American Journal of Plant Sciences
Vol.3 No.1(2012), Article ID:16631,11 pages DOI:10.4236/ajps.2012.31015
Nodulation Capacity of Argentinean Soybean (Glycine max L. Merr) Cultivars Inoculated with Commercial Strains of Bradyrhizobium japonicum
![]()
1Instituto de Fisiología Vegetal (INFIVE)-CONICET, Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, La Plata, Argentina; 2Instituto Fitotécnico de Santa Catalina, Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, Buenos Aires, Argentina; 3Embrapa, Centro Nacional de Pesquisa de Soja, Londrina, Brazil; 4Centro de Investigaciones de Fitopatología (CIDEFI), Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, La Plata, Argentina.
Email: *pbalatti@gmail.com
Received August 22nd, 2011; revised September 20th, 2011; accepted October 13th, 2011
Keywords: Nitrogen Fixation; Genetics of Nodulation; Soybean-BNF
ABSTRACT
The purpose of this research was to evaluate the nodulation potential of 31 Argentinean soybean commercial cultivars. Those with the highest nodulation capacity developed twice the amount of nodules than the low nodulating ones, which is the variation contained in soybean genotypes. Furthermore, this was not due to bacterial promiscuity, since the response was independent of the bradyrhizobia strain inoculated. The ability of cultivars to develop a larger number and biomass of nodules was unrelated with the maturity group they belong to and also was not a response to quorum sensing effects. Our results suggest that breeding programs can be aimed at improving the nodulation capacity of soybean and that cultivars from different maturity groups can be a source of nodulation QTLs.
1. Introduction
Nitrogen (N) is one of the most important nutrients that often limits plant growth and therefore yield. In modern agriculture, it is usually provided either by chemical or biological fertilization, being the latter one the plausible or less contaminating alternative for the environment and a sustainable agricultural management system.
Legumes belong to the third largest family among flowering plants (Fabaceae), which includes approximately 20,000 species [1,2]. Most of them, within the Papilionoidea subfamily, have the ability to establish a symbiotic association with soil gram (-) bacteria commonly known as rhizobium. The host plant nurse the bacteria with carbohydrates in structures known as nodules, where nitrogenase, a bacterial enzyme sensitive to high oxygen tensions, like that of the atmosphere, encounters the appropriate environment to fix atmospheric nitrogen into 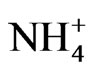 [3].
[3].
Soybean (Glycine max L. Merr) is the most important legume crop worldwide. Until 1980, Bradyrhizobium japonicum was considered the sole symbiont of soybean [4]. However, the isolation of rhizobia from new areas around the world and the availability of molecular techniques allowed researchers to identify and/or classify, already known rhizobia, as new symbionts of soybean. Among the genus Bradyrhizobium, three different species have been described, two slow growing and one extra slow growing rhizobia, Bradyrhizobium japonicum [4], Bradyrhizobium elkanii [5] and Bradyrhizobium liaoningense [6], respectively. In addition, in the early 1980s, several researchers also reported the isolation of fast growing organisms that developed nitrogen fixing nodules in soybean like Rhizobium sp. NGR234 [7,8] Sinorhizobium fredii [9], Sinorhizobium xinjiangensis [10,11]; and Mesorhizobium thianshanense [12].
There is a wide array of responses of soybean to rhizobium. While some cultivars are fully incompatible with rhizobia, others might exclude or restrict nodulation by bacteria belonging to certain serogroups of Bradyrhizobium japonicum [13]. Alternatively, soybean cultivars might be highly promiscuous like cultivar Mambloxi, which behaves like TGX African soybean cultivars [14]. In this regard, several genes controlling soybean nodulation have been described including those that emulate gene-for-gene relationships. While the rj1 allele [15] conditions a restriction of nodulation with a broad range of rhizobia, other genes like Rj2 [15], Rj3 [16], Rj4 [17], Rj5 and Rj6 [18] restrict nodulation only with defined members of bradyrhizobia serogroups. Furthermore, Nicolás et al. [19,20], Santos et al. [21] found that nodulation of soybean cultivars is also under the control of QTL (quantitative traits) such that soybean cultivars can be grouped in high and low nodulating ones.
Nodulation of soybean can also be restricted by the inoculum cell density, through a mechanism known as quorum sensing, response that is attenuated by the soil [22]. Quorum sensing is related to the synthesis and perception of signal molecules, known as autoinducers, which are produced by elevated population density that leads to the expression of a set of genes [23]. Takats [24] found that nodulation of plants was inhibited when they were inoculated with high density cell inoculum. More recently, bradyoxetin an autoinducer that is produced by Bradyrhizobium japonicum at high population densities, were found to repress nod gene [25]. Taken together these findings suggest that nod gene expression in Bradyrhizobium japonicum is regulated by autoinducers in a quorum sensing manner that might alter nodulation of soybean plants.
Though the role of the soybean genome in the soybean-rhizobia interaction has been known for a while, selection for nodulation and nitrogen fixation has not been a main objective of breeding programs and may be because of this, the symbiotic performance of most of the new cultivars might have been impaired. The biological nitrogen fixation capacity of soybean depends mostly on nodule mass [26-28]; and the specific activity of the bacterial nitrogen fixing enzyme, nitrogenase of the inoculated organisms [29]. Bohrer and Hungria [27] evaluated the nodulation capacity of North American and Brazilian soybean cultivars belonging to maturity groups VI-VIII. They found that cultivars not only differed in their nodulation response but also that they can be grouped in cultivars with high and low nodulation capacity.
In Argentina, like in Brazil soybean is an exotic species and it has to be inoculated, since rhizobia were not present in the soils. While the industry of inoculants is well developed in Argentina and companies and researchers have been looking for new highly efficient nitrogen fixing strains, not much work has been done from the plant side, in terms of selection for nodulation and nitrogen fixation. Therefore the purpose of this research was to evaluate the nodulation capacity of 31 Argentinean soybean commercial cultivars and identify among them those with contrasting nodulation capacity, being the most important aim the identification of cultivars with high nodulation and therefore nitrogen fixation potential.
2. Materials and Methods
2.1. Bacteria Maintenance and Inoculum Production
Bradyrhizobium japonicum strain SEMIA 5079, SEMIA 5080 and E109 were used as inoculant in the experiments. Stock cultures were kept at –70˚C in 10% glycerol [30]. Cultures were initiated by inoculating a loop of bacteria taken from agar slants that were kept in the fridge at 4˚C. Bacteria were grown in yeast extract mannitol (YEM) medium to late log phase [31] in an orbital shaker at 150 rev·min–1 at 28˚C. Cell density of the rhizobium suspension culture was adjusted by reading the O.D. at 625 nm of a serial dilution and plate counting aliquots. Cell density was adjusted as required with PBS buffer (7.2 g NaCl, 2.79 g Na2PO4H, 0.43 g PH2 per liter) [30].
2.2. Plant Materials
Seeds of commercial cultivars of soybean belonging to maturity groups II to VIII (Table 1) were provided by Nidera Company, Argentina. Seeds were surface sterilized by immersing them for 5 min in 20.83 M ethanol and 5 min in 1.5 M Clorox and then were rinsed 10 times with sterile distilled water.
They were transferred to petri plates containing 15 ml of 1% water agar and were incubated for 48 h at 30˚C in the darkness. Seedlings were inoculated by immersing the roots in commercial inoculants containing either ~109 CFU/ml cells of a mixture of B. japonicum strains SEMIA 5079 (CPAC 15) and SEMIA 5080 (CPAC 7) (both used in commercial inoculants in Brazil) or cells of B. japonicum E109 (USDA 138 derivative) (used in commercial inoculants in Argentina), as required. Plants were inoculated at the moment they were transplanted to sterile modified Leonard jars filled with vermiculite and were watered with N free Jensen nutritive solution [31]. After 3 days, plants were thinned to one per pot and were grown in the greenhouse at 25˚C, under a 14 h photoperiod. Humidity in the pots was maintained by refilling the bottom of the jars with deionized water. Since the evaluation of nodulation assays on a large number of cultivars with different bacterial strains is laborious we developed a cascade strategy. First we analyzed the nodulation ability of 30 cultivars inoculated with a mixture of strains B. japonicum SEMIA 5079 (CPAC 7) and SEMIA 5080 (CPAC 15). Based on the results from this experiment we selected fourteen cultivars for a second experiment, half of them with high and half with low nodulation capacity. In the third experiment, based on the preivous experiments we included two cultivars with high and two with low nodulation capacity. They were inoculated with B. japonicum strain E109.
