Food and Nutrition Sciences
Vol. 2 No. 10 (2011) , Article ID: 16359 , 8 pages DOI:10.4236/fns.2011.210153
Phytochemical Compositions, Antioxidant Properties, and Colon Cancer Antiproliferation Effects of Turkish and Oregon Hazelnut
![]()
Agricultural Research Station, Virginia State University, Petersburg, USA.
Email: *jparry@vsu.edu
Received July 30th, 2011; revised September 20th, 2011; accepted September 28th, 2011.
Keywords: Hazelnut, Antioxidant, Free Radical Scavenging, ORAC, DPPH, TPC, HT-29
ABSTRACT
Roasted and raw Turkish and Oregon hazelnuts were examined. Whole nuts, skins, and skinless nuts of both hazelnut varieties were tested for fat contents, fatty acid profiles. Hazelnut and other byproducts were extracted with 5% acetone and examined for total phenolic contents (TPC), antioxidant activities against the peroxyl (ORAC) and DPPH radicals, and were also administered in vitro to the human colon cancer HT-29 cell line to determine antiproliferative effects. The Turkish hazelnuts contained over 65% total oil while the Oregon roasted variety contained 43.8%. The primary fatty acid in both was oleic acid (18:1n-9) comprising 76.7 g/100g oil in the Oregon variety and 83.3 g/100g oil in the Turkish variety. The TPC were 91.4 and 102.16 mg gallic acid equivalents/g sample for the Turkish roasted hazelnut skin and Oregon roasted hazelnut skin respectively, at least 30-folds as high as the hazelnut without skin. Turkish roasted hazelnut skin had the highest ORAC value of 1166.27 Trolox equivalents (TE) mmol/g sample (TE mmol/g), it is 38 times as high as the Oregon roasted hazelnut no skin which as a value of 30.2 TE mmol/g sample. The range of ED50 of DPPH· is from 118.22 to 0.075 mg sample equivalents/mL among the samples, Oregon roasted hazelnut skin and Turkish raw hazelnut no skin exhibit the weakest and strongest ability to reduce DPPH· respectively. At 6 mg/mL media Oregon roasted hazelnut skin extract significantly inhibited the growth of the HT-29 cells by 96 h following 4 days of treatment, and no effect was seen from the Turkish roasted skinned hazelnut extract. The Turkish raw hazelnut had significantly higher antioxidant activities compared to the Oregon roasted variety which may be explained by chemical changes during heating or possibly the total oil to flour ratio.
1. Introduction
The total worldwide production of in shell hazelnut in 2009 was 765,666 tonnes, and 500,000 tonnes was supplied by Turkey. Other countries with significant hazelnut production in 2009 include Italy, the United States, and Spain that produced 104,900, 42,640, and 10,500 tonnes, respectively [1].
Hazelnut seed primarily consists of oil which generally comprises about 60% of the total weight but has been shown to contain over 75% oil and oil contents can vary greatly from year to year harvests [2]. Because of its high fatty acid composition, hazelnut is a very rich source of energy providing approximately 6 to 6.5 kcals/g fresh seed [3]. Recently, the understanding of the relationship between dietary fatty acids and their effect on health has been growing significantly, and the public is aware of its importance now more than ever.
The consumption of specific fatty acids such as omega- 3 fatty acids and oleic acid may provide health benefits. Increasing the intake of dietary omega-3 fatty acids such as EPA (20:5n-3) and DHA (22:6n-3) may reduce the risk of several diseases including arteriosclerosis, cancer, autoimmune disorders, and hypertension [4-6]. A previous studie from Tey et al., showed that the consumption of hazelnut in the diet can improve the lipoprotein profile and a-tocopherol concentration in human subjects having mild hypercholesterolemia [7]. Oleic acid (18:1n-9) has been associated with lowering the risk of heart disease. Several previous studies demonstrated that diets containing high levels of oleic acid lowered LDL and total cholesterol when experimentally replaced for saturated fat [8], and in 2004, the FDA published a qualified health claim for olive oil stating that the daily consumption of 2 tablespoons of olive oil (70% - 75% oleic acid) in place of saturated fat without increasing total calories can possibly decrease one’s overall risk for heart disease due to its high content of monounsaturated fat.
