Agricultural Sciences
Vol.3 No.4(2012), Article ID:20168,5 pages DOI:10.4236/as.2012.34067
Zinc chemical forms and organic acid exudation in non-heading Chinese cabbages under zinc stress
![]()
College of Resources and Environment, Shandong Agricultural University, Taian, China; *Corresponding Author: xiumincui@sdau.edu.cn
Received 3 March 2012; revised 9 April 2012; accepted 16 May 2012
Keywords: Non-Heading Chinese Cabbages; Zn Stress; Chemical Forms; Root Exudation
ABSTRACT
As an essential element, zinc also is a heavy metal. Non-heading Chinese cabbage showed obvious tolerance to Zn stress in former research. To further understand the mechanisms involved in Zn adaptability and detoxification, two genotypes Suzhouqing and Aijiaohuang were selected to investigate the chemical forms of Zn and root exudation. Zinc stress obvious strained the plant growth, and Aijiaohuang was more injured than Suhouqing under Zn stress. Under normal Zn levels, the chemical forms of Zn were diverse in three organs between genotypes. Results showed extractions of 2% HAc, 80% ethanol and 1 M NaCl were separately dominant in roots, petioles and leaves. However, under Zn stress (13 mg·L–1 and 52 mg·L–1) most of the Zn was extracted by 1M NaCl, and the subdominant amount of Zn was extracted by 80% ethanol. In the control only four types of organic acid could be detected. While under Zn stress, oxalic acid, tartaric acid, malic acid, lactic acid, acetic acid, citric acid and amber acid were all detected, so it could be speculated Zn detoxification with organic ligands or integrated with pectates and proteins in cells might be responsible for the adaptation of Zn stress in Chinese cabbage.
1. INTRODUCTION
Non-heading Chinese cabbge (Brassica campestris ssp. chinensis Makino), is one of the most popular leaf vegetables, widely cultivated in north and south China. As an essential element, zinc is also a heavy metal. In recent years, unreasonable emissions of zinc galvanized Industrial waste, such as zinc mining, smelting and processing which led to extraordinary accumulation of zinc in the soil. Zinc in contaminated soil was easily absorbed by plants, which easily caused zinc toxicity. Subsequently, agricultural products may be contaminated and through the food chain human health will be threatened [1-3]. Heavy metal pollution has caused worldwide concern. This program was to investigate chemical form of zinc and the root exudation in non-heading Chinese cabbage under Zn stress, which provides some theoretical evidence for Zn tolerance mechanisms in plants.
2. MATERIALS AND METHODS
2.1. Plants Materials
The experiments were carried out in hydroponic trial. Two genotypes Suzhouqing and Aijiaohuang were selected as plant materials. The seeds were sterilized by 0.3% NaClO for 10 min, washed by ditilled water H2O and germinated at 25˚C. The germinating seeds were then sown in vermiculite. After sprouting, the seedlings were irrigated by half-strength nutrient solution.
When the seedlings grew to five true leaves, the most uniform and healthy seedlings were chosen to transport into plastic pots, covered by 3 cm thick foam. A week later, the seedlings were supplied with Hoagland nutrient solution, which was renewed every two days.
After pre-cultivation for one week, the seedlings were treated with Zn stress. Zn2+ (ZnSO4·7H2O) concentration was 0.43 mg·L–1 (normal level), 13 mg·L–1 (mildly stress), 52 mg·L–1 (highly stress), recorded as CK, Zn1, Zn2. The experiments were arranged in a randomized block design with three replicates. The pH of nutrient solution was then adjusted to 5.5 ± 0.2 with KOH or HCl (low concentration). During the period, the enviromental conditions 32/15˚C (day/night) with a 12:12 h (light: dark). Treated with Zn stress for 12 days, the plants were harvested for analysis.
