American Journal of Plant Sciences
Vol.4 No.8(2013), Article ID:35470,6 pages DOI:10.4236/ajps.2013.48200
Antioxidant Capacity and Phenolic Content of Some Nepalese Medicinal Plants
![]()
1Natural Products Research Laboratory, Nepal Academy of Science and Technology (NAST), Kathmandu, Nepal; 2Biotechnology Laboratory, Nepal Academy of Science and Technology (NAST), Kathmandu, Nepal.
Email: utpalbodhi@gmail.com, bikubaral@yahoo.com
Copyright © 2013 Bijaya Laxmi Maharjan, Bikash Baral. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 1st, 2013; revised June 2nd, 2013; accepted July 15th, 2013
Keywords: DPPH; FRAP; Fritillaria; Lichens; Phytochemicals; Rhododendron; TPC
ABSTRACT
Antioxidant capacities and phenolic contents of medicinal plants namely Usnea longifolia, Cetraria nepalensis, Parmelia minarum, Everniastrum nepalense, Rhododendron anthopogon and Fritillaria delavayi were analyzed via FolinCiocaltau assay, Ferric reducing activity power assay and 2,2-Diphenyl-1-picrylhydrazyl assay. All the tested plants depicted the antioxidant activity with variation in extent of activity among the plants. The FRAP (F-value: 387.4), DPPH (F-value: 89.684) and TPC (F-value: 559.163) values between the extracts showed the highly significant differences (P < 0.01). This study indicated the strong antioxidant potential of R. anthopogon among the plants tested.
1. Introduction
The world populations, mainly of the developing countries (70% - 95%) rely primarily on traditional medicine for their healthcare [1]. In Nepal, >85% of total populations are benefited from Ayurveda, a traditional medicinal system. The altitudinal variation starting from almost sea level (ca. 70 m asl) to the top of the world (8848 m asl), climatic differences, varied topography and abundant ecological habitats offer rich floral and faunal life to Nepal [2] adding more to the diversification. However, still a lack of scientific evidence justifying the anecdotal claims regarding these Nepalese medicinal plants persists. Additionally, there is an increased global commercial interest in the use of these plant species for their proposed health benefits.
Oxidation, the most fundamental essence of many living organisms, can be exploited in producing energy to fuel biological process. However, the reactive oxygen species may cause severe oxidative stress if accumulated within body in excess amount. This may further enhance the risk in building several disorders in humans viz., cardiovascular disease, cancer, diabetes, neurodegenerative diseases, pulmonary diseases and ageing [3,4].
Antioxidants are pivotal substances, capable of scavenging reactive oxygen species (including superoxide free radical, hydrogen peroxide, hydroxyl free radical and singlet oxygen) and protecting from oxidative damage [5], finally being an important tools in obtaining and preserving good health. These antioxidants can basically be categorized into two groups; namely synthetic and natural ones. Restriction on the use of synthetic antioxidants is being imposed because of their carcinogenicity [6]. Thus, the interest in natural antioxidants has been increased considerably [7].
The search and high demand for raw materials containing potent antioxidants continue to attract the attention of scientific researchers, of which medicinal plants are the key sources for a wide variety of natural antioxidants [8]. Generally, antioxidant compounds like phenolic acids, polyphenols and flavonoids scavenge free radicals. These are known to prevent oxidative damage caused by free radical (interfers the oxidation process by reacting with free radicals, chelating, catalytic metals) [9, 10].
The plants namely Usnea longifolia, Cetraria nepalensis, Parmelia minarum, Everniastrum nepalense, Rhododendron anthopogon and Fritillaria delavayi are well known for their medicinal values but their antioxidant potential is not still revealed. The sole objective of this study was to investigate and evaluate the antioxidant capacities of crude extracts of medicinal plants by two different methods viz. DPPH free radical scavenging assay and ferric reducing antioxidant power (FRAP) assay. The total phenolic contents of these plants were also estimated with comparison to their antioxidant capacities.
2. Materials and Methods
2.1. Chemicals
All the chemicals used in the study were of analytical grade and were purchased from Sigma Chemical Co. (St. Louis, USA).
2.2. Study Site and Sample Collection
The medicinal plants viz., Usnea longifolia (aerial part), Cetraria nepalensis (whole plant), Parmelia minarum (whole plant), Everniastrum nepalense (whole plant), Rhododendron anthopogon (twigs) and Fritillaria delavayi (seeds) were collected in August 2011 (permission granted by the Department of National Parks and Wildlife Conservation, Babarmahal, Nepal) from Manaslu Conservation Area (27˚39'15.7"N, 85˚18'43.4"E; Elevation: 4600 m asl). Plant specimens were identified with the aid of available herbarium specimens and different literatures. Voucher samples (Accession codes: UL3, C2, PM1, EN6, RA2 and FD1) were deposited in the NAST laboratory for future reference. The plant materials were dried at room temperature and were ground to powder with the aid of grinder.