Important phytochemicals found in plant foods include tocopherols, carotenoids, and other antioxidant phenolic compounds. Alpha-tocopherol is a well known fat soluble vitamin antioxidant that protects unsaturated fatty acyls in lipid membranes from free radical oxidation and can stop lipid radical propagation that leads to the development of fatty streaks in the physiopathological process of arteriosclerosis. In a recent study, tocopherols including aand g-tocopherol were detected in tree nuts including hazelnut, macadamia, walnut, and almond, with almond containing the highest level of a-tocopherol at 31 mg/100g flesh and walnut having the highest g-tocopherol content of 30 mg/100g flesh [9]. Carotenoids are important beneficial dietary compounds. Beta-carotene can be converted to vitamin A when needed, and other dietary carotenoids are capable of quenching free radicals and singlet oxygen which is a free radical initiator [10]. Carotenoids have been detected in several seed oils including onion, parsley, cardamom, mullein, pumpkin, milk thistle, red raspberry, blueberry, marionberry, and blueberry [11,12]. Reactive oxygen species (ROS) are the major free radical byproducts of metabolism and are implicated in many diseases including cancer, heart disease, and many others because they may oxidize important biological molecules such as nucleic acids, and proteins [13]. Dietary compounds other than antioxidant vitamins may provide a critical role in protecting against ROS induced free radical injury. Phenolic compounds are found in virtually all plant foods and many phenols can act as powerful antioxidants that may reduce free radical damage. Individual phenolic compounds with known antioxidant activities have been identified in hazelnut including gallic acid, 4-OH benzoic acid, p-coumaric sinaptic acid, and quercetin [14,15]. Antioxidant activities have been detected in many foods and food byproducts including fruits, vegetables, grains, seed oils, and seed flours [11,12,16-19]. Hazelnut extracted with 80% EtOH has also demonstrated antioxidant activities against ABTS, hydrogen peroxide, superoxide, DPPH·, and b-carotene linoleate system. The hazelnut extract also inhibited human LDL oxidation and DNA scission [15]. The oxygen radical absorbance capacity (ORAC) assay is a physiologically relevant antioxidant test because it measures the ability of an antioxidant system to inhibit the oxidative damage to susceptible molecules of peroxyl radicals. Previous results have shown that tree nuts have significant antioxidant activities with pecan having the highest ORAC value of 175 trolox equivalent micromoles per g whole nut compared to eight other angiosperm tree nuts [20]. Transition metals may act as radical generators in food products and biological systems by removing an electron from a molecule present. Chelating agents may bind transition metals and reduce radical-mediated oxidative damage that may prevent the deterioration of food products and molecular damage to biological systems [13]. The chelating capacity assay measures the ability of a sample to bind ferrous iron (Fe2+). A recent study determined the chelating capacity of seed oils extracted with methanol and found significant chelating ability in hemp, caraway, and carrot seed oils with the carrot having the best capacity of 25.5 EDTA equivalent mg/g oil. Chelating capacity has not previously been examined in hazelnut.
Tree nuts, including hazelnut, have been examined for their ability to inhibit the proliferation of human cancer cell lines including Hep G2 (liver) and Caco-2 (colon) in vitro and several nuts including walnut, pecan, almond, macadamia and cashew significantly inhibited cancer cell proliferation [21]. To date, there is very limited information regarding the effect of hazelnut on the proliferation of cancer cell lines and none on the HT-29 human colon cancer line.
In the current study, Turkish raw hazelnuts and Oregon roasted hazelnuts (Corylus avellana L.) were examine for their total fat content and fatty acid profiles, tocopherols including aand g-tocopherol, and b-carotene. Defatted hazelnut flours extracted with 50% acetone were analyzed for antioxidant activities using the ORAC assay and DPPH·; they were also examined for their total phenolic content (TPC), and chelating capacity against Fe2+. The extracts were also examined for their ability to inhibit the proliferation of HT-29 human colon cancer cells in vitro.
2. Materials and Methods
2.1. Materials
Whole raw and roasted Turkish and Oregon hazelnuts were gifts from the Hazelnut Council, Oregon, USA. 2,2’-azobis (2-amino-propane) dihydrochloride (AAPH) was obtained from Wako Chemicals USA (Richmond, VA). 2,2-Diphenyl-1-picrylhydrazyl radical (DPPH·), Folin-Ciocalteu reagent, gallic acid, and 6-hydroxy-2,5,7, 8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (St. Louis, MO), b-cyclodextrin (RMCD) was purchased from Cyclolab R & D Ltd. (Budapest, Hungary). Cell culture media (McCoy’s 5A Medium Modified with L-glutamine, antibiotic/antimycotic, and fetal bovine serum (FBS), 0.25% trypsin with 0.9 mM EDTA) was purchased from Invitrogen (Carlsbad, CA). HT-29 human colon cancer cells were purchased from American Type Culture Collection (Rockville, MD). All other chemicals and solvents were of the highest commercial grade and used without further purification.
2.1.1. Extraction
Shelled hazelnuts were ground into meal, and oils were extracted using a Soxhlet apparatus with hexanes the solvent. The residual remaining following oil extraction was the flour, and flours were extracted using 10 mL 50% acetone per 1 g flour. Extracts were obtained by vortexing for 3 min 3 times at 1 h intervals then were allowed to stand overnight. The mixture was centrifuged at 500 g, and the supernatant was collected. Both oils and flours were kept under nitrogen and in the dark until analyzed.
2.1.2. Beta-Carotene
Concentrations of b-carotene were measured following a previously described method [11,22,23]. Briefly, 0.1 mL of hazelnut oil was dissolved in 0.9 mL of methanol/ tetrahydrofuran (1:1, v/v) and analyzed for b-carotene using HPLC-DAD-ESI-MSMS (high performance liquid chromatography-diode array detector-electron spray ionization-tandem mass spectrometry). A TSQ quantum tandem mass spectrometer (Thermo-Finnigan, San Jose, CA) was equipped with an ESI interface and an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) with a Zorbax SB C18 column, 50 mm ´ 1.0 mm i.d. with a 3.5-mm particle size (Agilent Technologies, Palo Alto, CA, USA), was used to determine b-carotene. Identification was accomplished by comparing HPLC retention time and selected reactant monitoring (SRM) analyses of the sample peak with that of authorized pure b-carotene. Quantifications were conducted using the total ion counts with an external standard. Data were obtained using Xcalibur software system (Thermo-Finnigan, San Jose, CA, USA).