2.2. Chemical Forms Extraction
To determine chemical forms of Zn in Chinese cabbage, the experiment was carried out by designated solution in the following order [3,4]: 1) 80% ethanol, extracting inorganic Zn giving priority to nitrate/nitrite, chloride, and aminophenol Zn. 2) Dual distilled water (ddH2O), extracting water soluble Zn-organic acid complexes and Zn(H2PO4)2. 3) 1.0 mol·L–1 NaCl, extracting pectates and protein integrated Zn. 4) 2% acetic acid (HAC), extracting undissolved zinc phosphate including ZnHPO4 and Zn3(PO4)2 and other Zn-phosphate complexes. 5) 0.6 mol·L–1 HCl, extracting zinc oxalate. The above extractants were recorded respectively as: FE, FH2O, FNaCl, FHAC, FHCl.
Fresh tissues were homogenized in extraction solution with a mortar and a pestle, diluted at the ratio of 1:100 (w/v), and vibrating for 22 hours at 25˚C. Afterwards the homogenate was centrifuged at 5000 g for 10 min, obtaining the first supernatant solution in a conical beaker. The sedimentation was suspended twice in extraction solution and shaken for two hours at 25˚C, centrifuged at 5000 g for 10 minutes. Then pooled the supernatant of the three suspending and centrifuge steps for each of the five extraction solutions. The residue was recorded as FR., and each of the pooled supernatant solutions were then evaporated on an electric plate at 70˚C to constant weight. The above six parts were then digested at 145˚C with an acid oxidative mixture of HNO3:HClO4 (2:1, v/v). Atomic absorption spectrometry (AA 370 MC) were used to test Zn content.
2.3. Root Exudates Collection
Root exudates were collected as described by Rengel [5]: The roots were first rapidly washed with ddH2O to remove the surface adhesion from nutrient solution. The plants were then transferred into 20 ml ddH2O, meanwhile all the roots should be assured to submerge in the water. After four hours the whole plants were moved away, and some thymols were added to the remaining solutions (to cease the activity of microorganism), and then condensed to 5 ml by a vacuum rotary evaporator at 40˚C.
The organic acids were analyzed using High Performance Liquid Chromatography (HPLC). The samples were filtered through 0.45 μm membrane to remove impurities.
The chromatograph conditions were as followed: mobile phase KH2PO4, flow rate 0.9 ml·min–1, detection wavelength 213 nm, sensitivity 0.8, manual injection volume 10 μl. The target time for the standard solutions (from Sigma Company) was 20 minutes, and 50 minutes for the exudate samples.
Least significant difference (LSD) was used for multiple comparisons between different treatment means.
3. RESULTS AND ANALYSIS
As a trace element, excessive Zn in media would lead to phytotoxicity. After Zn stress for 12 days, Suzhouqing and Aijiaohuang showed signs of chlorisis, and were withered along the leaf margin, although still alive. Seen from Table 1, Zn stress restrained the plant growth, and obviously decreased the biomass of leaves, petioles and roots. Compared with CK, the shoot biomass of Suzhouqing and Aijiaohuang (including the leaves and petioles) decreased 44.4% and 59.0% respectively under Zn1 stress. The root biomass of Suzhouqing was remarkablely higher than that of Aijiaohuang, compared with CK, Suzhouqing and Aijiaohuang respectively decreased 44.4% and 52.63% under Zn1 stress. Under Zn2 stress, the toxicity symptoms were much more visible, and the total biomass of Suzhouqing and Aijiaohuang reduced 66.5% and 84.8%, which indicated Aijiaohuang had sustained more injury under higher Zn stress.
3.1. The Zn Chemical Forms in Plants under Zn Stress
As Table 2 showed, Zn content extracted by different extractants were markedly diverse in different organs. Zinc stress led to zinc content obviously rise in nonheading Chinese cabbage. The Zn form extracted by NaCl was predominant in leaves, stems and roots.
In leaves of Suzhouqing, the Zn form extracted by NaCl were increased from 1.90 mg·kg–1 (CK) to 42.43 mg·kg–1 (Zn2), almost up to 20 times. The following chemical form was extracted by 80% ethanol, the highest concentration under CK level. Then the extraction of H2O, was much higher than FHAC, FR , FHCl, which were

Table 1. The biomass of non-heading Chinese cabbage under zinc stress (mg·plant−1, DW).