2.3. Extraction
Each powdered sample was extracted by Soxhlet (Borosil, Gujarat Borosil Ltd., India) extraction using methanol as an extracting solvent of analytical grade (99% Assay). The extracts obtained were concentrated to dryness using a rotary vacuum evaporator (Hahnvapor, Hahnshin Scientific Co., HS-2005V-N) [11].
The yield of each extract was calculated as:

2.4. Antioxidant Capacity Assays
Total phenolic content by Folin-Ciocaltau and antioxidant capacities by Ferric reducing activity power and 2,2-diphenyl-1-picrylhydrazyl assays were carried out of the selected medicinal plants. An aqueous stock solution of concentration 50 mg/mL was prepared by dissolving 0.25 g of each extract in 5 mL double distilled water (ddH2O).
(1) Folin-Ciocaltau Assay
Two different working solutions (2 and 10 µg/mL concentrations) of all the methanolic extracts were freshly prepared from the stock solution. 2.0 M Folin-Ciocaltau phenol reagent (0.5 mL) was added to 1 mL of each working solution (in a test tube) and vortex for 3 min, followed by the addition of aqueous Na2CO3 solution (2 mL; 75 g/L) with incubation in dark at room temperature for 1 h. The absorbance (760 nm) against ddH2O was recorded and the antioxidant concentration value was determined to gallic acid equivalent (GAE).
A linear curve of standard gallic acid concentration vs. absorbance was constructed employing a series of gallic acid solutions (12.5, 25, 50, 75, 100 and 125 µg/mL). The total phenolic content in the sample solutions was considered the slope of the linear curve derived from the constructed calibration graph and expressed in GAE/100 g dry mass [12].
(2) Ferric Reducing Activity Power (FRAP) Assay
The total antioxidant potential of each extract was determined using the ferric reducing antioxidant power (FRAP) assay using standard procedure [13]. The FRAP reagent was prepared freshly by mixing 25 mL of 300 mM acetate buffer (pH 3.6), 2.5 mL of 10 mM 2,4,6- tripyridyl-s-triazine (TPTZ) in 40 mM HCl solution and 2.5 mL of 20 mM FeCl3·6H2O solution. The acetate buffer was prepared by mixing 7.5 mL of 30 mM sodium acetate solution with 92.5 mL glacial acetic acid solution (1.7 mL/L). Thus prepared FRAP reagent was prewarmed at 37˚C before use.
The working solutions of 2 and 4 µg/mL concentrations of extracts were prepared. To each working solution (200 µL) taken in a test tube, 2800 µL of FRAP reagent was added, mixed homogeneously and incubated at 37˚C for 4 min. The analyses were performed in three replicates for each extract. The change in absorbance due to formation of the colored product (ferrous tripyridyltriazine complex) was determined (593 nm) using a UV/Visspectrophotometer (6715, Jenway) against ddH2O (employed as control).
A standard curve was plotted using aqueous solutions of FeSO4·7H2O with known concentrations (0.1, 0.2, 0.3, 0.4 and 0.5 µg/mL). The FRAP value obtained was considered as the slope of the linear curve derived from the constructed graph and expressed in µmol Fe(II)/100 g dry mass.
(3) 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
The antioxidant capacity of the extracts was also evaluated using DPPH• (a stable free radical) [14]. To generate the free DPPH• radical, 3.9 mg of DPPH in methanol (100 mL) was stirred overnight at 4˚C and then stored at −20˚C until use. Thus prepared solution was used as 0.1 mM DPPH• solution.
Two working solutions of concentrations 10 and 50 µM were prepared and 0.1 mM methanolic DPPH• solution (2.5 mL) was added to each working solution (0.5 mL). A control was prepared by mixing distilled water (0.5 mL) and 0.1 mM methanolic DPPH• solution (2.5 mL). These solutions were mixed homogeneously and then incubated at room temperature in dark (ca. 30 min). The absorbance was measured at 517 nm against the blank solution consisting MeOH (2.5 mL) and ddH2O (0.5 mL).
The inhibition of initial absorbance of the DPPH• solution was calculated by the equation as under:
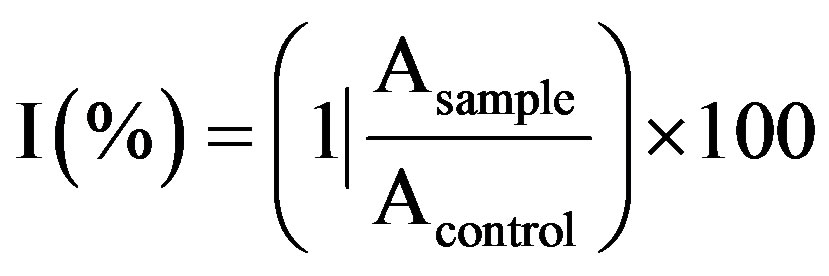
where, Asample and Acontrol are the respective absorbance values of the reaction mixture with and without sample.