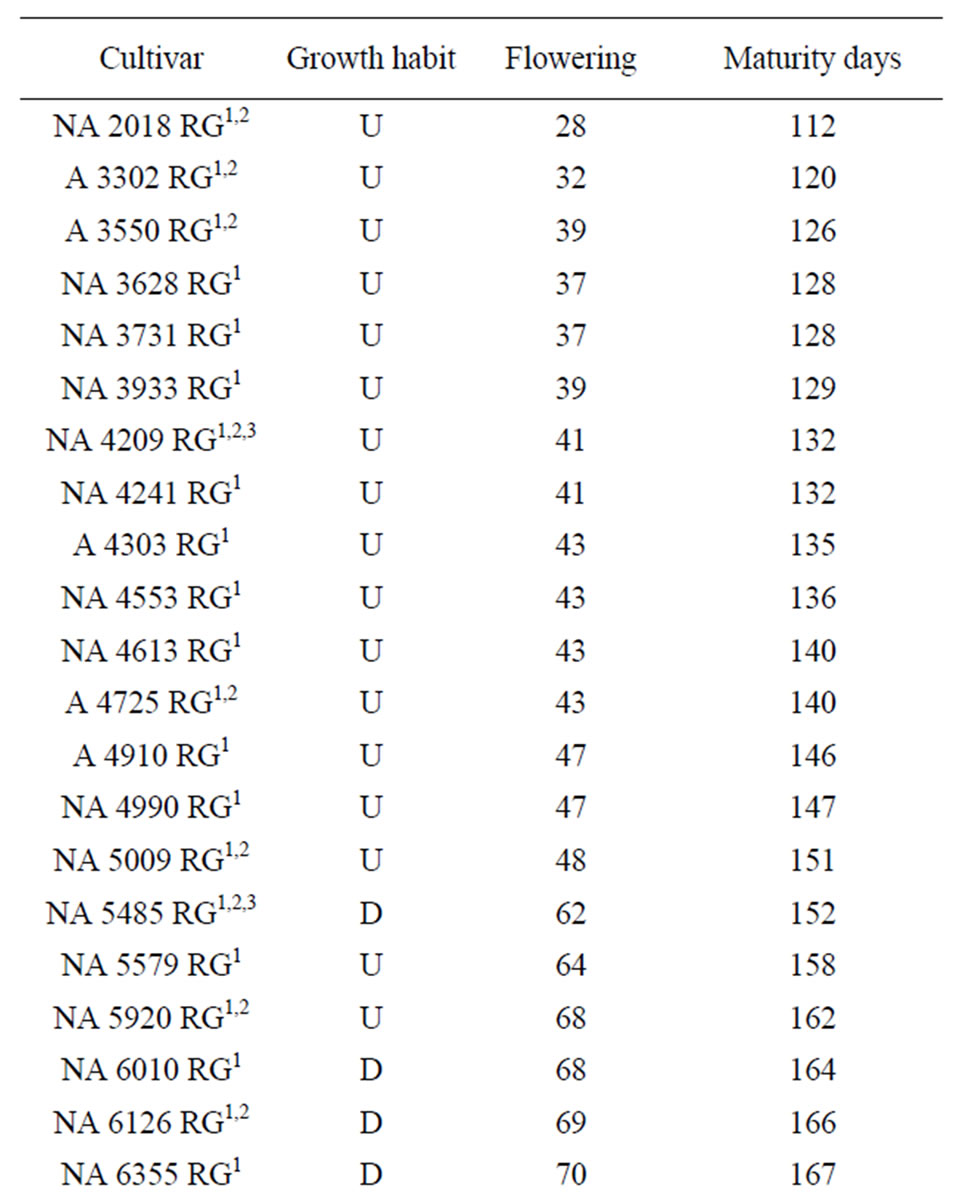
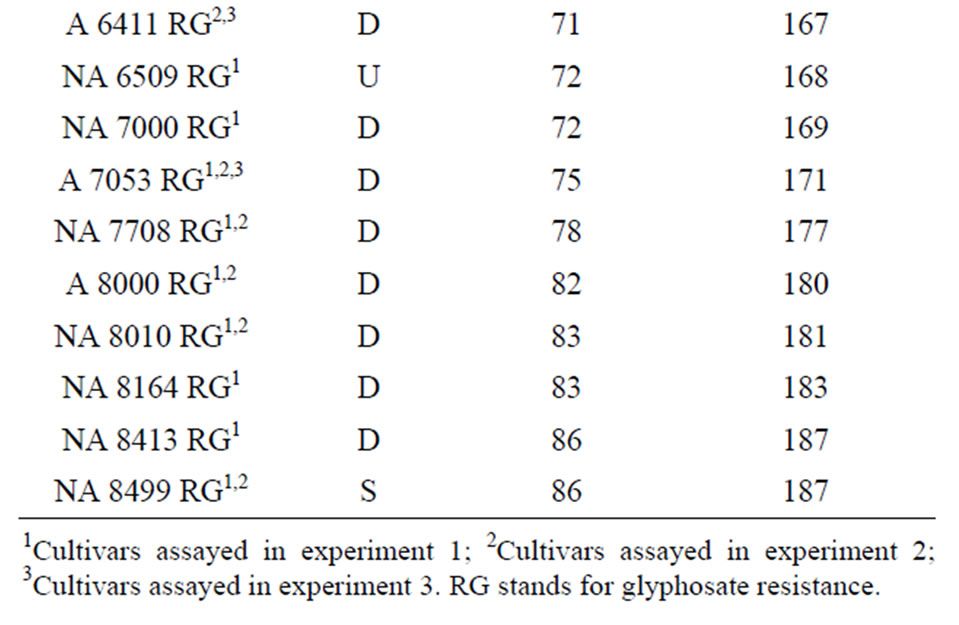
Table 1. Soybean cultivars included in these studies (Nidera Semillas). Maturity Groups: early (100 to 150 days to maturity), medium (116 a 150 days), late maturity more than 150 days to maturity. U: undeterminate growth, D: determinate growth, S: semideterminate growth; Flowering stands for the number of days from seeding to flowering; Maturity days are the number of days from seeding to R8.
In each experiment, plants were harvested and separated into shoots, roots and nodules thirty five days after germination. Nodule number (NN) was determined and shoot (SDW) and nodule (NDW) dry weight were evaluated after drying them at 65˚C until constant dry weight (approximately 48 h).
In the experiments with lower number of treatments (cultivars) a higher number of replicates were used. This is the reason that explains the different number of replicates used in each experiment.
2.3. Nodulation of Soybean Cultivars
2.3.1. First Experiment
Seedlings of soybean cultivars were inoculated with a mixture of strains SEMIA 5079 and SEMIA 5080 at a concentration of 109 cells·ml–1 [30]. We cultivated the plants under controlled conditions for 5 weeks [20] and evaluated the nodulation capacity of 30 commercial cultivar of soybean belonging to early, intermediate and late maturity group; which were marketed or ready to release cultivars (Table 1). With the aim of showing that there was no contamination of the plants and to avoid enlarging the number of pots, we included as uninoculated controls one cultivar belonging to each maturity group, which were mock inoculated with sterilized distilled water. The experiment was performed in a completely randomized design with four replicates per treatment [32].
2.3.2. Second Experiment
A new cultivar A 6411 RG was added to the fourteen cultivars that were selected from the first experiment and that according to nodule number and dry weight were clustered in three distinguishable groups, high, medium and low nodulating cultivars (Table 1). The whole procedure was identical to experiment I. The experiment was performed in a completely randomized design with 12 replicates.
2.3.3. Third Experiment
In this experiment only four cultivars were included, two with low (A 6411 RG and A 7053 RG) and two with high nodulation capacity (NA 5485 RG and NA 4209 RG). They were inoculated with strain E109 and the experimental design was completely at random with 10 replicates per cultivar. Cultivars NA 4209 RG and A 7053 RG were inoculated with sterile distilled water and considered as non-inoculated controls.
2.4. Nodulation Response to Bacterial Cell Concentration
Bradyrhizobium japonicum was grown in YEM [31] to late log phase. Cultures were serially diluted to 102, 104, 106 and 108 cells·ml–1 in sterile YEM and 1 ml aliquots of these dilutions were added to surface sterilized seeds of Glycine max (L.) Merr. Plant assays were done in sterile Leonard Jars assemblies containing vermiculite. After inoculation, seeds of cultivar NA 5485 RG and NA 6411 RG were covered with a one cm layer of vermiculite and after germination were thinned to two seedlings per jar. Plants were watered with N free nutrient solution [31] or distilled water in a growth chamber under a 16 h photoperiod and 26˚C. The number and dry weight of nodules and shoot dry weight were determined 35 days after inoculation. Nodules and shoots were dried by placing them in an oven at 60˚C until constant weight. The design was completely at random with 10 replicates per treatment.