Table 2. The chemical forms of Zn in non-heading Chinese cabbage under Zn stress (mg·kg−1).
rather low. Under control conditions, Zn forms extracted by 80% ethanol and ddH2O were the predominant, respectively representing 35.30% and 27.35% of the total amount. Under Zn stress, the Zn form extracted by NaCl and 80% ethanol were predominant form.
In petioles of Suzhouqing, the extraction of 1 M NaCl was always much higher than other forms regardless of stress. As followed was extraction of 80% ethanol, ddH2O, and 2% HAc. Zinc stress made the above four chemicals sharply increase, and the other forms ranged a little. Most forms in petioles that were extracted by NaCl and ethanol, accounted for 55.87% and 21.16% of the total amount respectively.
In roots of Suzhouqing, Zn form extracted by 1 M NaCl were increased from 11.07 mg·kg–1 (CK) to 193.2 mg·kg–1 (Zn2), almost up to 18 times. As followed was extracted by 2% HAc, the highest concentration at CK level, increased three times under Zn2 stress. The most diverse form was extraction of 1 M NaCl, the following was extraction of ddH2O, which were more sensitive to Zn stress. This indicated that they might play more important role in tolerance faced Zn stress. Differently from the aboveground, under CK level in roots extraction of 2% HAc was the highest, then extraction of 80% ethanol, and accounted for 33.08% and 21.58% of the total amount.
Zinc stress enabled the roots to take up and accumulate much more Zn than the leaves and petioles. The similar trend was obtained in Aijiaohuang.
3.2. The Organic Acids in Root Exudates of Non-Heading Chinese Cabbage under Zn Stress
Seven types of low molecular weight organic acids were detected in this trial: oxalic acid, tartaric acid, succinic acid, malic acid, citric acid, acetic acid and lactic acid. The secretion of each plant ranged from 569.3 μg to 1709 μg. Under the control level, four kinds of organic acids were detected in Suzhouqing: oxalic acid, tartaric acid, malic acid and succinic acid. Among them, the oxalic acid was the most, followed the tartaric acid and malic acid, succinic acid the least. Similarly, also four types of organic acids were detected in Aijiaohuang, different from the former, malic acid replaced of lactic acid (Table 3).
Under the different degree of Zn stress for 12 days, the composition and content of organic acids changed. Compared with the control, Zn1stress enhanced two kinds of organic acids (acetic acid and citric acid) in Suzhouqing, however, the total content of organic acids detected decreased by 50.32%. Under Zn2 stress, seven types of organic acids could be detected but the total amount of the organic acids increased by 49.15%. For Aijiaohuang, only four organic acids were identified in control, while
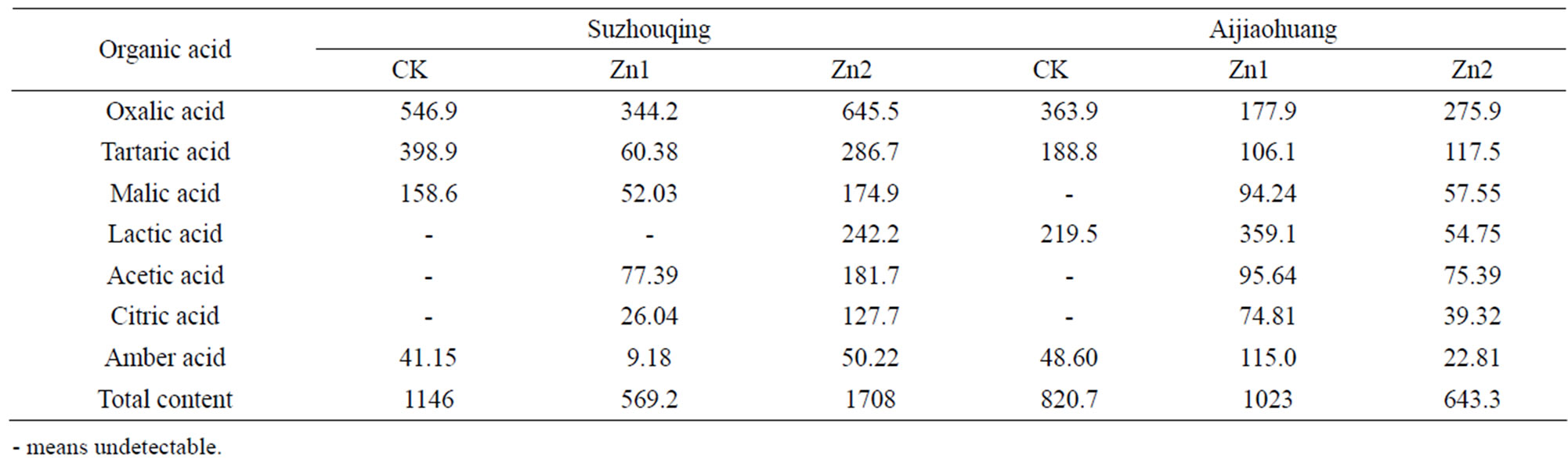
Table 3. The organic acid exudated in non-heading Chinese cabbage under Zn stress (ug·plant−1).