2.1.3. Tocopherols
The methanol/tetrahydrofuran solutions prepared for bcarotene composition were also used to quantify aand g-tocopherol concentrations by a previously described method [11]. HPLC with a Zorbax SB C18 column, 30 mm × 1.0 mm i.d. with a 3.5-mm particle size (Agilent Technologies, Palo Alto, CA, USA), was used to separate the tocopherols. The individual tocopherols were identified by peak retention time and selected reactant monitoring with those of the pure commercial compounds, quantification was determined using the total ion counts with external standards of the individual compounds.
2.1.4. Fatty Acid Composition
Fatty acid methyl esters (FAME) were prepared from hazelnut oil according to the previously described method [11]. Fatty acid profiles were analyzed by GC-FID using a Shimadzu GC-2010 with a FID and a Shimadzu AOC- 20i autosampler (Shimadzu, Columbia, MD). The column was a Supelco 2380, 30 m ´ 0.25 mm i.d. with a 0.20 µm film thickness (Supelco Inc., Bellefonte, PA). Helium was the carrier gas and was set at a flow rate of 0.8 mL/min. Injection volume was 1 mL at a split ratio of 10/1. Initial oven temperature was 142˚C and increased 6˚C/min to 184˚C and held for 3 min, then 6˚C/min to 244˚C. Fatty acids were identified by retention times compared to individual commercial standard FAMEs.
2.1.5. Oxygen Radical Absorbance Capacity (ORAC)
ORAC was determined using the protocol previously described [24]. Fluorescein was used as the fluorescent probe. The assay mixture contained 0.067 µM of fluorescein, 60 mM of AAPH, 300 µL of flour extract or 50% acetone for the reagent blank. The fluorescence of an assay mixture was recorded every minute, and the area under the curve of fluorescence vs time plot was calculated and compared against a standard curve prepared with trolox. ORAC value was expressed as trolox equivalents (TE) in mmol per gram of the fruit seed flour. Triplicate measurements were conducted.
2.1.6. DPPH• Scavenging Activity
The stable DPPH (2,2-diphenyl-1-picryhydrazyl) radical scavenging capacities of the cold-pressed seed flour extracts were analyzed following a previously described procedure [25]. A DPPH·-50% acetone solution was freshly prepared and was mixed with 1 mL seed flour extracts at different concentrations to start the radicalantioxidant reaction. The final DPPH· concentration was 100 mM and the final reaction volume was 2.0 mL. Absorbance readings were measured at 517 nm against a blank of 50% acetone and used to estimate the remaining radical levels according to the standard curve. The seed flour extracts were tested for their ED50-DPPH concentrations at 20 minutes of reaction. The ED50-DPPH is the concentration of extract needed to reduce 50% of the DPPH radicals under experimental conditions. Time and dose dependencies of extracts and DPPH· reactions were demonstrated by plotting the percent of DPPH· remaining against time for each level of the seed oil extract tested.
2.1.7. Total Phenolic Content (TPC)
The TPC of hazelnut flour extracts was estimated using Folin & Ciocalteu’s (FC) reagent [25]. Briefly, the reaction mixture contained 250 mL of freshly prepared FC reagent, 50 mL of hazelnut flour extract, 0.75 mL of 20% sodium carbonate, and 3 mL of pure water. Absorbance was read at 765 nm after 2 h of reaction at ambient temperature, and gallic acid was the standard used to calculate TPC.
2.1.8. HT-29 Colon Cancer Cell Proliferation Inhibition
HT-29 human colorectal adenocarcinoma cell line characterized by Fogh et al., 1975 were propagated in T-150 flasks in Mcoy’s 5A media containing 10% FBS and 1% antibiotic/antimycotic. Flasks were incubated at 37˚C in a humidified atmosphere at 5% CO2 [26,27].
Cell proliferation was examined following a modified procedure using 12-well plates [27]. After 24 h of incubation in the control media at 37˚C in a humidified atmosphere containing 5% CO2, cells were treated with media containing the DMSO solution of the hazelnut flour extracts at two levels, while the control cells were treated with same volume of DMSO. The two dose levels were 2.5 and 5 mg flour equivalents per mL culture media. Media and treatments were changed daily, and live cells were counted on day 1 through day 4 of treatment.
2.2. Statistical Analysis
Data were reported as mean ± standard deviation (SD). ANOVA with Tukey’s post hoc test was used to identify differences among the means. Statistical significance was declared at P < 0.05.
3. Results and Discussion
Roasted and raw Turkish and Oregon hazelnuts were examined. Whole nuts, skins, oils, and skinless meat were examined.