2.5. Statistical Analysis
One-way ANOVA was used to analyze differences among cultivars followed by Tukey HSD test. In addition to this, a Dunnet test was used to estimate differences between cultivars and the controls [32].
The data obtained from the 14 cultivars that were included in the two first experiments were used to perform a factorial combined analysis of variance. It allowed us to determine the cultivars and environmental effects (assays) and the cultivar x environment interaction effect on nodulation ability [33]. Expected mean squares were estimated as it is shown:
Environment (E):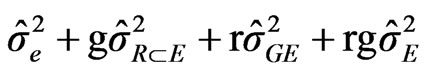 (1)
(1)
Blocks in Environment (Rep/E):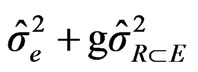 (2)
(2)
Cultivars (G): (3)
(3)
Cultivar × Environment (GxE):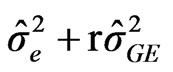 (4)
(4)
Error: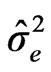 (5)
(5)
where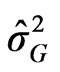 : represents variance among cultivars,
: represents variance among cultivars,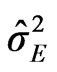 : variance among environment,
: variance among environment,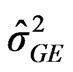 : variance due to cultivars by environment interaction,
: variance due to cultivars by environment interaction,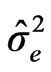 : variance due to error, g = number of cultivars, e = number of environments and r = the number of blocks or repetition/per experiment.
: variance due to error, g = number of cultivars, e = number of environments and r = the number of blocks or repetition/per experiment.
The calculated variances (Equation (1) to Equation (5)) allowed us to estimate the broad sense heritability ratio (He) of the traits [34] as:
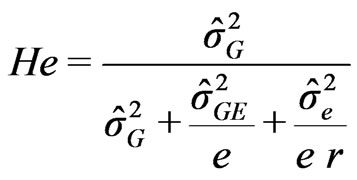 (6)
(6)
In order to get a measure of the relative importance of each factor studied (“Cultivar”, “Environment” and the interaction “Cultivar x Environment”), we determined the percentage of variation for each effect as the ratio between the sum of squares of the source of corresponding variation and the sum of squares of treatments [32].
Nodulation-related variables and life cycle were associated by means of the Pearson’s correlation coefficient [32].
3. Results
One of the problems of experiments evaluating cultivars is the number of plants included in the experiment, since we should include an adequate number of replicates. Because of this, when we evaluated the nodulation capacity among 30 soybean cultivars we included a low number of replicates. Since we wanted to increase the number of replicates to confirm the results we needed to reduce the number of cultivars, based on the results obtained in the first experiment. Therefore, in the second experiment the number of cultivars was reduced and we included those with high, medium and low nodulation capacity. The third experiment was aimed at evaluating if the soybean nodulation response was unrelated to the species. In this experiment, we evaluated the ability of two high and two low nodulating cultivars to develop nodules in association with strain E109. Cultivars were analyzed with regard to their ability to develop nodules, two had a high and two other ones low nodulation capacity. By using only these four cultivars we were able to increase substantially the number of replicates, which resulted in more reliable data.
3.1. First Experiment
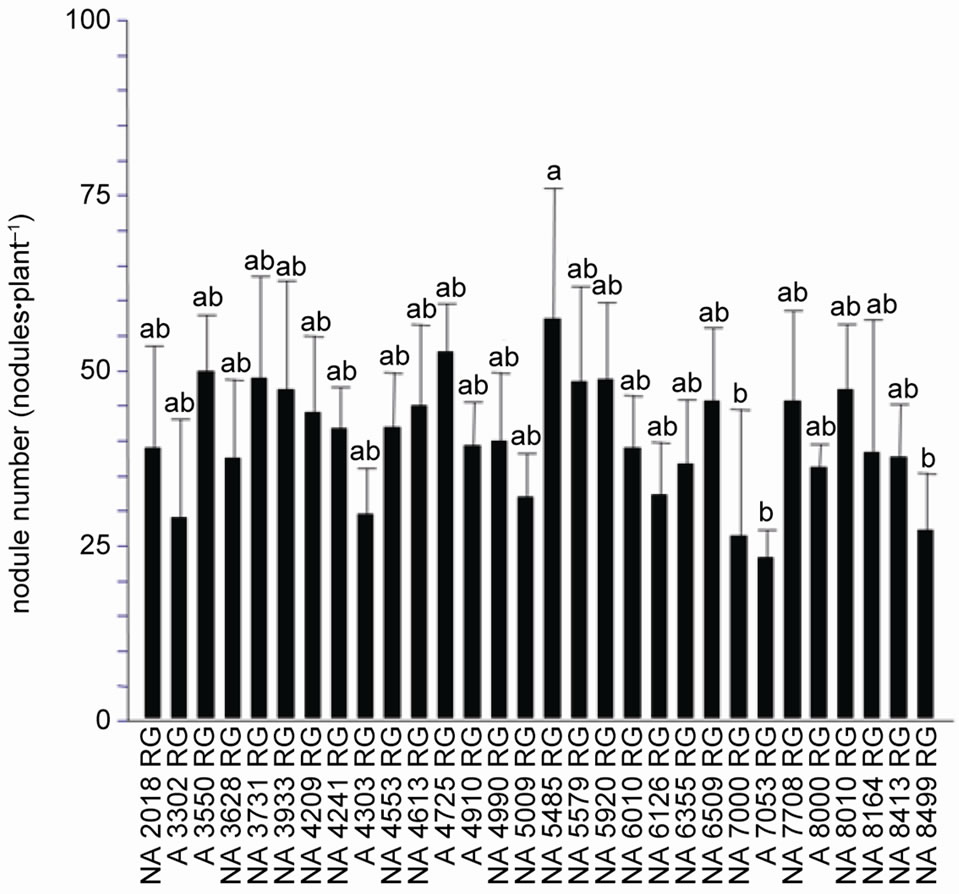
Soybean response to inoculation with Bradyrhizobium varied widely among cultivars. We found that nodule number (F = 2.33, p < 0.01) and dry weight (F = 1.96, p < 0.01) as well as shoot dry weight were significantly different between cultivars (F = 1.84, p < 0.05). Based on the analysis of NN and NDW, cultivars were clustered in two groups. One included cultivars with high nodulation capacity and the other one those with low. Cultivar NA 5485 RG had the highest nodulation capacity (Figure 1(a)) and cultivars NA 7000 RG, A 7053 RG and NA 8499 RG showed the lowest nodule number. NA 4209 RG presented the largest nodule biomass (NDW) while cultivars A 3302 RG and NA 8000 RG the lowest one (Figure 1(b)). The rest of the cultivars developed a number and nodule dry weight between these two extremes (Figure 1(a)). Cultivar A 7053 RG that presented the lowest nodule number developed 23 nodules·pl–1, which is a quantity approximately 60% lower than the number of nodules developed by high nodulation cultivar NA 5485 RG (57.5 nodules·pl–1). Based on the above results, genotypes were classified as low, middle and high nodulating ones (Figure 1(a)).
While the lowest nodule mass observed was 24.5 mg·pl–1, the highest one was 57.6 mg·pl–1 and corresponded to cultivar NA 4209 RG (Figure 1(b)).