The data obtained for % inhibitions at different concentrations were computed to calculate IC50. A standard linear curve was constructed using methanolic solutions of gallic acid with known concentrations (50, 75, 100, 125 and 150 µM). The DPPH value was then expressed in g GAE/100 g dry mass.
2.5. Statistical Analysis
The results of the extracts are expressed as the mean ± SE of three replicates (n = 3) in each test. A test for normality was performed on all data. Since normality was confirmed for all data, a parametric statistical test (twoway anova) was chosen for this study; the analysis of data between groups and pair-wise mean separations were carried out using Duncan’s Multiple Range Test (DMRT) at α = 0.05 to assess the statistical significance using SPSS version 16.0 (SPSS, Inc., USA) and Excel 2007 (Microsoft Office).
3. Result and Discussion
The antioxidant capacity of the studied medicinal plants were assessed by FRAP and DPPH assays (Table 1). The difference between two could be explained by different mechanisms of analytical methods. FRAP assay measures the ability to reduce ferric tripyridyltriazine (Fe3+TPTZ) to a ferrous form (Fe2+-TPTZ) while DPPH assay was used on the capability to donate a hydrogen radical or an electron to DPPH. Also, the total phenolics present in the sample or their reducing capacity were determined by FC assay. The beneficial effects that are derived from phenolic compounds have been attributed to their antioxidant activities [15].
Results showed that total phenolic concentration in extract of R. anthopogon was found to be high (10.9 GAE/100 g dried plant material), while it was lowest in F. delavayi (0.4 GAE/100 g dried plant material). In plants, phenolic compounds are one of the major groups of compounds with multiple biological effects including primarily acting as antioxidants or free terminators [16]. They are able to scavenge reactive oxygen species due to their electron donating properties. Their effective antioxidant properties depend on stability in different systems along with number and location of hydroxyl groups [17]. FC assay, despite being a rough estimation of total phenolics, it is rather a simple, rapid and a most popular method to evaluate plants antioxidant activities [18].
The phenolic compounds are well known to contribute towards quality and nutritional value through modifying color, taste, aroma, and flavor and also in providing health beneficial effects. They also serve in plant defense mechanisms to counteract reactive oxygen species (ROS) in order to survive and prevent molecular damage and damage by microorganisms, insects, and herbivores [19].
R. anthopogon had the highest antioxidant capacity analyzed by FRAP assay, followed by U. longifolia, Cetraria nepalensis, E. nepalense, P. minarum and F. delavayi (Figure 1). DPPH radical scavenging activity was observed in all the extracts (Figure 2). Generally, FRAP assay was used due to its simplicity and reproducibility. R. anthopogon extract showed dominant activity with lowest IC50 value (41.6 mg/mL) followed by P. minarum (62.2), Cetrarianepalensis (62.8), E. nepalense (64.7), F. delavayi (77.6) and U. longifolia (94.0) respectively. Since IC50 is a measure of inhibitory concentration, a
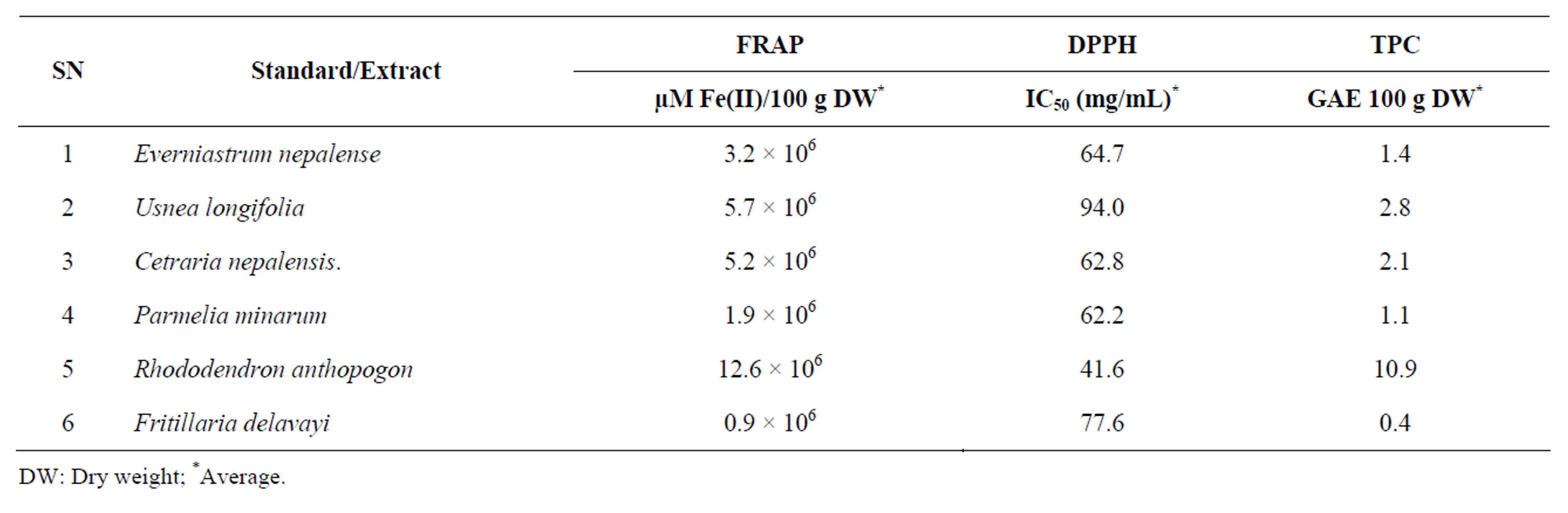
Table 1. Antioxidant capacity and total phenolic content estimation.