Beta-Carotene. Beta-carotene was detected in both Oregon and Turkish hazelnut oil samples in approximate equal amounts (Table 1). The experimental values of 9.9 and 10.0 mg per 100 g oil are similar to the value of 11 mg b-carotene in 100 g of unroasted hazelnut [28]. In one previous study on 10 different nuts, including hazelnut, b-carotene was not detected in the hazelnut sample and was only detected in pistachio nuts [29]. Another study by Alasalvar et al. [30] did not detect a carotenoid isomer in Turkish Tombul hazelnut.
Tocopherols. Both aand l-tocopherol were detected in the hazelnut oil samples. The Turkish hazelnut oil contained a-tocopherol and l-tocopherol at concentrations of 26.8 and 3.7 mg/100g oil, respectively. These values were approximately 1.3 and 15 times higher than the Oregon hazelnut oil (Table 1). Previous investigations have demonstrated hazelnut oil to have a range of atocopherol concentrations from 10.6 to 65.5 mg/100g oil [31,32], and both sample concentrations fell nearer to the lower end of this range.
Total Fat. The two hazelnut samples had very different total fat contents (Table 1). The Turkish hazelnut contained 65.7% oil which was more than 20% higher than the Oregon hazelnut. Previous studies demonstrated that hazelnuts contain from 46.7% to 76.8% oil and contain approximately 60% oil per nut on average [2,3,18,32,33].
Fatty Acid Profile. The fatty acid profiles of and Oregon and Turkish hazelnut oils demonstrated very high concentrations of oleic acid which was consistent with the literature. The Turkish hazelnut oil contained the highest amount of oleic acid (18:1n-9) at a concentration of 83.3 g/100g oil (Table 2). Linoleic acid was the second most prevalent fatty acid and its concentration was inversely associated to oleic acid concentration. From previous investigations, the oleic acid concentration in hazelnut oil has been in the range of 70.5% to 85.3% of total fatty acids and has been shown to be significantly and inversely related to its linoleic acid concentration [33]. Total unsaturated fatty acids for the Turkish and Oregon hazelnut oils were similar at 92.2 and 93.0 g/ 100g oil, respectively (Table 2). These results are very consistent with other studies demonstrating that total unsaturated fatty acid concentrations among many hazelnut varieties grown in many different locations have a very tight compositional range of unsaturated fatty acids from approximately 89% to 92% of the total fatty acids [3,33,34,9,2,35]. The ratio of the percent of total fat to linoleic acid from this study was consistent with previous results that found a significant negative correlation between total fat percent and linoleic acid [33]. This may indicate a negative relationship between growing temperature and total fatty acid percent considering cell membranes may increase the level of unsaturation at

Table 1. Nutritional content of hazelnut seeds.
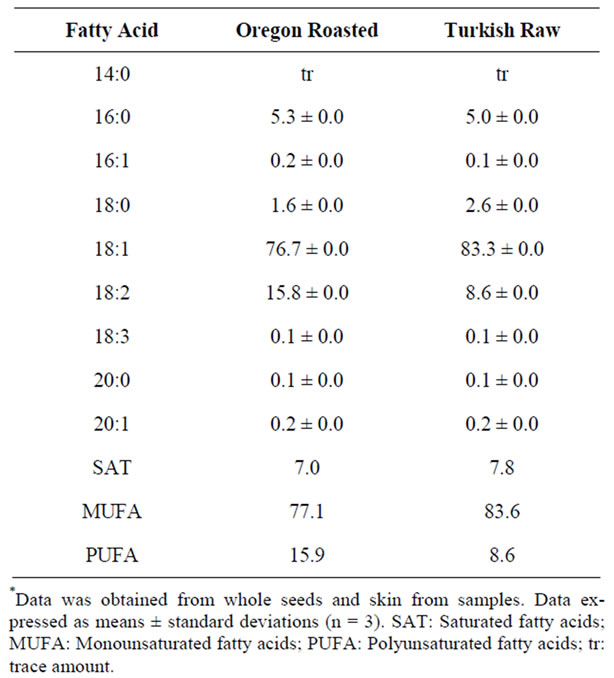
Table 2. Fatty acid profiles of hazelnut oils (g/100g oil)*.
lower temperatures to maintain membrane fluidity.
ORAC. ORAC values of the tested hazelnut samples are shown in Table 3. The ORAC value of the samples ranged from 1166.2 - 30.7 trolox equivalents (TE) in micromoles per g sample (TE eq mmol/g) The ORAC values of roasted Turkish hazelnut skin was significantly higher than the rest of the samples. It was 40-fold higher than Oregon roasted hazelnut without skin. Previously, 8 samples of whole hazelnut kernel were evaluated for ORAC values by testing for both lipophilic and hydrophilic antioxidant components and averaged 96.45 TE eq mmol/g whole kernel meal [20].
DPPH. DPPH values were determined as ED50-DPPH, which is the concentration of an extract required to decrease the amount of DPPH radicals to 50% of the initial concentration under experimental conditions. Oregon roasted hazelnut skin extract demonstrated greater antioxidant activity than the other samples. The ED50-DPPH value of the Oregon roasted hazelnut skin extract was 0.075 mg sample equivalent per ml (mg flour equiv/ml) and was approximately 235.5 times lower than that of the Oregon raw hazelnut ’without skin’ extract.