under Zn1 and Zn2 stress, the other three types like malic acid, citric acid and acetic acid could even be detected. The total amount of organic acids under Zn1 stress were increased by 24.61% than the control, however they reduced by 21.63% at Zn2 level.
4. DISCUSSION
Though the biomass of non-heading Chinese cabbage was decreased by Zn stress, they were still alive, which indicates some tolerant mechanism is produced. Chemical speciation of heavy metals is closely related to their biological function, and different chemical forms of Zn extracted by different designated extraction solutions have distinct toxicity degrees and migration of Zn. For instance, inorganic and organic water-soluble Zn (extracted by 80% ethanol and ddH2O, respectively), with higher capacity to migrate, is more deleterious to plant cells than the undissolved Zn phosphate (extracted by 2% HAc) and zinc oxalate (extracted by 0.6 M HCl) [6]. Meanwhile, for plants containing high concentration of Zn that showed no or little toxicity, Zn should be in a chemical form that causes low or no phytotoxicity [7,8]. In our study, Zn existed in different chemical forms among different organs.
Under control conditions the Zn form extracted by ethanol in leaves has the highest concentration, followed by NaCl in petioles and by HAc in root (Table 1). The majority of Zn was in inorganic form suggesting high capacity to be transported to leaves (Table 2), most Zn was integrated with pectates and protein (extracted by 1 M NaCl) in petioles, in roots the majority of Zn was not dissolved Zn phosphate and other Zn-phosphate complexes.
Zn was hypothesized to be chelated by some specific polar material, such as hydroxyl or carboxyl, to form a non-toxic complex under Zn stress. It may also be assumed that higher concentration and larger percentages of NaCl-extractable Zn in organs were responsible for the adaptation of Chinese cabbage to Zn stress, which stands for the point of view that compartmentation of vacuolar and sequestration in cell wall may be crucial for the detoxification of Zn and thus tolerance to metal stress [4].
Exudating organic compounds to the external environment was an adaptive response in plants. Under heavy metal stress, plant roots promote the secretion of citric acid, succinic acid, malic acid and other organic acids to alleviate heavy metal toxicity [9,10]. Under Al stress, buckwheat roots secreted large amounts of oxalic acid and combined with Al3+ to form oxalic acid aluminum compound to reduce the Al toxicity on roots [11]. Furthermore under Zn stress, different genotypic Chinese cabbage released oxalic acid, tartaric acid, malic acid and other organic acids respectively [12]. The above results indicated that the non-heading Chinese cabbage under Zn stress secreted more oxalic acid, tartaric acid, malic acid, lactic acid, acetic acid, citric acid and succinic acid than the control. The variety of organic acid between Suzhouqing and Aijiaohuang were mainly citric acid, malic acid, acetic acid and lactic acid, which perhaps was one of the reasons that Zn absorption and tolerance varied significantly.