DW: Dry weight; *Average.
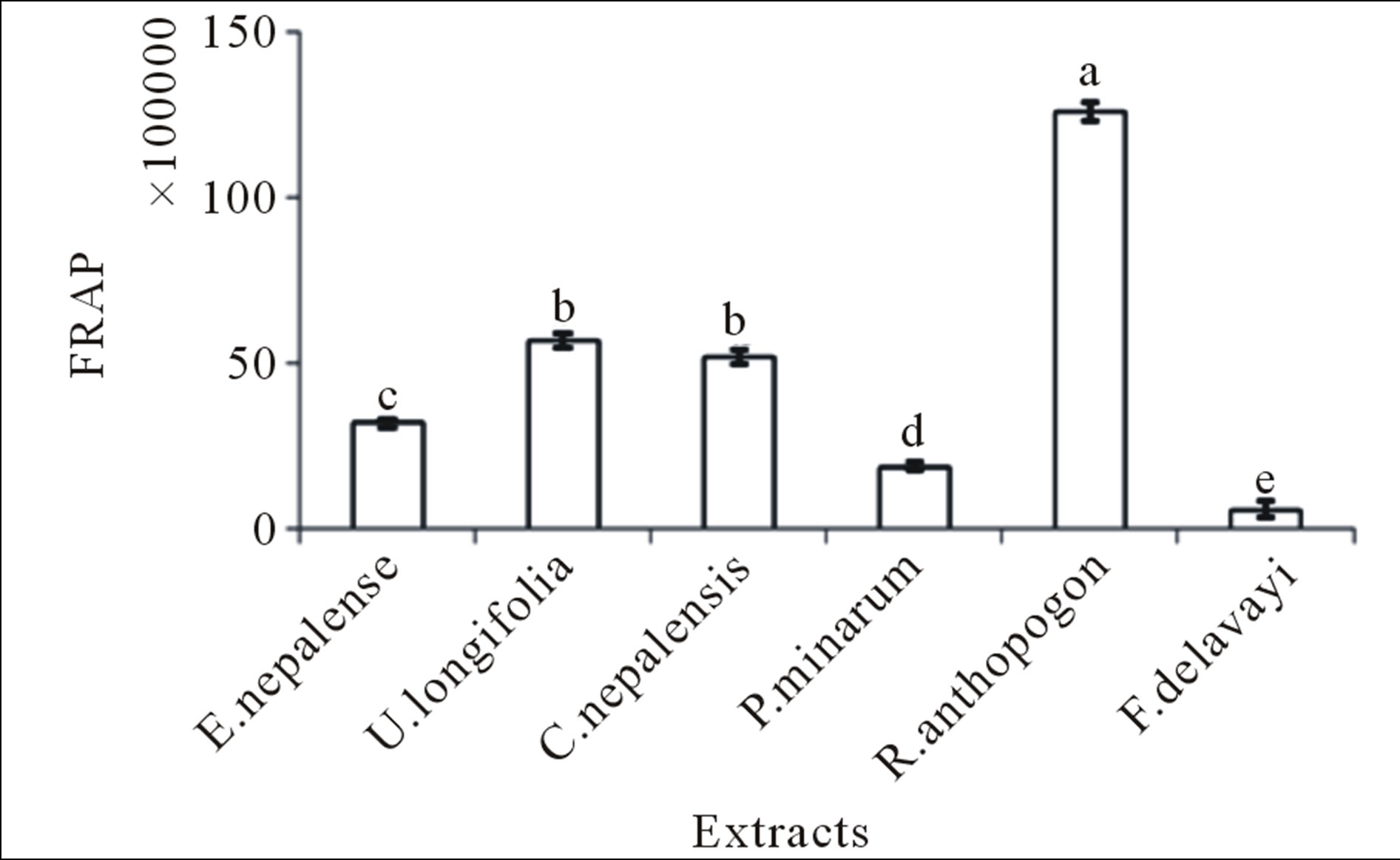
Figure 1. Total FRAP values for different plant extracts Mean ± SE (n = 3) with different superscripts at the top of bar represent that they are significantly different (P < 0.01) according to DMRT at α = 0.05. Superscript “a” indicates the highest value while “e” indicates the lowest value. The error bar in the figure represents the standard error (±1 SE).