As expected, there was a positive and highly significant correlation between nodule number and dry weight (r = 0.69, p < 0.01). If cultivars are organized based on the
 (a)
(a)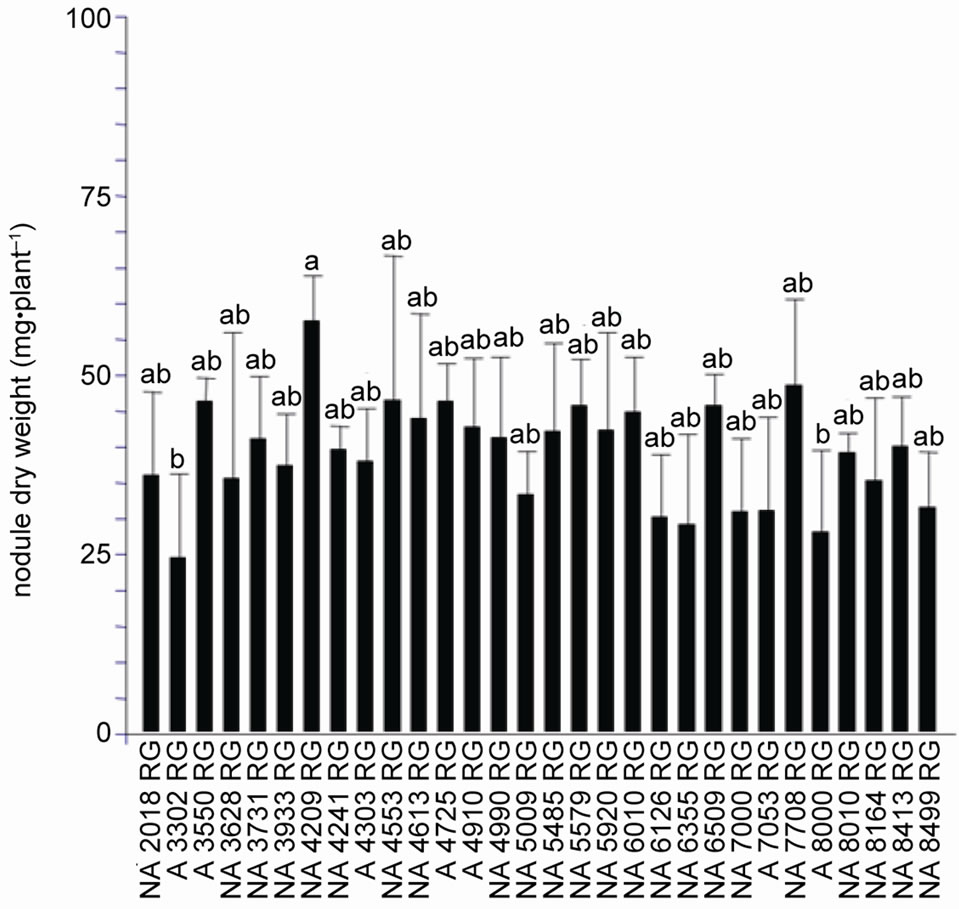 (b)
(b)
Figure 1. Nodulation response of thirty soybean cultivars to inoculation with a mixture of Bradyrhizobium japonicum strains SEMIA 5079 and SEMIA 5080 (average values ± standard deviation), the cell concentration of the inoculum was 1 × 108 rhizobia·ml–1. (a) Nodule number; (b) Nodule dry weight. Same letter indicates no significant difference (Tukey 5%).
nodule number they developed, those within the uppermost and lowermost values coincidentally presented a higher and lower nodule dry weight, respectively. Furthermore, shoot dry weight was also highly correlated with both, nodule number and nodule dry weight (r = 0.53, p < 001 and r = 0.67, p < 0.01, respectively).
However no correlation was found between nodulation capacity-related variables such as NN and NDW and SDW and days to maturity (MD) (p > 0.01).
3.2. Second Experiment
In the second experiment, fourteen cultivars (Table 1) were evaluated (see Materials and methods), and they included representatives of the groups with high, middle and low nodulation capacity, in addition to a new cultivar that was introduced, A 6411 RG.
Nodule number (F = 8.00, p < 0.01) and dry weight (F = 6.65, p < 0.01) and shoot dry weight (F = 13.00, p < 0.01) were significantly different among cultivars. While NA 5920 RG and NA 5485 RG presented the highest values either in nodule number, nodule dry weight or shoot dry weight (Figure 2), cultivar NA 2018 RG only presented a significantly higher nodule biomass. On the contrary, cultivars A 6411 RG, A 7053 RG, NA 8010 RG and A 3302 RG developed the lowest nodule biomass (Figure 2(b)). In addition to this, A 6411 RG and NA 7053 RG did not differed significantly from the uninoculated controls for SDW as shown by the Dunnet (5%) test (Figure 2(c)). Furthermore, both cultivars were the least responsive to inoculation, since they developed the lowest number of nodules and as a result of this the plants had a low shoot dry weight.
Although the experiments with soybean cultivars showed a wide and diverse array of responses to inoculation with rhizobia, the results of the second experiment allowed us to classify genotypes based on their differential response into a higher number of groups. While NDW and NN presented a lower range of variation, SDW presented a higher one. Differences within the uppermost or lowermost values of number and dry weight of nodules were of 34.2 nodules and 33.1 mg versus 15.1 nodules and 27.3 mg per plant, for the first and the second experiment, respectively. Shoot dry weight showed a range of 594.1 mg and 688.9 mg for the first and second experiment, respectively.
The analysis of experiments 1 and 2 together included those cultivars evaluated in both of them and showed that there was a significant effect of “experiment” and “cultivars” upon nodule number and dry weight as well as on plant dry weight. The effect of the “Cultivar × Environment” interaction was significant except for plant shoot dry weight. This means that although the difference between experiments was significant, the relation between cultivars responses within experiments was similar. Biomass traits; NDW and SDW, both presented experimental errors higher than 40% of the total variation (Table 2).
As a result of this and considering the average of the simple effect “Cultivars” in both assays, those with the
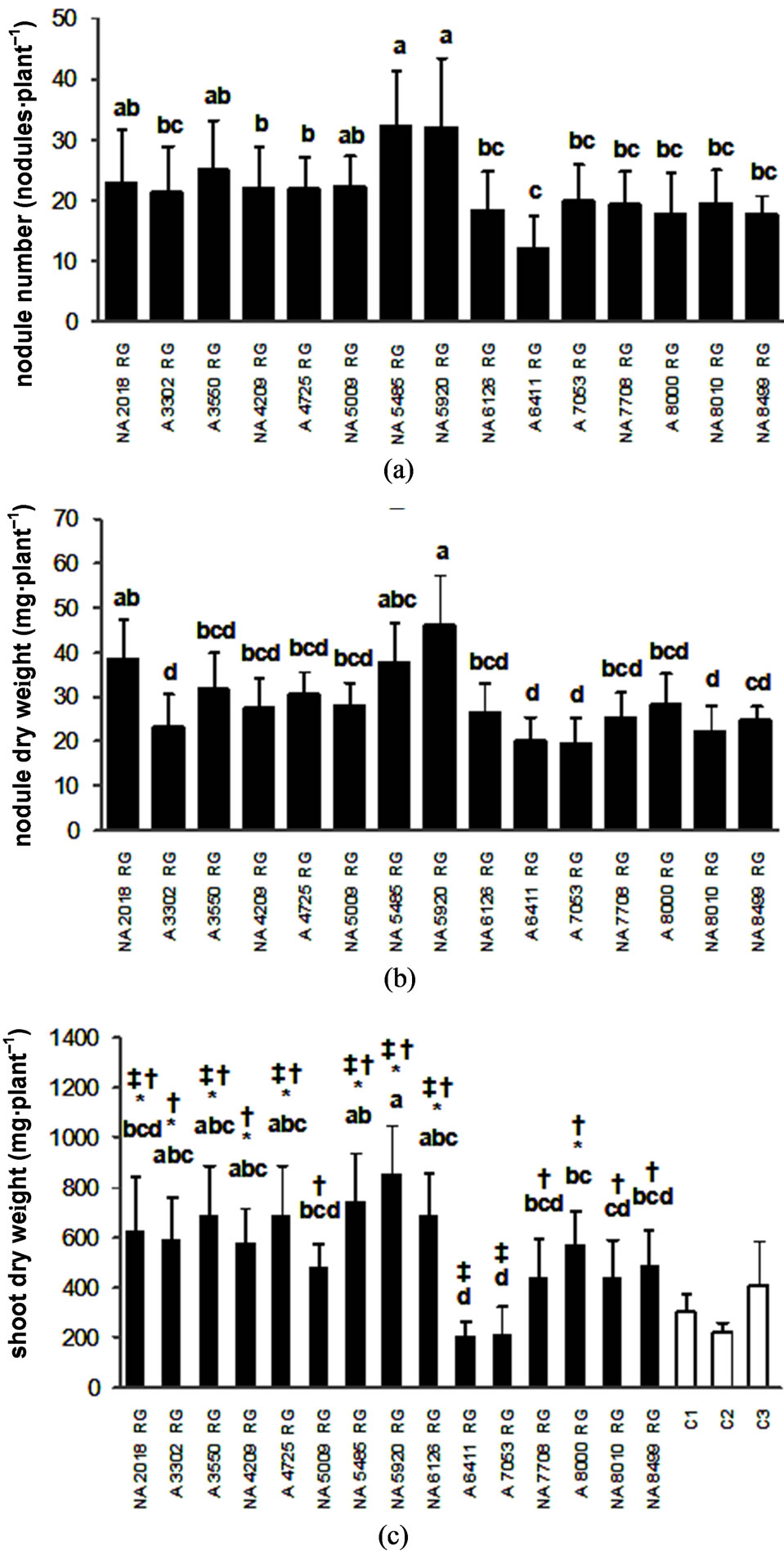
Figure 2. Response of Soybean genotypes to the inoculation with a mixture of Bradyrhizobium japonicum strains SEMIA 5079 and SEMIA 5080 (average values ± standard deviation). The concentration of the inocula was 1 × 108 rhizobia·ml–1. (a) Nodule number; (b) Nodule dry weight and (c) Shoot dry weight. Same letter indicates no significant difference (Tukey 5%). Controls were mock inoculated with sterile distilled water, control 1 (C1) corresponds to mock inoculated of early maturity group soybean plants (cultivar NA 3731 RG), control 2 (C2) corresponds to mock inoculated of middle maturity group soybean plants (cultivar NA 5579 RG) and control 3 (C3) corresponds to mock inoculated of late maturity group soybean plants (cultivar A 8000 RG). (*) significant difference between cultivar and C1 by Dunnet test. (†) significant difference between cultivar and C2 by Dunnet test. (‡) significant difference between cultivar and C3 by Dunnet test.