A similar result was shown in the Turkish hazelnut. The ED50-DPPH of the Turkish roasted hazelnut skin extract was 1074.7 lower than that of the Oregon raw hazelnut without skin extract was observed (Table 3).
Total Phenolic Content (TPC). TPC values showed a similar trend as compared to the ORAC test. The highest TPC value was in the skin extract of the Oregon and Turkey hazelnuts. The TPC values were 102.16 and 91.40 mg gallic acid equivalents (GAE) per g flour (mg GAE/g) respectively, and was significantly higher than the TPC of the other hazelnut extracts (Table 3). In a previous investigation of several tree nuts, hazelnut demonstrated a TPC of 2.91 mg GAE/g edible nut and was higher than other tested nuts including almonds, Brazil nuts, cashews, macadamias, and pines but lower than [29]. The roasted hazelnut skin samples have higher antioxidant capacities than roasted hazelnut with skin and without skin samples in both Oregon and Turkey varieties. Similar result was observed in the raw hazelnut samples.
Comparative results among ORAC, DPPH, TPC were correlated. The highest overall antioxidant capacities were observed in the roasted hazelnut skin samples extracts, and the lowest antioxidant values were seen in both raw hazelnut samples without skin. The skin showed the highest ORAC, DPPH and TPC value in both Turkey and Oregon, roasted and raw hazelnut samples. A result of a study by Monagas et al. 2009 showed that flavan-3-ol composition of roasted peanut, hazelnut and almond nut skins tested for total phenolic contents, flavan-3-ol found that 90% of flavan-3-ol was a combination of monomeric flavan-3-ols and hazelnuts were mainly contained B-type proanthoc-yanidins in the polymeric flavan-3-ols [36].
Anti-Proliferation. The effects of the hazelnut flour extracts on HT-29 colon cancer cell proliferation are shown in Table 4. The hazelnuts extracts are potent scavengers of free radicals and inhibit cancer cell proliferation. At 6 mg/mL media Oregon roasted hazelnut skin extract significantly inhibited the growth of the HT-29 cells by 96% and 89% of the cancer cells were inhibited by Turkish roasted hazelnut skin extract following 4 days of treatment. No effect was observed in both Turkey and Oregon roasted hazelnut with or without skin.
The Turkish hazelnut flour extract significantly inhibited the proliferation of HT-29 cells from day 2 to day 4 at 5 mg flour equivalents per ml media (mg equiv/mL) but only slightly inhibited proliferation at 2.5 mg equiv/ mL (data not shown). The Oregon roasted hazelnut did not show any proliferation inhibitory effects at either concentration. The reason for the difference between the Turkish and Oregon hazelnut flour extract’s effectiveness in inhibiting proliferation is not completely clear. However, previous studies have demonstrated that individual phenolic acids commonly found in plant foods, and plant food extracts with strong antioxidant activities can significantly reduce the proliferation of cancer cell growth in vitro, and the Turkish hazelnut had significantly