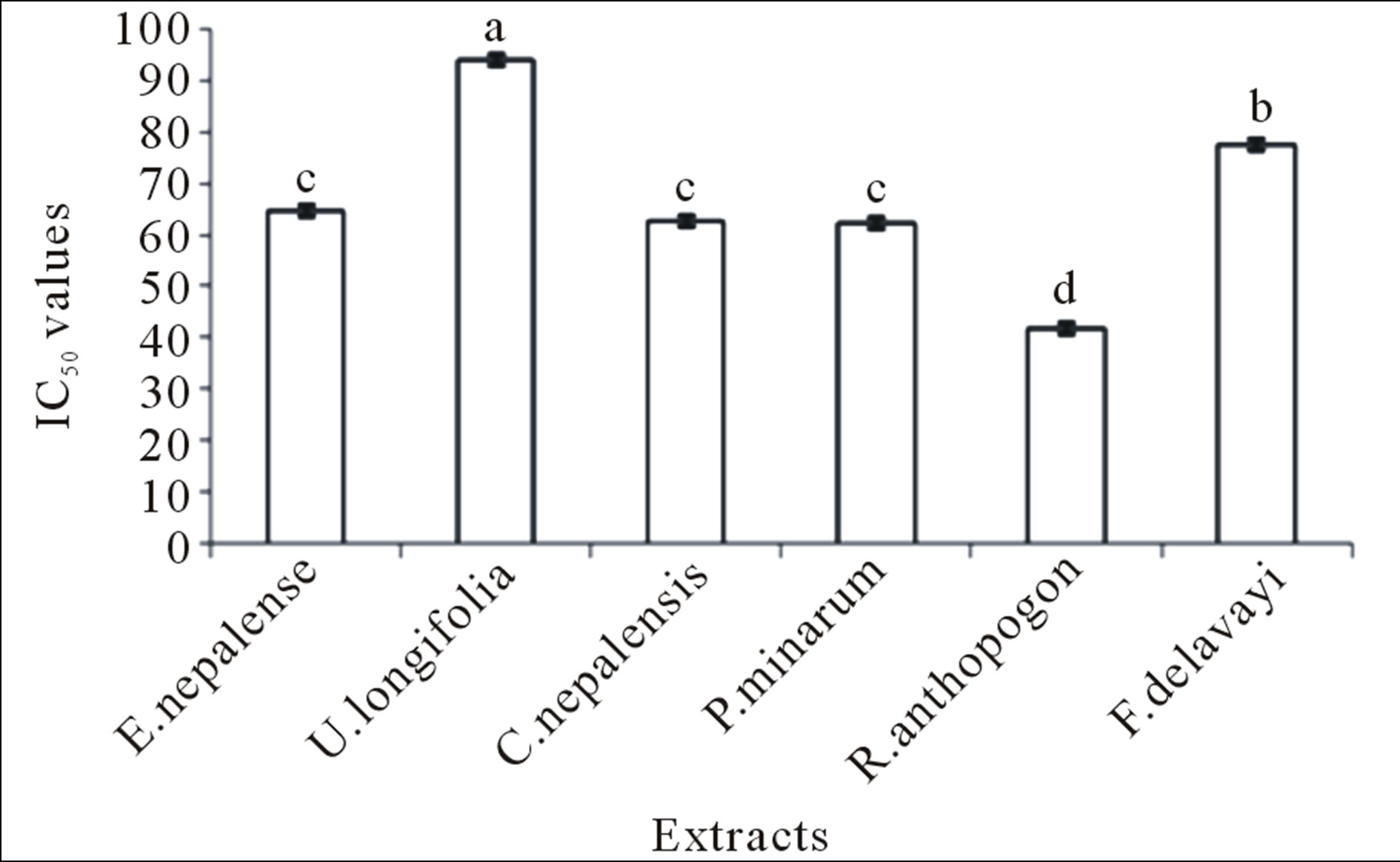
Figure 2. Total DPPH values for different plant extracts. Mean ± SE (n = 3) with different superscripts at the top of bar represent that they are significantly different (P < 0.01) according to DMRT at α = 0.05. Superscript “a” indicates the highest value while “d” indicates the lowest value. The error bar in the figure represents the standard error (±1 SE).
