. (1995) Psicología social de la salud. Editorial Síntesis, S. A., Madrid.
- Pimentel, B. (2007) Ansiedad, depresión y funcionalidad familiar en embarazo de alto riesgo obstétrico en el hospital materno infantil de la C.N.S, La Paz-Bolivia. Revista Paceña de Medicina Familiar, 4, 15-19.
- Orr, S.T., Reiter, J.P., Blazer, D.G. and James, S.A. (2007) Maternal prenatal pregnancy-related anxiety and spontaneous preterm birth in Baltimore, Maryland. Psychosomatic Medicine, 69, 566-570. doi:10.1097/PSY.0b013e3180cac25d
- Teixeira, C., Figueiredo, B., Conde, A., Pacheco, A. and Costa, R. (2009) Anxiety and depression during pregnancy in women and men. Journal of Affective Disorders, 119, 142-148. doi:10.1016/j.jad.2009.03.005
- Ruiz, R.J., Fullerton, J. and Dudley, D.J. (2003) The interrelationship of maternal stress, endocrine factors and inflammation on gestational length. Obstetrical & Gynecological Survey, 58, 415-428. doi:10.1097/01.OGX.0000071160.26072.DE
- Arck, P., Hansen, P., Jericevic, B. M., Piccini, M.P. and Szekeres-Bartho, J. (2007) Progesterone during pregp nancy: endocrine-immune cross talk in mamalian species and the role of stress. American Journal of Reproductive Immunology, 58, 268-279. doi:10.1111/j.1600-0897.2007.00512.x
- Diego, M., Jones, N., Field, T., Hernandez-Reif, M., Schanberg, S., Kuhn, C. and Gonzalez-Garcia, A. (2006) Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosomatic Medicine, 68, 747-753. doi:10.1097/01.psy.0000238212.21598.7b
- Rini, C.K., Dunkel-Schetter, C., Wadhwa, P.D. and Sandman, C.A. (1999) Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context in pregnancy. Health Psychology, 18, 333-345. doi:10.1037/0278-6133.18.4.333
- Diario Oficial de la Federación (1995) NOM-007- SSA2-1993.
- Spielberg, C., Gorsuch, R. and Lushene, R. (1968) State Trait Anxiety Inventory: Preliminary test manual form X. Florida State University, Tallahassee.
- Spielberger, C.D. (1983) The state-trait anxiety inventory for adults sampler set manual, test, scoring, key. Mind Garden, Menlo Park.
- Morales, F. and González, G. (1990) Normalización del Instrumento de Ansiedad (IDARE) en Mujeres Embarazadas. Revista Mexicana de Psicología, 7, 75-80.
- Carr, B., Parker, C., Madden J., McDonald, P. and Porter, J. (1981) Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. American Journal of Obstetrics and Gynecology, 139, 416- 422.
- Lustyk, M., Olson, K., Gerrish, W., Holder, A. and Widman, L. (2009) Psychophysiological and neuroendocrine responses to laboratory stressors in women: Implications of menstrual cycle phase and stressor type. Biological Psychology, 83, 84-92. doi:10.1016/j.biopsycho.2009.11.003
- Matalka, K. and Ali D. (2005) Stress-induced versus preovulatory and pregnancy hormonal levels in modulating cytokine production following whole blood stimulation. Neuroimmunomodulation, 12, 366-374. doi:10.1159/000091130
- Field, T. and Diego, M., (2008) Cortisol: The culprit prenatal stress variable. International Journal of Neuroscience, 118, 1181. doi:10.1080/00207450701820944
- Benyo, D.F., Smarason, A., Redman, C.W., Sims, C., Conrad, K.P. (2001) Expression of inflammatory cytokines in placentas from women with preeclampsia. Journal of Clinical Endocrinology & Metabolism, 86, 2505- 2512. doi:10.1210/jc.86.6.2505
- Wilfinger, W.W., Mackey, M. and Chomczynski, P. (1997) Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques, 22, 474.
- Kleiboeker, S.B. (2003) Applications of competitor RNA in diagnostic reverse transcription-PCR. Journal of Clinical Microbiology, 5, 2055-2061. doi:10.1128/JCM.41.5.2055-2061.2003
- Figuero-Ruiz, E., Prieto, I. and Bascones-Martínez, A. (2006). Cambios hormonales asociados al embarazo. Afectación gingivo-periodontal. Avances en Periodoncia, 18, 101-113.
- Arck, P., Hansen, P., Jericevic, B.M., Piccini, M.P. and Szekeres-Bartho, J. (2007) Progesterone During Pregnancy: Endocrine-Immune Cross Talk in Mamalian Species and the Role of Stress. American Journal of Reproductive Immunology, 58, 268-279. doi:10.1111/j.1600-0897.2007.00512.x
- Chan, J., Rabbitt, E.H., Innes, B.A., Bulmer, J.N., Stewart, P.M., Kilby, M.D. and Hewison, M. (2007). Glucocorticoid-induced apoptosis in human decidua: A novel role for 11beta-hydroxysteroid dehydrogenase in late gestation. The Journal of Endocrinology, 195, 7-15. doi:10.1677/JOE-07-0289
- Chang, K. and Zhang, L. (2008). Steroid hormones and uterine vascular adaptation to pregnancy. Reproductive Sciences, 15, 336-348. doi:10.1177/1933719108317975
- Gambino, Y.P., Maymó, J.L., Pérez-Pérez, A., Dueñas, J.L., Sánchez-Margalet, V., Calvo, J.C. and Varone, C.L. (2010). 17-Beta-estradiol enhances leptin expression in human placental cells through genomic and nongenomic actions. Biology of reproduction, 83, 42-51. doi:10.1095/biolreprod.110.083535
- Rodriguez-Sierra, J.F., Howard, J.L., Pollard, G.T. and Hendricks, S.E. (1984) Effect of ovarian hormones on conflict behavior. Psychoneuroendocrinology, 9, 293-300. doi:10.1016/0306-4530(84)90008-8
- Gallo, M.A. and Smith, S.S. (1993). Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: A possible rat model of PMS anxiety. Pharmacology Biochemistry & Behavior, 46, 897- 904. doi:10.1016/0091-3057(93)90219-J
- Rodriguez-Sierra, J.F., Howard, J.L., Pollard, G.T. and Hendricks, S.E. (1984) Effect of ovarian hormones on conflict behavior. Psychoneuroendocrinology, 9, 293-300. doi:10.1016/0306-4530(84)90008-8
- Stites, D.P. and Siiteri, P.K. (1983). Steroids as immunosuppressants in pregnancy. Immunological Reviews, 75, 117-138. doi:10.1111/j.1600-065X.1983.tb01093.x
NOTES
*Corresponding author.