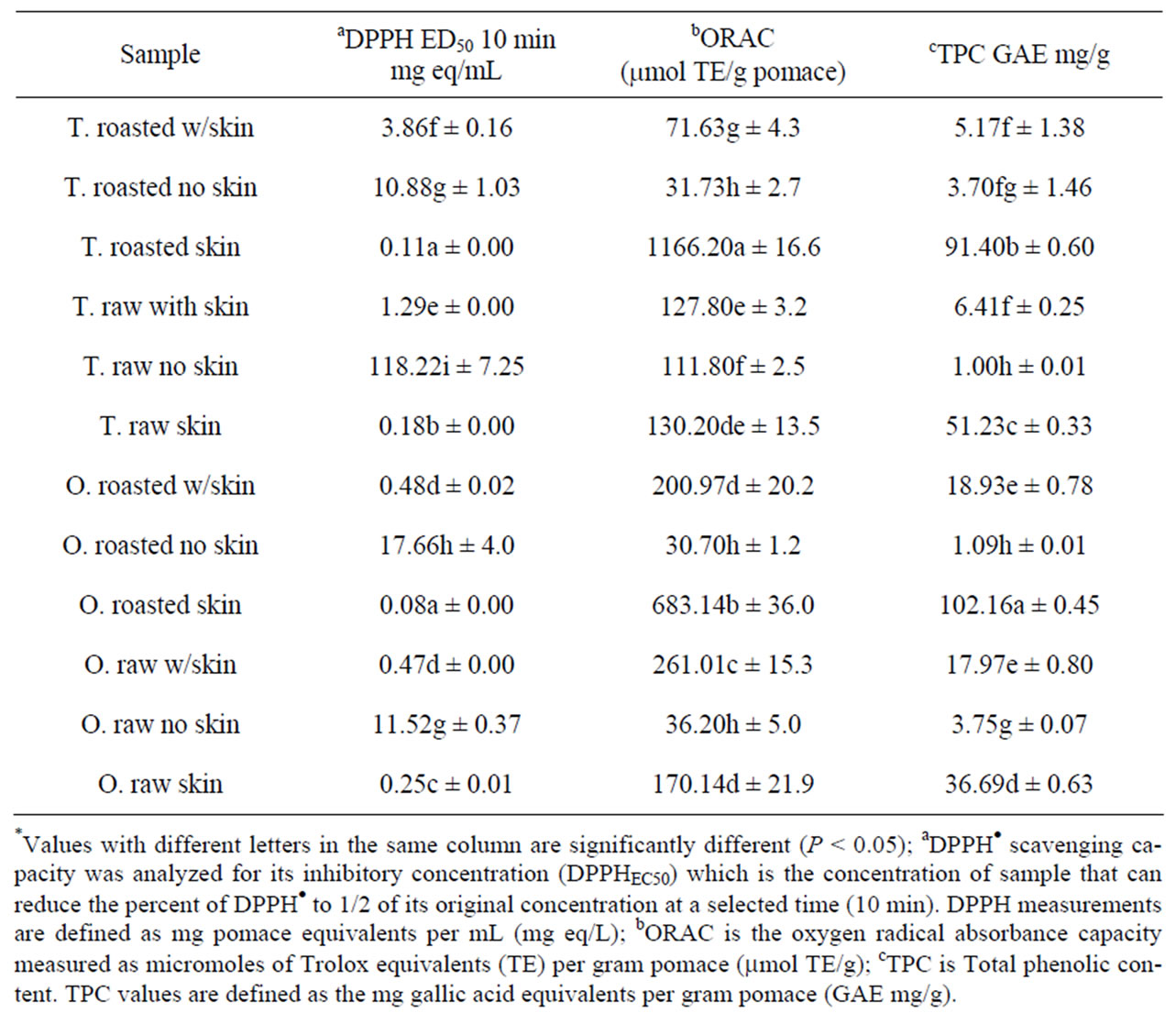
Table 3. Antioxidant properties of hazelnut flours*.

Table 4. Percent antiproliferation of hazelnut extracts on HT-29 cells following 96 h of treatment.
stronger antioxidant activities than the Oregon hazelnut. In a previous study of common phenolic food components, ferulic acid and coumaric acid both significantly inhibited the proliferation of Caco-2 human colon cancer cells at 1500 mmol at least in part by inhibiting cell cycle progression [37]. Silibinin is a phenolic compound isolated from milk thistle and it is well documented for its ability to inhibit the proliferation of several cancer cell models in vitro. A recent study of fruit seed flours determined that their extracts could significantly inhibit the proliferation of HT-29 human colon cancer cells in a dose dependent manner, and antiproliferation was positively correlated to antioxidant activity [12]. It is also possible that the roasting process may have effectively modified and inactivated chemicals responsible for antiproliferation activity in the Oregon hazelnut.
Hazelnut has the potential to be a bioactive food ingredient and increase the profits for growers as a valueadding byproduct.
4. Conclusions
Whole nuts, skins, and skinless nuts of both Turkish and Oregon hazelnuts varieties were tested for fat contents and fatty acid profiles, antioxidant capacities of ORAC, DPPH, TPC were evaluated. Hazelnut kernel contains a high concentration of total weight as oil, and may be used to increase the dietary consumption of oleic acid for those individuals looking to increase the level of monounsaturated fats. The hazelnuts extracts were potent scavengers of free radicals and inhibited cancer cell proliferation. The roasted Turkish and Oregon hazelnut skin extracts had significantly higher antioxidant activities compared to the other extracted. At 6 mg/mL media Oregon roasted hazelnut skin extract significantly inhibited the growth of the HT-29 cells by 96% following 4 days of treatment, and a similar result was seen from the Turkish roasted skinned hazelnut extract with an inhibition of growth of the HT-29 cells by 89%.
REFERENCES
- Food and Agriculture Organization of the United Nations, FAOSTAT data, 2011. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor
- M. Ozdemir, F. Ackurt, M. Kaplan, M. Yildiz, M. Loker, T. Gurcan, G. Biringen, A. Okay and F. G. Seyhan, “Evaluation of New Turkish Hybrid Hazelnut (Corylus avellana L.) Varieties: Fatty Acid Composition, a-Tocopherol Content, Mineral Composition and Stability,” Food Chemistry, Vol. 73, 2001, pp. 411-415.
- A. I. Koksal, N. Artik, A. Simsek and N. Gunes, “Nutrient Composition of Hazelnut (Corylus avellana L.) Varieties Cultivated in Turkey,” Food Chemistry, Vol. 99, No. 3, 2006, pp. 509-515. doi:10.1016/j.foodchem.2005.08.013
- W. E. Connor, “Importance of n-3 Fatty Acids in Health and Disease,” American Journal of Clinical Nutrition, Vol. 71, Suppl. 1, 2000, pp. 171S-175S.