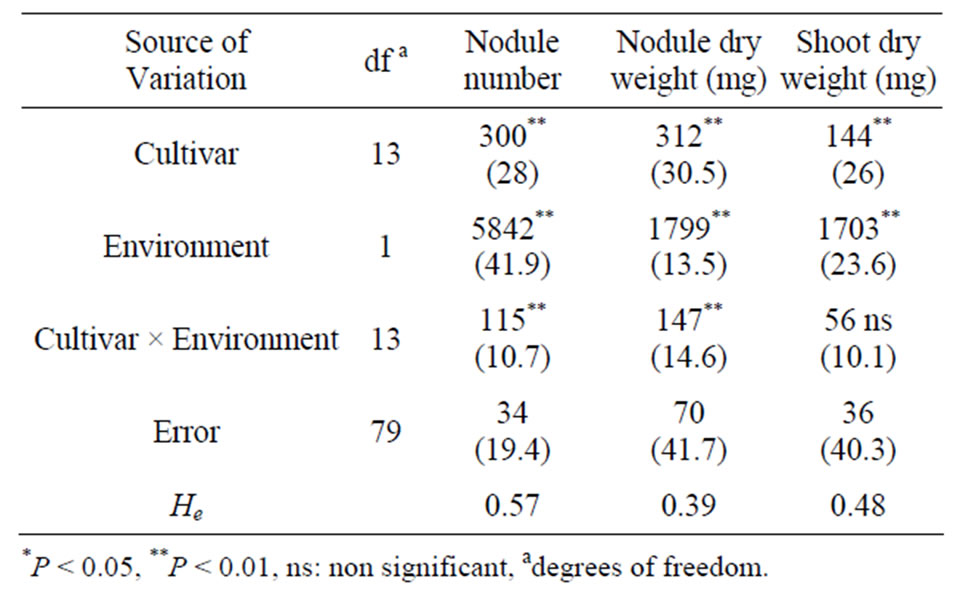
Table 2. Statistical analysis of nodule number, nodule dry weight and shoot dry weight of soybean genotypes inoculated with a mixture of Bradyrhizobium japonicum strains. Mean squares and percentage (%) of total variation explained by each source of variation. He stands for broadsense heritability values.
highest and lowest nodule dry weight were selected for further studies.
3.3. Third Experiment
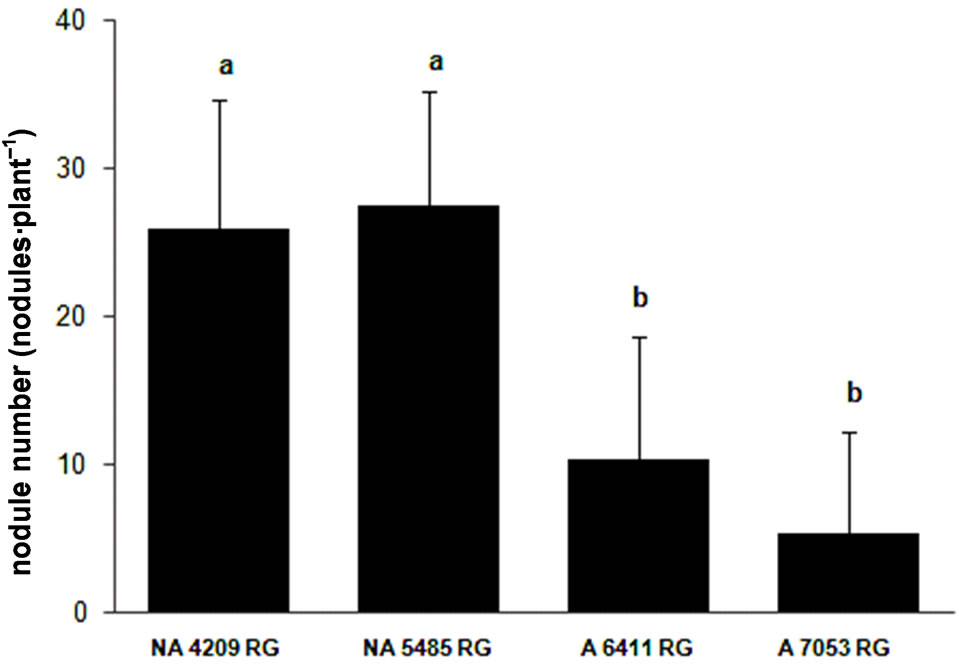
The third experiment was aimed not only at confirming the differential nodulation capacity of the cultivars but also at evaluating if nodulation was a genotype characteristic and was not due to a specific effect of bacterial strains. We included four cultivars, two with high (NA 5485 RG and NA 4209 RG) and two other ones (A 6411 RG and A 7053 RG) with low nodulation capacity, which were inoculated with only an Argentinean commercial strain E109.
The statistical analysis of the data (ANOVA) showed that the cultivars differed in NDW (F = 9.64, p < 0.01), NN (F = 4.94, p < 0.01) and SDW (F = 5.08, p < 0.01). The cultivars that were selected based on their high nodulation capacity with Brazilian strains SEMIA also developed high NN and dry weight when they were inoculated with strain E109 (Figure 3). Furthermore, the findings were confirmed by the plant biomass that was significantly higher in the cultivars that produced the highest number and dry weight of nodules.