lower IC50 value would reflect greater antioxidant activity ofthe sample (Table 1). Scavenging of the stable radical (DPPH) is considered as a valid and easy assay to evaluate scavenging activity of antioxidants [20]. Hence, the results clarified that R. anthopogon has the greatest antioxidation potential among the plants tested. There was a linear increase in % inhibition with concentration of the sample (Figure 3)
The FRAP, DPPH and TPC values between the extracts showed the highly significant differences (P < 0.01) with F-values 387.4, 89.684 and 559.163 respectively (Figures 1-3). The obtained results indicated that, the antioxidant activity of the sample tested was not only dependent on the total phenolic content, instead the presence of different amounts of potent free radical scavengers imparts pronounced effect. Hence, it can be con-
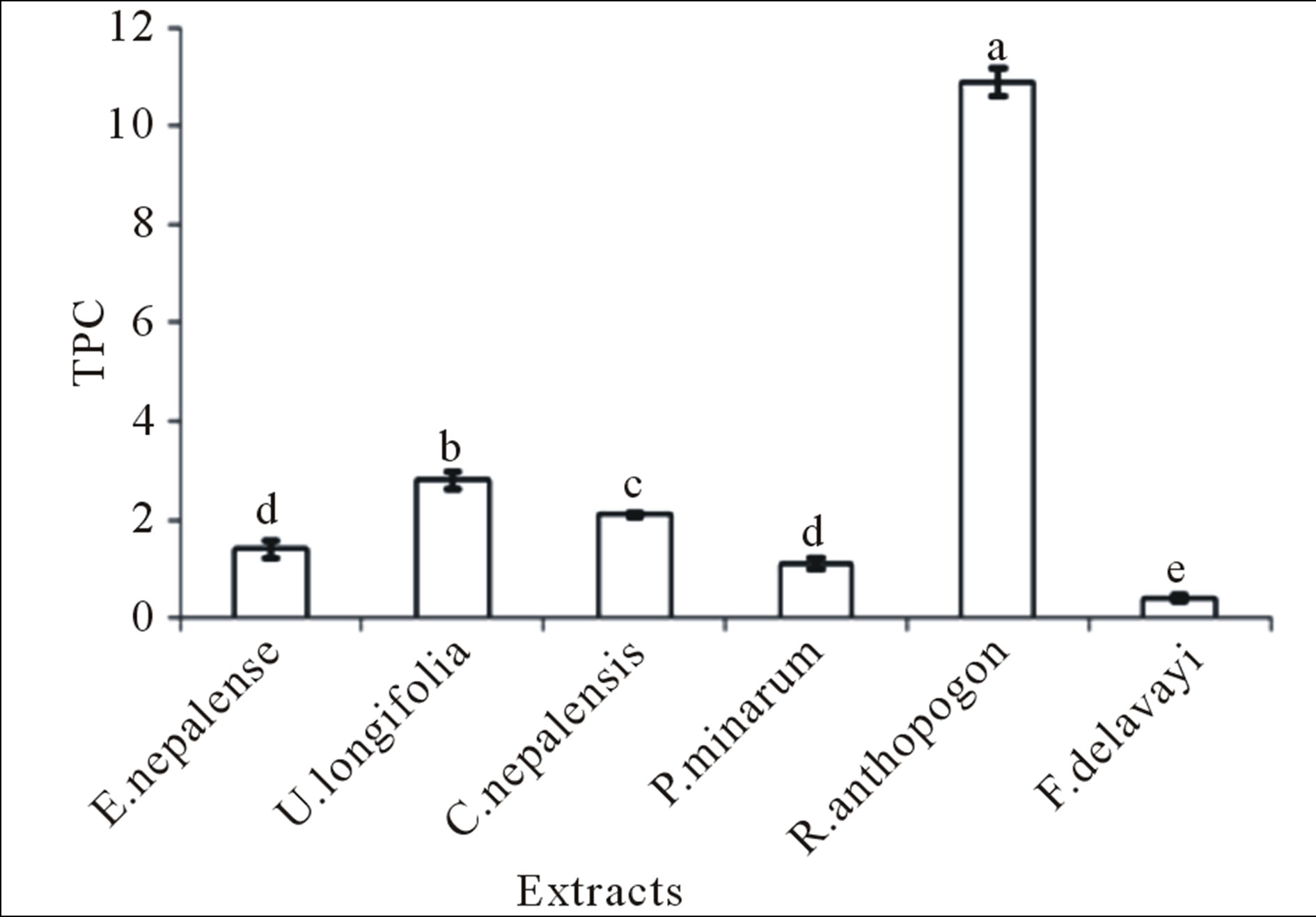
Figure 3. Total TPC values for different plant extracts. Mean ± SE (n = 3) with different superscripts at the top of bar represent that they are significantly different (P < 0.01) according to DMRT at α = 0.05. Superscript “a” indicates the highest value while “e” indicates the lowest value. The error bar in the figure represents the standard error (±1 SE).
cluded that R. anthopogon possesses the highest antioxidant capacity. Also, as R. anthopogon exhibited better free radical scavenging ability, these plants bear potent therapeutic potential and could highly be considered as potential source for drug discovery.
Several studies [21-23] reported that phenolic compounds in spices and herbs significantly contributed to their antioxidant properties. Secondary metabolites produced by lichens are mainly phenolic compounds [24]. Bahera et al reported the presence of phenolics with trihydroxy groups in the methanolic extract of lichen Usnea ghattensis [25]. And these phenolic compounds present in lichens are able to scavenge free radicals and active oxygen species [26,27]. In the overall, the synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) have been widely used for many years to retard lipid oxidation. However, the safety of using these synthetic antioxidants in food industry has become a concern among scientists and leading to current interest in uncovering natural antioxidants [28]. The results in this investigation have shown that medicinal plants may be good source of natural antioxidants. The potential of natural medicinal plants as an antioxidant in reducing such free radical induced tissue injury, suggests that many plants have antioxidant activities that can be therapeutically useful [29].