- W. J. Aronson, J. A. Glaspy, S. T. Reddy, D. Reese, D. Heber and D. Bagga, “Modulation of Omega-3/Omega-6 Polyunsaturated Ratios with Dietary Fish Oils in Men with Prostate Cancer,” Urology, Vol. 58, No. 2, 2001, pp. 283-288. doi:10.1016/S0090-4295(01)01116-5
- H. Iso, S. Sato, U. Umemura, M. Kudo, K. Koike, A. Kitamura, H. Imano, T. Okamura, Y. Naito and T. Shimamoto, “Linoleic Acid, Other Fatty Acids, and the Risk of Stroke,” Stroke, Vol. 33, 2002, pp. 2086-2093. doi:10.1161/01.STR.0000023890.25066.50
- S. L. Tey, R. C. Brown, C. M. Chisholm, C. M. Delahunty, A. R. Gray and S. M. Williams, “Effects of Different Forms of Hazelnuts on Blood Lipids and a-Tocopherol Concentrations in Mildly Hypercholesterolemic Individuals,” European Journal of Clinical Nutrition, Vol. 65, 2011, pp. 117-124. doi:10.1038/ejcn.2010.200
- C. D. Gardner and H. C. Kraemer, “Monounsaturated versus Polyunsaturated Dietary Fat and Serum Lipids. A Meta-Analysis,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 15, 1995, pp. 1917-1927. doi:10.1161/01.ATV.15.11.1917
- L. S. Maguire, S. M. O’Sullivan, K. Galvin, T. P. O’Connor and N. M. O’Brien, “Fatty Acid Profile, Tocopherol, Squalene and Phytosterol Content of Walnuts, Almonds, Peanuts, Hazelnuts and the Macadamia Nut,” International Journal of Food Sciences & Nutrition, Vol. 55, No. 3, 2004, pp. 171-178. doi:10.1080/09637480410001725175
- S. Oshima, F. Ojima, H. Sakamoto, Y. Ishiguro and J. Terao, “Supplementation with Carotenoids Inhibits Singlet Oxygen-Mediated Oxidation of Human Plasma LowDensity Lipoprotein,” Journal of Agricultural and Food Chemistry, Vol. 44, No. 8, 1996, pp. 2306-2309. doi:10.1021/jf950350i
- J. Parry and L. Yu, “Fatty Acid Content and Antioxidant Properties of Cold-Pressed Marionberry, Boysenberry, Red Raspberry, and Blueberry Seed Oils,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 3, 2005, pp. 566-573. doi:10.1021/jf048615t
- J. Parry, L. Su, J. Moore, Z. Cheng, M. Luther, J. N. Rao, J. Y. Wang and L. Yu, “Chemical Compositions, Antioxidant Capacities, and Antiproliferative Activities of Selected Fruit Seed Flours,” Journal of Agricultural and Food Chemistry, Vol. 54, No. 11, 2006, pp. 3773-3778. doi:10.1021/jf060325k
- L. Yu, K. Zhou and J. Parry, “Antioxidant Properties of Cold-Pressed Black Caraway, Carrot, Cranberry, and Hemp Seed Oils,” Food Chemistry, Vol. 91, No. 4, 2005, pp. 723-729. doi:10.1016/j.foodchem.2004.06.044
- H. C. Yurttas, H. W. Schafer and J. J. Warthesen, “Antioxidant Activity of Nontocopherol Hazelnut (Corylus spp.) Phenolics,” Journal of Food Chemistry, Vol. 65, No. 2, 2000, pp. 276-280. doi:10.1111/j.1365-2621.2000.tb15993.x
- F. Shahidi, C. Alasalvar and M. Liyana-Pathirana, “Antioxidant Phytochemicals in Hazelnut Kernel (Corylus avellana L.) and Hazelnut Byproducts,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 4, 2007, pp. 1212-1220. doi:10.1021/jf062472o
- W. Kalt, C. F. Forney, A. Martin and R. Prior, “Antioxidant Capacity, Vitamin C, Phenolics, and Anthocyanins after Fresh Storage of Small Fruits,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 11, 1999, pp. 4638-4644. doi:10.1021/jf990266t
- P. Ninfali and M. Bacchiocca, “Polyphenols and Antioxidant Capacity of Vegetables under Fresh and Frozen Conditions,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 8, 2003, pp. 2222-2226. doi:10.1021/jf020936m
- J. S. Bonvehi and F. V. Coll, “Oil Content, Stability and Fatty Acid Composition of the Main Varieties of Catalonian Hazelnuts (Corylus avellana L.),” Food Chemistry, Vol. 48, No. 3, 1993, pp. 237-241. doi:10.1016/0308-8146(93)90133-Z
- L. Yu, L. Scanlin, J. Wilson and G. Schmidt, “Rosemary Extracts as Inhibitors of Lipid Oxidation and Color Change in Cooked Turkey Products during Refrigerated Storage,” Journal of Food Chemistry, Vol. 67, No. 2, 2002, pp. 582-585. doi:10.1111/j.1365-2621.2002.tb10642.x
- X. Wu, G. R. Beecher, J. M. Holden, D. B. Haytowitz, S. E. Gebhart and R. Prior, “Lipophilic and Hydrophilic Antioxidant Capacities of Foods in the United States,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 12, 2004, pp. 4026-4037. doi:10.1021/jf049696w
- J. Yang, L. Halim and R. H. Liu, “Antioxidant and Antiproliferative Activities of Common Nuts,” IFT Annual Meeting, New Orleans, 2005.