3.4. Nodulation Response to Bacterial Cell Concentrations
Nodulation in the Bradyrhzobium japonicum-soybean response symbiosis might be restricted and/or suppressed in a density dependent manner known as quorum sensing. To determine if soybean cultivar nodulation was differentially affected by high cell population, Glycine max L. Merr cultivars NA 5485 RG and A 6411 RG were inoculated with 102, 104, 106 and 108 cells·ml–1 of B. japoni-
 (a)
(a)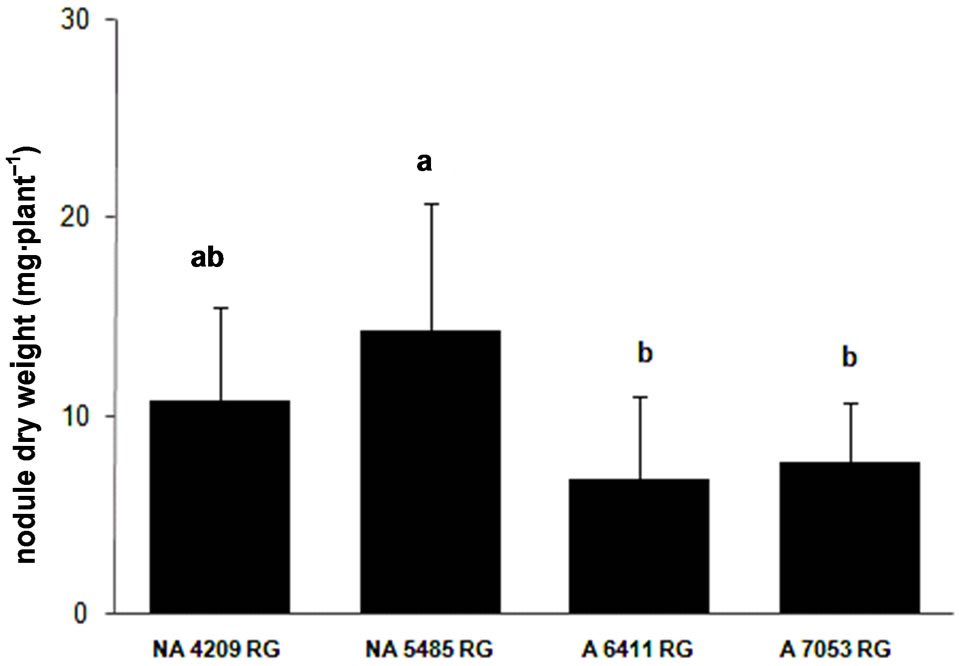 (b)
(b)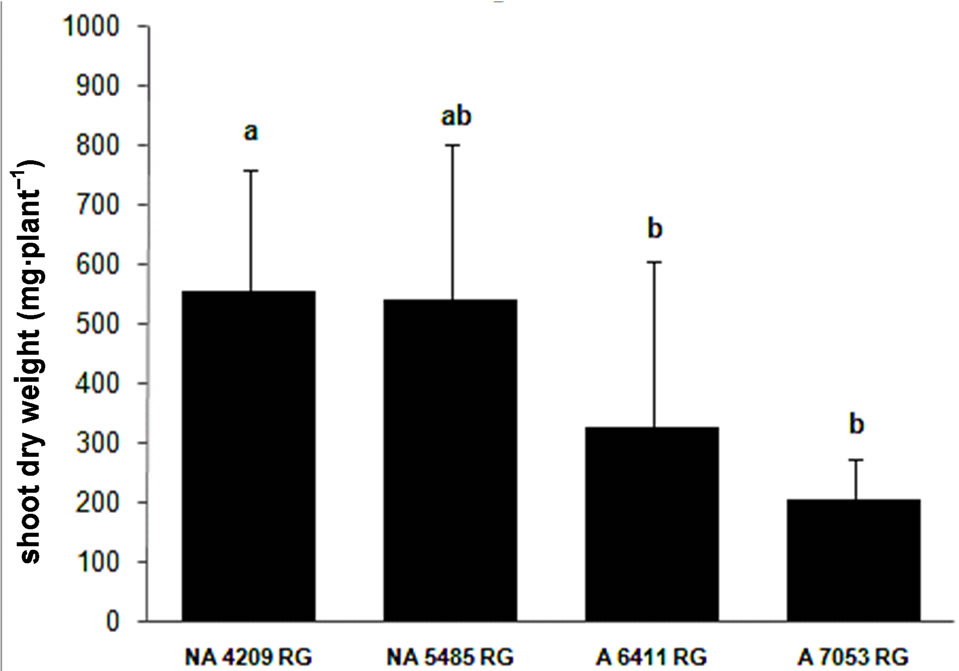 (c)
(c)
Figure 3. Nodulation response of four cultivars of soybean to inoculation with Bradyrhizobium japonicum strain E109 the commercial strain used in inoculants in Argentina (average values ± standard deviation). (a) Nodule number; (b) Nodule dry weight and (c) Shoot dry weight. The concentration of the inocula was 1 × 108 rhizobia·ml–1. Same letter indicates no significant difference (Tukey 5%).
cum E109. The results are presented in Figure 4. It can be seen that the rhizobium cell concentration did not alter the soybean cultivar response to inoculation. This is to say cultivar NA 5485 RG developed a much higher number of nodules than A 6411 RG independently of the rhizobium cell concentration of the inoculum. However, the results showed that when a high cell density was inoculated it induced a reduction on nodulation on both high and low nodulating cultivars.
4. Discussion
Argentina is one of the main producers of soybean. The cultivated area is of approximately 19 million ha and considering an average yield of approximately 2,800 kg·ha–1 the total production is approximately 50 million tons [35]. Since the N harvest index of soybean is 60% the total amount of N required by such production represents an insurmountable amount of money in terms of fertilizer. Because of this, biological nitrogen fixation appears as the alternative N source, that turns out to be particularly important in developing countries.
In Argentina there are several companies that produce inoculants of high quality with selected nitrogen fixing strains, however to our knowledge studies regarding the nodulation capacity of plant genotypes are lacking.
The interaction between soybean and B. japonicum, like other plant microbe interactions such as diseases caused by pathogens, can be represented by a triangle formed by the plant and microorganism’s genome under the influence of the environment [13]. This means that the interaction is under the effect of these three components plus their interactions [36]. The Bradyrhizobium-soybean symbiosis has been largely studied, and there are many studies upon the effect of environmental variables upon the process and the same holds true for B. japonicum strain selection. However, even though the role of the plant genotype on nodulation has been known for a while [37-39], only recently it has gained importance [19,20]. This is particularly relevant considering that the development of nitrogen fixing nodules is a complex and highly regulated process that requires the synthesis and exchange of molecular signals between partners, in this case soybean and Bradyrhizobium.
Soybean genotypes from Argentina presented a differential response to inoculation most probably due to differences at the genetic level, much alike the results obtained with Brazilian cultivars [27,28]. If we arrange the 30 soybean cultivars tested based on the number or dry weight of nodules, the group of bars corresponding to the response of each cultivar made up a straight line within the lowest and highest values, which were 23 and 57 (nodules), 25 and 57 mg (nodule dry weight). The lack of discontinuities in the distribution frequency might be
 (a)
(a)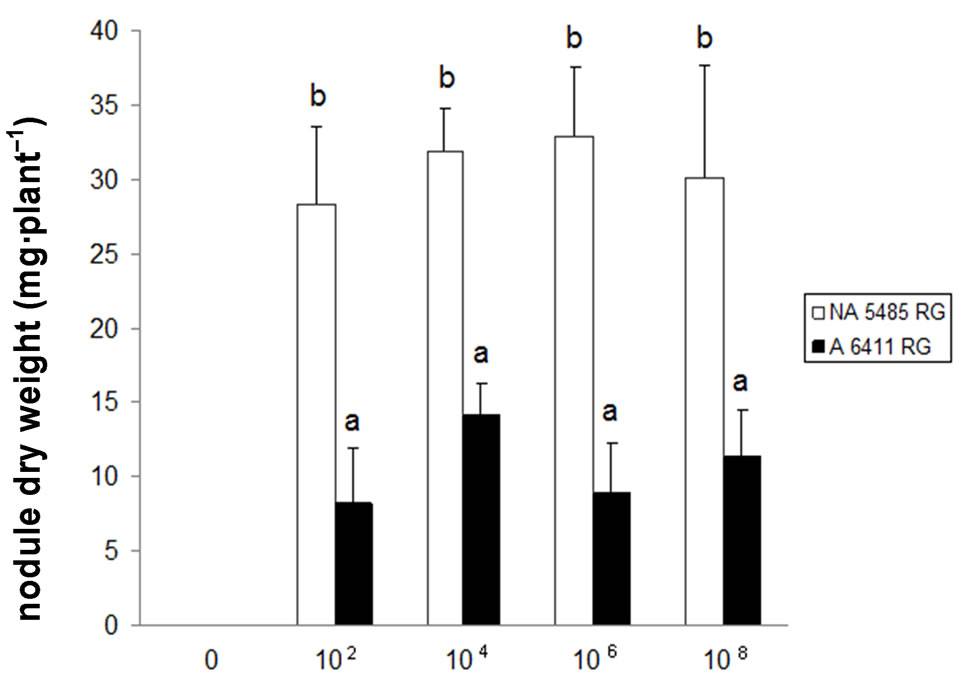 (b)
(b) (c)
(c)
Figure 4. Bradyrhizobium japonicum cell density effect upon nodulation of Glycine max L. Merr cultivar NA 5485 RG (white) and A 6411 RG (black), average values ± standard deviation. (a) Nodule number; (b) Nodule dry weight and (c) Shoot dry weight. Values of the X axis stands for number of bradyrhizobium cells·ml–1. The concentration of the inoculums was adjusted by resuspending rhizobia on sterile PBS as required. Control plants were mock inoculated with sterile PBS buffer.
indicative of polygenic inheritance, which is in agreement with other authors findings [19,39]. Furthermore, those cultivars that developed a higher number of nodules presented a twofold nodule number over those with low nodulation capacity, which was confirmed in the following experiments. Sinclair et al. [39] and Hungria and Bohrer [28] also found that high nodulation cultivars presented a twofold increment in nodule number over those that nodulate the least. Therefore it appears that the twofold difference in nodule number might be the diversity contained in today’s soybean cultivars in terms of nodulation potential.