4. Conclusion
Based on the results, it can be concluded that methanolic extracts of the studied medicinal plants had different level of antioxidant potential. But among them, R. anthopogon was found to have higher antioxidant activity as determined by DPPH and FRAP assay and TPC. Further, isolation and identification of active components and evaluation of possible synergism among them for their antioxidant activity can be done.
5. Acknowledgements
We are highly indebted to the efforts of Rokesh Maharjan for his keen help in the research activities. We are grateful to different concerned authorities (Department of National Parks and Wild-life Conservation, Babarmahal, Nepal) for giving us permission to collect plants from the conservation area. Thanks are also due to NAST, who, despite harsh environmental constraints, whole heartedly supported us with the effective management of field trip to high Himalaya.
REFERENCES
- WHO, “World Health Organization: The World Medicines Situation 2011,” Traditional Medicines: Global Situation, Issues and Challenges, Geneva, 2011.
- LRMP—Land Resources Mapping Project, Survey Department, HMGN and Kenting Earth Sciences, Kathmandu, Nepal, 1986.
- B. Halliwell and J. M. C. Gutteridge, “Free Radicals in Biology and Medicine,” Oxford University Press, Oxford, 1999.
- K. J. Barnham, C. L. Masters and A. I. Bush, “Neurodegenerative Diseases and Oxidative Stress,” Nature Reviews Drug Discovery, Vol. 3, No. 3, 2004, pp. 205-214. doi:10.1038/nrd1330
- G. G. Duthie, S. J. Duthie and J. A. M. Kyle, “Plant Polyphenols in Cancer and Heart Disease: Implications as Nutritional Antioxidants,” Nutrition Research Reviews, Vol. 13, No. 1, 2000, pp. 79-106. doi:10.1079/095442200108729016
- N. Ito, S. Fukushima, A. Hasegawa, M. Shibata and T. Ogiso, “Carcinogenicity of Butylated Hydroxylanisole in F344 Rats,” Journal of National Cancer Institute, Vol. 70, 1983, pp. 343-344.
- M. Couladis, O. Tzakou and E. Verykokidou, “Screening of Some Greek Aromatic Plants for Aromatic Activity,” Phytotherapy Research, Vol. 17, 2003, pp. 194-196. doi:10.1002/ptr.1261
- R. Y. Gan, L. Kuang, X. R. Xu, Y. Zhang, E. Q. Xia and F. L. Song, “Screening of Natural Antioxidants from Traditional Chinese Medicinal Plants Associated with Treatment of Rheumatic Disease,” Molecules, Vol. 15, No. 9, 2010, pp. 5988-5997. doi:10.3390/molecules15095988
- F. Shahidi, P. K. Janitha and P. D. Wanasundara, “Phenolic Antioxidants,” Critical Reviews in Food Science and Nutrition, Vol. 32, No. 1, 1992, pp. 67-103. doi:10.1080/10408399209527581
- M. E. Buyukokuroglu, I. Gulcin, M. Oktay and O. I. Kufrevioglu, “In-Vitro Antioxidant Properties of Dantrolene Sodium,” Pharmacological Research, Vol. 44, No. 6, 2001, pp. 491-494. doi:10.1006/phrs.2001.0890
- K. S. Tiwari, S. N. Malhotra and N. K. Vishnoi, “A Textbook of Organic Chemistry,” 2nd Edition, Vikas Publishing House pvt. Ltd., Noida, 1992.