- C. Pinzino, B. Nanni and M. Zandomeneghi, “Aging, Free Radicals, and Antioxidants in Wheat Seeds,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 4, 1999, pp. 1333-1339. doi:10.1021/jf980876d
- V. Hentschel, K. Kranl, J. Hollmann, M. G. Lindhauer, V. Bohm and R. Bitsch, “Spectrophotometric Determination of Yellow Pigment Content and Evaluation of Carotenoids by High-Perfomance Liquid Chromatography in Durum Wheat Grain,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 23, 2002, pp. 6663-6668. doi:10.1021/jf025701p
- J. Moore, Z. Hao, K. Zhou, M. Luther, J. Costa and L. Yu, “Carotenoid, Tocopherol, Phenolic Acid, and Antioxidant Properties of Maryland-Grown Soft Wheat,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 17, 2005, pp. 6649-6657. doi:10.1021/jf050481b
- L. Yu, J. Perret, M. Harris, J. Wilson and S. Haley, “Antioxidant Properties of Bran Extracts from ‘Akron’ Wheat Grown at Different Locations,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 6, 2003, pp. 1566-1570. doi:10.1021/jf020950z
- S. Yoshida, A. Honda, Y. Matsuzaki, S. Fukushima, N. Tanaka, A. Takagiwa, Y. Fujimoto, H. Miyazaki and G. Salen, “Anti-Proliferative Action of Endogenous Dehydroepiandrosterone Metabolites on Human Cancer Cell Lines,” Steroids, Vol. 68, No. 1, 2003, pp. 73-83. doi:10.1016/S0039-128X(02)00117-4
- L. Qiao, M. Koutsos, L. L. Tsai, V. Kozoni, J. Guzman, S. J. Shiff and B. Rigas, “Staurosporine Inhibits the Proliferation, Alters the Cell Cycle Distribution and Induces Apoptosis in HT-29 Human Colon Adenocarcinoma Cells,” Cancer Letter, Vol. 107, No. 1, 1996, pp. 83-89. doi:10.1016/0304-3835(96)04346-7
- USDA National Nutrient Database for Standard Reference, Release 19, 2007. http://riley.nal.usda.gov/NDL/cgi-bin/list_nut_edit.pl
- M. Kornsteiner, K.-H. Wagner and I. Elmadfa, “Tocopherols and Total Phenolics in 10 Different Nut Types,” Food Chemistry, Vol. 98, No. 2, 2006, pp. 381-387. doi:10.1016/j.foodchem.2005.07.033
- C. Alasalvar, F. Shahidi, C. M. Liyanapathirana and T. Ohshima, “Turkish Tombul Hazelnut (Corylus avellana L.) Compositional Characteristics,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 13, 2003, pp. 3790-3796. doi:10.1021/jf0212385
- J. S. Amaral, S. Casal, R. M. Seabra and B. P. Oliveira, “Effect of Roasting on Hazelnut,” Journal of Agricultural and Food Chemistry, Vol. 54, No. 4, 2006, pp. 1315-1321. doi:10.1021/jf052287v
- J. Parcerisa, D. G. Richardson, M. Rafecas, R. Codony and J. Boatella, “Fatty Acid, Tocopherol and Sterol Content of Some Hazelnut Varieties (Corylus avellana L.) Harvested in Oregon (USA),” Journal of Chromatography A, Vol. 805, No. 1-2, 1998, pp. 259-268. doi:10.1016/S0021-9673(98)00049-1
- J. Parcerisa, J. Boatella, R. Codony, A. Farran, J. Garcia, A. Lopez, M. Rafecas and A. Romero, “Influence of Variety and Geographical Origin on the Lipid Fraction of Hazelnuts (Corylus avellana L.) from Spain: I. Fatty Acid Composition,” Food Chemistry, Vol. 48, No. 4, 1993, pp. 411-414. doi:10.1016/0308-8146(93)90326-B
- C. Crews, P. Hough, J. Godward, P. Brereton, M. Lees, S. Guiet and W. Winkelmann, “Study of the Main Constituents of Some Authentic Hazelnut Oils,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 12, 2005, pp. 4843-4852. doi:10.1021/jf047836w
- C. Alasalvar, M. Karaamac, R. Amarowicz and F. Shahidi, “Antioxidant and Antiradical Activities in Extracts of Hazelnut Kernel (Corylus avellana L.) and Hazelnut Green Leafy Cover,” Journal of Agricultural and Food Chemistry, Vol. 54, No. 13, 2006, pp. 4826-4832. doi:10.1021/jf0601259
- M. Monagas, L. Garrido, L. Lebrόn-Aguilar, M. C. GmezCordov, A. Rybarczyk, R. Amarowicz and B. Bartolom, “Comparative Flavan-3-ol Profile and Antioxidant Capacity of Roasted Peanut, Hazelnut, and Almond Skins,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 22, 2009, pp. 10590-10599. doi:10.1021/jf901391a
- B. Janicke, G. Onning and S. M. Oredsson, “Differential Effects of Ferulic Acid and p-Coumaric Acid of S Phase Distribution and Length of S Phase in the Human Colonic Cell Line Caco-2,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 17, 2005, pp. 6658-6665. doi:10.1021/jf050489l
NOTES
#Both authors contributed equally to this work.