The environment has a profound impact upon genotypes nodulation [40,41], which can be verified by comparing the nodule number and mass developed by the cultivars included in the first and second experiments, since in the later one, nodule number was half of that observed in the first experiment. Even though the maximum and lowest nodule number developed by soybean cultivars in each experiment was different, the ability of the cultivars to develop nodules remained unchanged. This means that whether the environmental conditions favored or not nodulation, differences between cultivars were still evident.
The inoculated strain is not the explanation for the differential number of nodules developed by the cultivars, since soybean cultivars responded similarly to inoculation with different strains, as suggested by the nodule number and dry weight observed when they were inoculated either with a mixture of B. japonicum SEMIA 5079 and SEMIA 5080 or B. japonicum E109 alone. This confirms that ability of the plant to nodulate is defined by the plant genotype.
Nodulation is unrelated with the life cycle of the genotype. Cultivars either with the ability to develop high or low number of nodules included genotypes with long and short life cycles (days to maturity) such as A 3550 RG and NA 5920 RG and A 7053 RG, which is in agreement with the findings of Sinclair et al. [39]. On the contrary, Balatti and Pueppke [42] while studying the efficiency of soybean genotypes to nodulate with fast growing Ensifer (Sinorhizobium fredii) found that the length of the genotype life cycle was correlated with the ability of the cultivars to develop nitrogen fixing nodules or inefficient nodules. Evidently the pathways involved in the plant response to infection and nodulation with Ensifer (Sinorhizobium) fredii might be different or might differ, at least in part, with those used by soybean to interact with Bradyrhizobium japonicum.
Based on both the simple correlation multivariate linear regression analysis (data not shown), NDW was the variable that mostly conditions shoot biomass, a variable highly dependent on nitrogen availability. Furthermore the heritability of this trait was found to be 0.39. Altogether these findings suggest that nodule dry weight is a character that might help breeders to select genotypes based on N fixation. While Nicolás et al. [19] found narrow-sense heritability for the traits NN and NDW (39% to 77%). Greder et al. [43] found nodule mass heritability values ranging from 54% to 67% in soybeans inoculated with B. japonicum. Our results confirm these findings though the broad-sense heritability estimation was measured in a controlled environment. Altogether previous results and ours show a complex nature of growth and nodulation related traits because of their interaction with the environment.
Cell density of rhizobia did not alter the cultivars response to inoculation suggesting this that nodulation was not the result of suboptimal bacterial numbers or the inefficiency of the strains to induce nodules on soybean. Furthermore, the suppression of nodulation observed on both high and low nodulating cultivars, probably due to the high cell density of the inoculant suggest that the phenomenon might be a general response of soybean cultivars as has already been suggested by Jitacksorn and Sadoswsky [22].
No previous studies regarding the nodulation capacity of soybean genotypes from Argentina have been performed. Although, it cannot be assessed if selection against nodulation and therefore nitrogen fixation occurred, our findings suggest that the materials bred in Argentina are highly diverse materials in terms of their ability to nodulate. The use of soybean cultivars with high nodulation capacity would be desirable mainly if symbiotic efficient N fixing associations can be induced with highly efficient strains, which should lead to high levels of N fixed that might impact positively upon plant growth and yield. Alternatively, the energetic cost of a larger nodule mass might be deleterious to the C harvest index of soybeans, as it was found for supernodulating legumes [44].
Considering the economical importance of soybean for Argentina and the large demand of N by the plant and that inoculation is a widely accepted practice among farmers, the ability of genotypes to nodulate and fix nitrogen should be considered by breeders as an important aim while evaluating soybean genotypes that are going through the last steps of evaluation to be released to the market. We confirmed the broad sense heritability of NDW, which also appear to be the trait that varied the most, not to mention that cultivars from different maturity group can be used as sources of nodulation QTLs. Therefore, breeding for nodule dry weight might lead to a faster genetic gain. Cultivars that develop a larger nodule mass in association with highly efficient N fixing bacteria will impact later on seed yield and also on the N content remaining in the soils. We are currently crossing plants and developing markers in order to be able to follow genes along progenies.
5. Acknowledgements
This work was supported by grants from Universidad Nacional de La Plata, Comisión de Investigaciones Científicas de la Provincia de Buenos Aires CICBA and Grant N5 2004 from CABBIO.
REFERENCES
- R. M. Polhill and P. H. Raven, “Advances in Legume Systematics,” Royal Botanic Gardens, Kew, 1981.
- J. J. Doyle, “Phylogeny of the Legume Family: An Approach to Understanding the Origins of Nodulation,” Annual Review of Ecology and Systematics, Vol. 25, No. 1, 1994, pp. 325-349. doi:10.1146/annurev.es.25.110194.001545
- R. H. Burris and G. P. Roberts, “Biological Nitrogen Fixation,” Annual Review of Nutrition, Vol. 13, No. 1, 1993, pp. 317-335. doi:10.1146/annurev.nu.13.070193.001533
- D. C. Jordan, “Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a Genus of Slow-Growing, Root Nodule Bacteria from Leguminous Plants,” International Journal of Systematic Bacteriology, Vol. 32, No. 1, 1982, pp. 136-139. doi:10.1099/00207713-32-1-136
- L. D. Kuykendall, B. Saxena, T. E. Devine and S. E. Udell, “Genetic Diversity in Bradyrhizobium japonicum Jordan 1982 and a Proposal for Bradyrhizobium elkanii sp. nov.,” Canadian Journal of Microbiology, Vol. 38, No. 6, 1992, pp. 501-505. doi:10.1139/m92-082doi:10.1139/m92-082
- L. M. Xu, C. Ge, Z. Cui, J. Li and H. Fan, “Bradyrhizobium liaoningense sp. nov., Isolated from the Root Nodules of Soybeans,” International Journal of Systematic Bacteriology, Vol. 45, No. 4, 1995 pp. 706-711. doi:10.1099/00207713-45-4-706
- M. J. Trinick, “Relationships Amongst the Fast-growing Rhizobia of Lablab purpureus, Leucaena leucocephala, Mimosa spp., Acacia farnesiana and Sesbania grandiflora and Their Affinities with Other Rhizobial Groups,” Journal of Applied Microbiology, Vol. 49, No. 1, 1980, pp. 39-53. doi:10.1111/j.1365-2672.1980.tb01042.x
- G. Saldaña, V. Martinez-Alcántara, J. M. Vinardell, R. Bellogín, J. E. Ruíz-Sainz and P. A. Balatti, “Genetic Diversity of Fast-Growing Rhizobia That Nodulate Soybean (Glycine max L. Merr),” Archives of Microbiology, Vol. 80, No. 1, 2003, pp. 45-52. doi:10.1007/s00203-003-0559-y
- H. H. Keyser, B. B. Bohlool, T. S. Hu and D. F. Weber, “Fast-Growing Rhizobia Isolated from Root Nodules of Soybean,” Science, Vol. 215, No. 4540, 1982, pp. 1631- 1632. doi:10.1126/science.215.4540.1631
- W. X. Chen, G. H. Yan and J. L. Li, “Numerical Taxonomic Study of Fast-Growing Soybean Rhizobia and a Proposal That Rhizobium fredii Be Assigned to Sinorhizobium gen. nov.,” International Journal of Systematic Bacteriology, Vol. 38, No. 4, 1988, pp. 392-397. doi:10.1099/00207713-38-4-392
- G. X. Peng, Z. Y. Tan, E. T. Wang, B. Reinhold-Hurek, W. F. Chen and W. X. Chen, “Identification of Isolates from Soybean Nodules in Xinjiang Region as Sinorhizobium xinjiangense and Genetic Differentiation of S. xinjiangense from Sinorhizobium fredii,” International Journal of Systematic and Evolutionary Microbiology, Vol. 52, No. 2, 2002, pp. 457-462. doi:10.1099/ijs.0.01921-0
- W. Chen, E. Wang, S. Wang, Y. Li, X. Chen and Y. Li, “Characteristics of Rhizobium tianshanense sp. nov., a Moderately and Slowly Growing Root Nodule Bacterium Isolated from an Arid Saline Environment in Xinjiang, People’s Republic of China,” International Journal of Systematic Bacteriology, Vol. 45, No. 1, 1995, pp. 153- 159. doi:10.1099/00207713-45-1-153
- K. Van, M. Y. Kim and S. H. Lee, “Genomic of Root Nodulation,” Genomics-Assisted Crop Improvement, Vol. 2, 2007, pp. 435-452. doi:10.1007/978-1-4020-6297-1_16
- P. A. Balatti, “Competition an Old Unsolved Problem of Rhizobia Nodulating Soybean. The Old Unsolved Problem of Competition,” Proceedings of the VII World Soybean Research Conference, Foz do Iguazú, 5 February 2004.