- A. Medina-Remon, R. Zamora-Ros, M. Rotches-Riballa, C. Andres-Lacueva, M. A. Martinez-Gonzalez, M. I. Covas, D. Corella, J. Salas-Salvado, E. Gomez-Gracia, V. Ruiz-Gutierrez, F. J. Garcia de La Corte, M. Fiol, M. A. Pena, G. T. Saez, E. Ros, L. Serra-Majem, X. Pinto, J. Warnberg, R. Estruch and R. M. Lamuela-Raventos, “Total Polyphenol Excretion and Blood Pressure in Subjects at High Cardiovascular Risk,” Nutrition, Metabolism and Cardiovascular Diseases, Vol. 21, No. 5, 2011, pp. 323- 331. doi:10.1016/j.numecd.2009.10.019
- I. F. F. Benzie and J. J. Strain, “The Reducing Ability of Plasma as a Measure of ‘Antioxidant Power’—The FRAP Assay,” Analytical Biochemistry, Vol. 239, No. 1, 1996, pp. 70-76. doi:10.1006/abio.1996.0292
- W. Brand-Williams, M. E. Cuvelier and C. Berset, “Use of a Free Radical Method to Evaluate Antioxidant Activity,” Food Science and Technology, Vol. 28, No. 1, 1995, pp. 25-30. doi:10.1016/S0023-6438(95)80008-5
- K. E. Heim, A. R. Tagliaferro and D. J. Bobilya, “Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships,” Journal of Nutritional Biochemistry. Vol. 13, No. 10, 2002, pp. 572-584. doi:10.1016/S0955-2863(02)00208-5
- N. C. Cook and S. Samman, “Flavonoids-Chemistry, Metabolism, Cardioprotective Effects and Dietary Sources,” Journal of Nutritional Biochemistry, Vol. 7, No. 2, 1996, pp. 66-76. doi:10.1016/S0955-2863(95)00168-9
- A. Podsedek, “Natural Antioxidants and Antioxidant Capacity of Brassica Vegetables: A Review,” LWT-Food Science and Technology, Vol. 40, No. 1, 2007, pp. 1-11. doi:10.1016/j.lwt.2005.07.023
- V. Roginsky and E. A. Lissi, “Review of Methods to Determine Chain-Breaking Antioxidant Activity in Food,” Food Chemistry, Vol. 92, No. 2, 2005, pp. 235-254. doi:10.1016/j.foodchem.2004.08.004
- J. Vaya, P. A. Belinky and M. Aviram, “Antioxidant Constituents from Licorice Roots: Isolation, Structure Elucidation and Antioxidative Capacity toward LDL Oxidation,” Free Radical Biology and Medicine, Vol. 23, No. 2, 1997, pp. 302-313. doi:10.1016/S0891-5849(97)00089-0
- M. Suhaj, “Spice Antioxidants Isolation and Their Antiradical Activity: A Review,” Journal of Food Composition and Analysis, Vol. 19, No. 6-7, 2006, pp. 531-537. doi:10.1016/j.jfca.2004.11.005
- B. Shan, Y. Z. Cai, M. Sun and H. Corke, “Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents,” Journal of Agriculture and Food Chemistry, Vol. 53, No. 20, 2005, pp. 7749-7759. doi:10.1021/jf051513y
- C. Q. Wu, F. Chen, X. Wang, H. J. Kim, G. Q. He, V. Haley-Zitlin and G. Huang, “Antioxidant Constituents in Feverfew (Tanacetum parthenium) Extractand Their Chromatographic Quantification,” Food Chemistry, Vol. 96, No. 2, 2006, pp. 220-227. doi:10.1016/j.foodchem.2005.02.024
- C. Wong, H. Li, K. Cheng and F. Chen, “A Systematic Survey of Antioxidant Activity of 30 Chinese Medicinal Plants Using the Ferric Reducing Antioxidant Power Assay,” Food Chemistry, Vol. 97, No. 4, 2006, pp. 705-711. doi:10.1016/j.foodchem.2005.05.049
- T. H. Nash, “Lichen Biology,” Cambridge University Press, Cambridge, 1996.
- B. C. Behera, N. Verma, A. Sonone and U. Makhija, “Optimization of Culture Conditions for Lichen Usnea ghattensis G. Awasthi to Increase Biomass and Antioxidant Metabolite Production,” Food Technology and Biotechnology, Vol. 47, No. 1, 2009, pp. 7-12.
- P. D. Duh, Y. Y. Tu and G. C. Yen, “Antioxidant Activity of Aqueous Extract of Harng Jyur (Chrysanthemum morifolium Ramat),” LWT-Food Science and Technology, Vol. 32, No. 5, 1999, pp. 269-277. doi:10.1006/fstl.1999.0548
- F. Atalay, M. B. Halici, A. Mavi, A. Cakir, F. Odabasoglu, C. Kazaz, A. Aslan and O. I. Kufrevioglu, “Antioxidant Phenolics from Lobaria pulmonaria (L.) Hoffm. and Usnea longissima Ach. Lichen Species,” Turkish Journal of Chemistry, Vol. 35, 2011, pp. 647-661.
- E. Karimi, H. Z. E. Jaafar and S. Ahmad, “Phenolics and Flavonoids Profiling and Antioxidant Activity of Three Varieties of Malaysian Indigenous Medicinal Herb Labisia pumila Benth,” Journal of Medicinal Plants Research, Vol. 5, No. 7, 2011, pp. 1200-1206.
- S. R. Kanatt, R. Chander and A. Sharma, “Antioxidant Potential of Mint (Mentha spicata L.) in Radiation-Processed Lamb Meat,” Food Chemistry, Vol. 100, No. 2, 2007, pp. 451-458. doi:10.1016/j.foodchem.2005.09.066
Abbreviations and Acronyms
ASL: above sea level;
GAE: Gallic acid equivalent;
FRAP: ferric reducing antioxidant power;
TPC: Total phenolic content;
FC: Folin-Ciocaltau;
TPTZ: 2,4,6-tripyridyl-s-triazine;
DPPH: 2,2-Diphenyl-1-picrylhydrazyl.