Organic acids could ligate metal ion into chelate complex, and participate compartment in vacuole in order to reduce excessive ion toxicity. Under Zn stress, plant secretions of large quantities of organic acids was one of the resistance mechanisms [7,13]. Therefore, we speculated that similar mechanism also existed in non-heading Chinese cabbage.
5. ACKNOWLEDGEMENTS
This work was supported by Sci-Tech Development Project of Taian city (32606) and China Agriculture Research System (CARS-25-D).
REFERENCES
- Bi, X.Y., Feng, X.B., Yang,Y.G., Qiu, G., Li, G., Li, F., Liu, T., Fu, Z. and Jin, Z. (2006) Environmental contamination of heavy metals from zinc smelting areas in Hezhang County, western Guizhou, China. Environment International, 32, 883-890. doi:10.1016/j.envint.2006.05.010
- Broadley, M.R., White, P.J., Hammond, J.P., Zelko, I. and Lux, A. (2007). Zinc in plants. New Phytologist, 173, 677-702. doi:10.1111/j.1469-8137.2007.01996.x
- Zhou, S.B., Xu, L.S., Wu, L.H., Li, Y.M., Li, N. and Cui, L.Q. (2008) Subcellular distribution and chemical forms of Cd and Zn in Sedum jinianu. Chinese Journal of Applied Ecology, 19, 2515-2520.
- Fu, X.P., Dou, C.M., Chen, Y.X., Chen, X.C., Sh, J.Y. and Yu, M.G. (2011) Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. Journal of Hazardous Materials, 186, 103-107. doi:10.1016/j.jhazmat.2010.10.122
- Rengel, Z., Romheld, V. and Marschner, H. (1998) Uptake of zinc and iron by wheat genotypes differing in tolerance to zinc efficiency. Journal of Plant Physiology, 152, 433-438. doi:10.1016/S0176-1617(98)80260-5
- Wu, Q., Du, S.J., Zeng, X.W., Fang, X.H., Yu, F.M. and Qiu, R.L. (2006) Subcellular distribution and chemical forms of Potentilla grifithii Hook. Ecology and Environment, 15, 40-44.
- Chen, Y.X. (2008) Plant contaminated chemistry of heavy metal in soil. Science China press, Beijing.
- Calhôa, C.F., Monteiro, M.S., Soares, A.M. and Mann, R.M. (2011) The influence of metal speciation on the bioavailability and sub-cellular distribution of cadmium to the terrestrial isopod, Porcellio dilatatus. Chemosphere, 83, 531-537. doi:10.1016/j.chemosphere.2010.12.055
- Pellet, D.M., Papemik, L.A. and Kochian, L.V. (1996) Multiple aluminum resistance mechanisms in wheat, the roles of root apical phosphate and malate exudation. Plant Physiology, 112, 591-597.
- Jones, D.L. (1998). Organic acids in the rhizosphere—a critical review. Plant and Soil, 205, 25-44. doi:10.1023/A:1004356007312
- Ma, J.F., Zheng, S.J. and Matsumoto, H. (1997) Specific secretion of citric acid induced by Al stress in Cassia tora L. Plant and Cell Physiology, 38, 1019-1025. doi:10.1093/oxfordjournals.pcp.a029266
- Xu, W.H., Liu, J.Z., Huang, H. and Xiong, Z.T. (2006) Study of Zn stress on plant growth, zn uptake and root exudates in different cultivars of Chinese cabbage. Chinese Agricultural Science Bulletin, 22, 458-463.
- Lombi, E., Zhao, F.J., Dunham, S.J. and McGrath, S.P. (2000) Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytologist, 145, 11-20. doi:10.1046/j.1469-8137.2000.00560.x