- B. E. Caldwell, “Inheritance of a Strain-Specific Ineffective Nodulation in Soybeans,” Crop Science, Vol. 6, No. 5, 1966, pp. 427-428. doi:10.2135/cropsci1966.0011183X000600050010x
- G. Vest, “Rj3 a Gene Conditioning Ineffective Nodulation in Soybean,” Crop Science, Vol. 10, No. 1, 1970, pp. 34-35. doi:10.2135/cropsci1970.0011183X001000010013x
- G. Vest and B. E. Caldwell, “Rj4—A Gene Conditioning Ineffective Nodulation in Soybean,” Crop Science, Vol. 12, No. 5, 1972, pp. 692-693. doi:10.2135/cropsci1972.0011183X001200050042x
- J. E. Pracht, C. D. Nickell and J. E. Harper, “Genes Controlling Nodulation in Soybean: Rj5 and Rj6,” Crop Science, Vol. 33, No. 4, 1993, pp. 711-713. doi:10.2135/cropsci1993.0011183X003300040014x
- M. Nicolás, C. A. Arias and M. Hungria, “Genetics of Nodulation and Nitrogen Fixation in Brazilian Soybean Cultivars,” Biology and Fertility of Soils, Vol. 36, No. 2, 2002, pp. 109-117. doi:10.1007/s00374-002-0511-3
- M. F. Nicolás, M. Hungria and C. A. A. Arias, “Identification of Quantitative Trait Loci Controlling Nodulation and Shoot Mass in Progenies from Two Brazilian Soybean Cultivars,” Field Crops Research, Vol. 95, No. 2-3, 2006, pp. 355-366. doi:10.1016/j.fcr.2005.04.012
- M. A. dos Santos, M. F. Nicolás and M. Hungria, “Identificação de QTL Associados à Simbiose Entre Bradyrhizobium japonicum, B. elkanii e soja,” Pesquisa Agropecuária Brasileira, Vol. 41, No. 1, 2006, pp. 67-75. doi:10.1590/S0100-204X2006000100010
- S. Jitacksorn and M. J. Sadowsky, “Nodulation Gene Regulation and Quorum Sensing Control Density-Dependent Suppression and Restriction of Nodulation in the Bradyrhizobium japonicum-Soybean Symbiosis,” Applied and Environmental Microbiology, Vol. 74, No. 12, 2008, pp. 3749-3756. doi:10.1128/AEM.02939-07
- C. M. Waters and B. L. Bassler, “Quorum Sensing: Cellto-Cell Communication in Bacteria,” Annual Review of Cell and Developmental Biology, Vol. 21, No. 1, 2005, pp. 319-346. doi:10.1146/annurev.cellbio.21.012704.131001
- S. T. Takats, “Suppression of Nodulation in Soybeans by Superoptimal Inoculation with Bradyrhizobium japonicum,” Physiologia Plantarum, Vol. 66, No. 4, 1986, pp. 669-673. doi:10.1111/j.1399-3054.1986.tb05597.x
- J. Loh and G. Stacey, “Nodulation Gene Regulation in Bradyrhizobium japonicum: A Unique Integration of Global Regulatory Circuits,” Applied and Environmental Microbiology, Vol. 69, No. 1, 2003, pp. 10-17. doi:10.1128/AEM.69.1.10-17.2003
- J. Döbereiner, “Evaluation of Nitrogen Fixation in Legumes by the Regression of Total Plant Nitrogen with Nodule Weight,” Nature, Vol. 210, No. 5038, 1966, pp. 850-852. doi:10.1038/210850a0
- T. R. J. Bohrer and M. Hungria, “Avaliação de Cultivares de soja Quanto à Fixação Biológica do Nitrogênio,” Pesquisa Agropecuária Brasileira, Vol. 33, No. 6, 1998, pp. 937-953.
- M. Hungria and T. R. J. Bohrer, “Variability of Nodulation and Dinitrogen Fixation Capacity among Soybean Cultivars,” Biology and Fertility of Soils, Vol. 31, No. 1, 2000, pp. 45-52. doi:10.1007/s003740050622
- J. Stougaard, “Regulators and Regulation of Legume Root Nodule Development,” Plant Physiology, Vol. 124, No. 2, 2000, pp. 531-540. doi:10.1104/pp.124.2.531
- A. Chatterjee, P. A. Balatti, W. Gibbons and S. G. Pueppke, “Interaction of Rhizobium fredii USDA257 and Nodulation Mutants Derived from it with the Agronomically Improved Soybean Cultivar McCall,” Planta, Vol. 180, No. 3, 1990, pp. 303-311. doi:10.1007/BF01160385
- J. M. Vincent, “Manual for the Practical Study of Root Nodule Bacteria,” Blackwell, Oxford, 1970.
- R. R. Sokal and F. J. Rohlf, “Biometry: The Principals and Practice of Statistics in Biological Research,” W.H. Freeman and Company, San Francisco, 1995.
- M. S. McIntosh, “Analysis of Combined Experiments,” Agronomy Journal, Vol. 75, No. 1, 1983, pp. 153-155. doi:10.2134/agronj1983.00021962007500010041x
- C. H. Hanson, H. F. Robinson and R. E. Comstock, “Biometrical Studies of Yield in Segregating Populations of Korean Lespedeza,” Agronomy Journal, Vol. 48, No. 6, 1956, pp. 268-272. doi:10.2134/agronj1956.00021962004800060008x
- Secretaria de Agricultura Ganadería y Pesca (SAGPyA), Argentina, 2009. http://www.sagpya.mecon.gov.ar/
- A. A. Yusuf, R. C. Abaidoo, E. N. O. Iwafor and O. O. Olufajo, “Genotype Effects of Cowpea and Soybean on Nodulation, N2-Fixation and N Balance in the Northern Guinea Savanna of Nigeria,” Journal of Agronomy, Vol. 7, No. 3, 2008, pp. 258-264. doi:10.3923/ja.2008.258.264
- J. H. Voorhees, “Variations in Soybean Inoculation,” Agronomy Journal, Vol. 7, No. 3, 1915, pp. 139-140. doi:10.2134/agronj1915.00021962000700030006x
- P. S. Nutman, “Genetics of Symbiosis and Nitrogen Fixation in Legumes,” Proceedings of the Royal Society of London. Series B. Biological Sciences, Vol. 172, No. 1029, 1969, pp. 417-437. doi:10.1098/rspb.1969.0030
- T. R. Sinclair, A. R. Soffes, K. Hinson, S. L. Albrecht and P. L. Pfahler, “Genotypic Variation in Soybean Nodule Number and Weight,” Crop Science, Vol. 31, No. 2, 1991, pp. 301-304. doi:10.2135/cropsci1991.0011183X003100020014x
- T. A. Lie, “Environmental Effects on Nodulation and Symbiotic Nitrogen Fixation,” In: A. Quispel, Ed., The Biology of Nitrogen Fixation, North Holland Publishing Company, Amsterdan, 1974, pp. 555-582.
- H. S. Lee and S. H. Lee, “Introduction, Development and Characterization of Supernodulating Soybean MutantNitrate Inhibition of Nodulation and Nitrogen Fixation in Supernodulating Soybean Mutant,” Korean Journal of Crop Science, Vol. 43, No. 1, 1998, pp. 23-27.
- P. A. Balatti and S. G. Pueppke, “Identification of North American Soybean Lines That Form Nitrogen-Fixing Nodules with Rhizobium fredii USDA257,” Canadian Journal of Plant Science, Vol. 72, No. 1, 1992, pp. 49-55. doi:10.4141/cjps92-006
- R. R. Greder, J. H. Orf and J. W. Lambert, “Heritabilities and Associations of Nodule Mass and Recovery of Bradyrhizobium japonicum Serogroup USDA 110 in Soybean,” Crop Science, Vol. 26, No. 1, 1986, pp. 33-37. doi:10.2135/cropsci1986.0011183X002600010007x
- K. Novák, E. Biedermannová and J. Vondrys, “Symbiotic and Growth Performance of Supernodulating Forage Pea Lines,” Crop Science, Vol. 49, No. 4, 2009, pp. 1227- 1234. doi:10.2135/cropsci2008.06.0341
NOTES
*Corresponding author.

